1. Introduction
The field of organofluorine chemistry is a vibrant research area in which the quest for innovation towards molecular diversity is steadily increasing [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]; for examples, see [13, 14]. Indeed, the unique properties of the most electronegative element of the periodic table to modify the physical and chemical properties of the molecule [15, 16], in which it is incorporated, render fluorinated molecules essential in various fields including materials, agrochemical, and pharmaceutical industries [17, 18, 19, 20]. Incorporating fluorine-containing motifs in the search for new drugs remains a powerful approach to developing new products for enhanced societal benefits. To push beyond the frontiers of knowledge, one paradigm was to design and study original functionalized fluorinated moieties as a potent alternative to the classical fluorine atom and the CF3 group generally found in molecules. Although the synthesis route to these newly designed fluorinated-group-containing molecules remains quite restricted compared to the large number of existing fluorinated and trifluoromethylated molecules, major advances have been made over the years. Recently, attention has been focused on the CF2H residue and related groups (e.g., SCF2H and OCF2H groups) [21]. In the quest to find original fluorinated moieties with unique properties, the design of straightforward tools for the synthesis of CF2FG- and SCF2FG-containing molecules has garnered significant interest [22, 23, 24, 25]. These fluorinated moieties are appealing thanks to their unique properties, reactivity, degradation pathways, and a high modularity potential (towards other fluorinated moieties), and provide medicinal chemists a powerful toolbox for new drug discovery. Hence, the research field has been rapidly growing over the past years. Retrosynthetic disconnections to produce these compounds might rely on different pathways, namely (1) building the fluorinated moieties and (2) its direct incorporation into the target molecules; we focused on the last approach with the synthesis of suitable reagents. The account provides an overview of our main contributions to the field with the design of original reagents and their applications. Therefore, the review is organized according to the nature of the fluorinated moieties and their applications.
2. Design of CF2SO2Ph- and CHFMe-containing reagents
2.1. Design of a bench-stable PhSO2CF2-containing reagent for C–H electrophilic (phenylsulfonyl)difluoromethylation [26]
In our quest towards emergent fluorinated groups, we focused on the PhSO2CF2 residue. The latter is an important precursor of fluorinated units of interest including difluoromethyl (HCF2), difluoromethylene (–CF2–), and difluoromethylidene (=CF2) groups. Different approaches have been developed to build up a PhSO2CF2–C bond. While most of them relied on the use of nucleophilic sources of the (phenylsulfonyl)difluoromethyl residue or via radical processes, the number of electrophilic sources is in sharp contrast rather limited. Pioneering works have been conducted by the groups of Hu [27, 28, 29] and Shibata [30]. With an electrophilic source derived from hypervalent iodine, Hu and co-workers developed an efficient reagent for the functionalization of thiols as well as in copper-catalyzed vinyl and allyl (phenylsulfonyl)difluoromethylation reactions. In the case of Shibata, the functionalization of C(sp3) centers of β-ketoesters, β-diketones, and dicyanoalkylidenes was achieved thanks to a sulfonium salt. Inspired by these major advances, we synthesized a S-([phenylsulfonyl]difluoromethyl)dibenzothiophenium salt (Scheme 1). Key features related to this electrophilic reagent are large-scale synthesis (an approximately 40 g scale without the need for ozone depleting reagents) [31, 32] and three-step/one-purification synthesis from suitable biaryl disulfide and (difluoromethyl)sulfonylbenzene in 51% overall yield with an estimated global cost of about 10.6 €/g. Thanks to a transition metal free process, the (phenylsulfonyl)difluoromethylation of various classes of compounds was successfully achieved. A panel of aniline derivatives and electron-rich (hetero)arenes as well as phenol and anisole derivatives underwent this transformation. Moreover, the potential of reagent I was further demonstrated by the functionalization of C(sp3)-centered nucleophiles and for S–CF2SO2Ph bond formation. The synthetic utility of this functionalized fluorinated moiety was illustrated by its conversion into the high-value-added HCF2 group, the late functionalization of bioactive molecules, and the synthesis of lidocaine analogues bearing either a CF2H or a CF2SO2Ph motif. To ensure a safe process, the stability of this reagent and key intermediates of its synthesis was studied by differential scanning calorimetry measurements [31, 32].
Design of a bench-stable PhSO2CF2-containing reagent I for C–H electrophilic (phenylsulfonyl)difluoromethylation. (a) Reaction conditions: aniline (3 equiv.), I (0.3 mmol, 1 equiv.), CH2Cl2, 21 °C, 24 h, Ar. (b) Reaction conditions: phenol derivatives or (hetero)arenes (1.5 equiv.), I (1.0 equiv.), DMF, 50 °C, 3 h, Ar.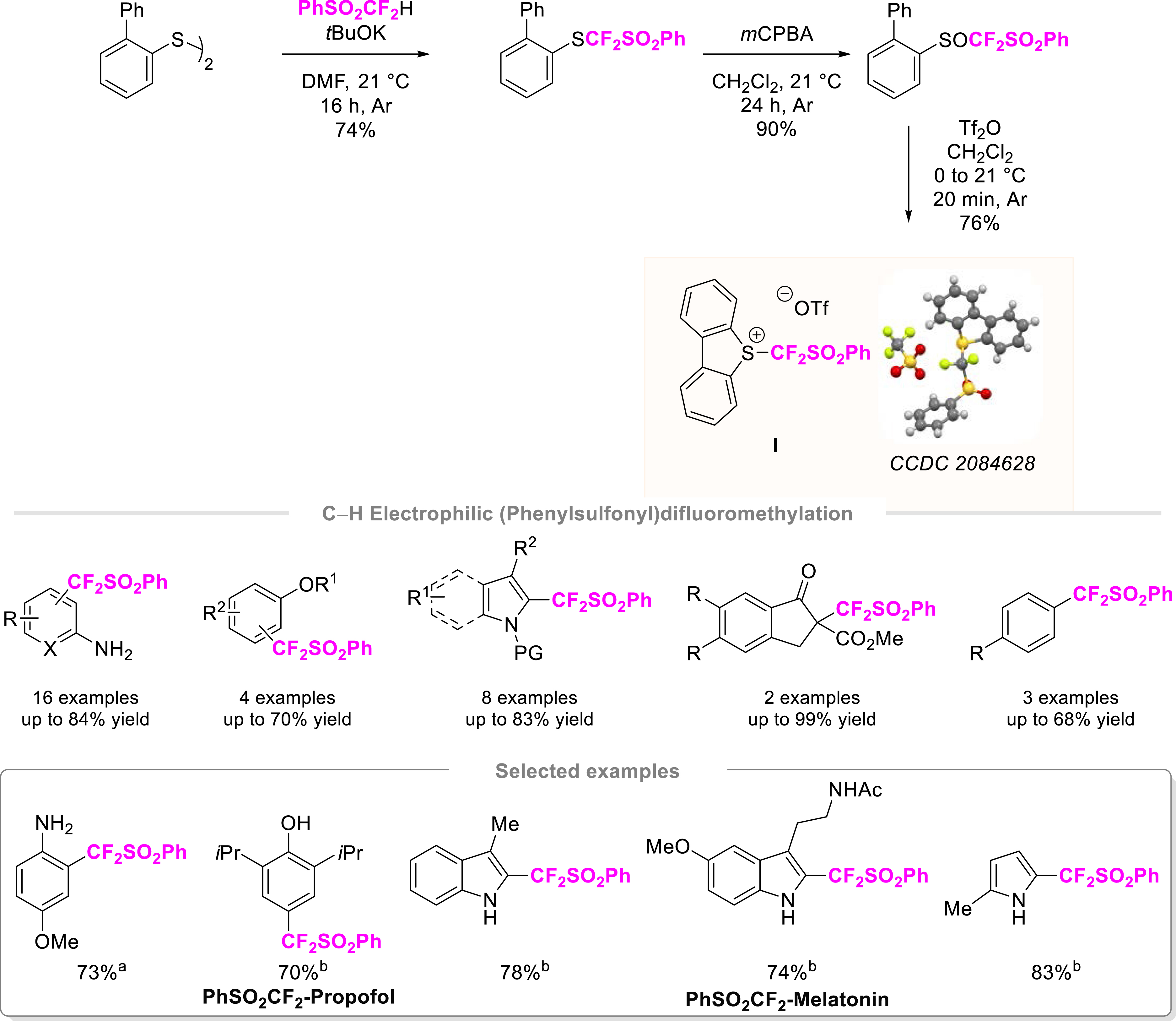
We then explored other applications of this reagent to delineate its potential [33]. As part of our ongoing research about transition-metal-catalyzed C–H bond functionalization, S-([phenylsulfonyl]difluoromethyl)dibenzothiophenium salt I was applied as an electrophilic source for the first copper-catalyzed (phenylsulfonyl)difluoromethylation of the vinylic-C(sp2)–H bond of acrylamides under robust reaction conditions (80 °C, air; Scheme 2). Using a native functional group, the transformation turned out to be completely Z-selective. To further showcase the interest in this method from an industrial perspective, a copper-mediated alternative was also smoothly developed. Mechanistic studies supported by DFT calculations enabled identifying the true nature of the active catalyst and shed light on the critical roles of TFA and NMF used as additives in the reaction.
Copper-catalyzed and mediated C–H (phenylsulfonyl)difluoromethylation of acrylamides.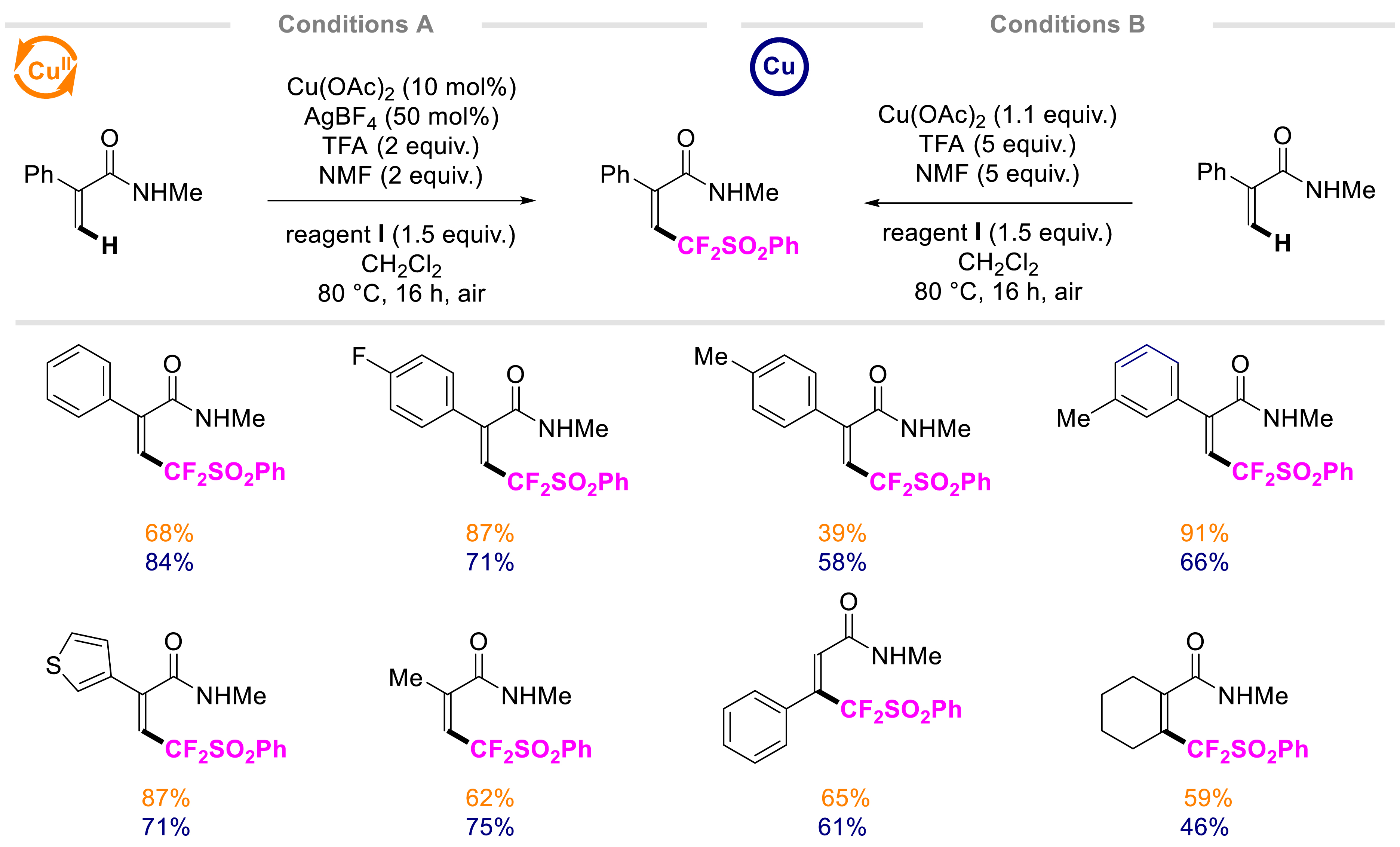
2.2. Original synthetic pathway for the synthesis of OCHFMe [34]
Due to the recent demand for the OCF3 group, there is strong interest in providing synthetic tools for the efficient synthesis of OCHFMe-containing molecules (Scheme 3). With this in mind, we developed the synthesis of novel reagent II, an electrophilic source of CHFMe. The bench-stable, air- and moisture-tolerant sulfonium salt II was prepared as a 3:1 mixture of diastereoisomers from thiophenol in five steps. The functionalization of a variety of phenols and alcohols was smoothly achieved with a good functional group tolerance (CN, ketone, ester, and nitro groups). The functionalization of heterocyclic derivatives is of high importance in pharmaceutical and agrochemical research as illustrated with 3-hydroxyquinoline. Under the same reaction conditions, homobenzylic alcohol was converted into the OCHFMe derivative in 59% yield. Interestingly, the approach was further applied to construct the SCHFMe motif. It is worth mentioning that these thiophenols can be further converted into the corresponding sulfones.
Synthesis of an electrophilic source II of CHFMe and applications. (a) Reaction conditions: reagent II (1.0 equiv.), ROH (1.2 equiv.), Cs2CO3 (1.2 equiv.), MeCN, rt, 16 h. (b) Reaction conditions: reagent II (1.0 equiv.), RSH (1.2 equiv.), Cs2CO3 (1.2 equiv.), MeCN, rt, 16 h. (i) mCPBA, CH2Cl2, rt, 2 h.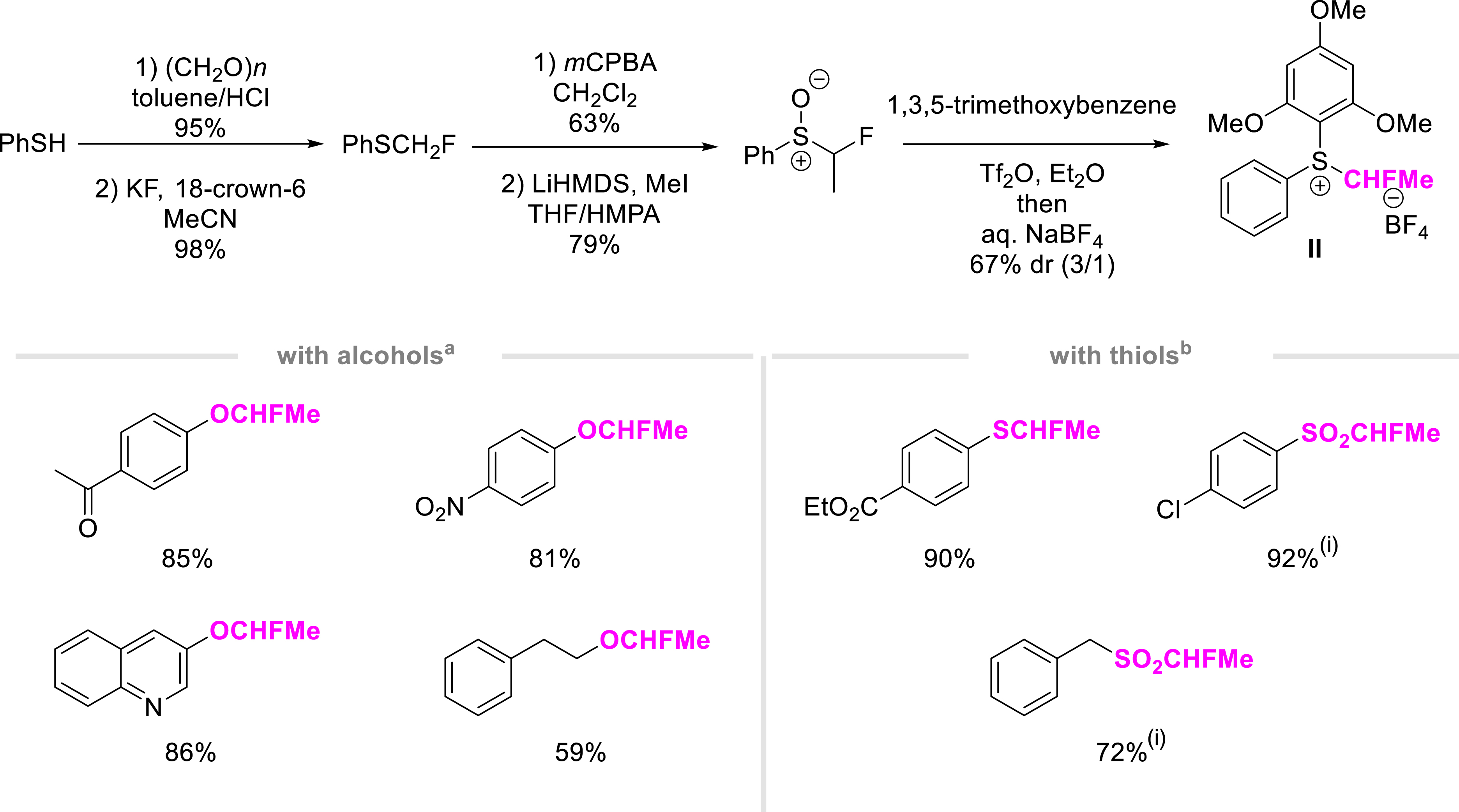
3. Original reagents as sulfur-containing fluorinated group sources
The design for unprecedented reagents as sources of sulfur-containing fluorinated groups has garnered interest, and key players in the field have been particularly active in providing new synthetic tools [22, 23, 24, 25].
In the past years, we have pursued our longstanding aim of developing synthetic routes to difluoromethylthiolated scaffolds. Hence, we have designed and synthesized a library of reagents, capable of acting as both electrophilic and radical sources of SCF2FG residues (FG = PO(OEt)2, CO2Et). These reagents have opened up new vistas in reactivity and possible transformations beyond what could historically be achieved using nucleophilic sources, widening further the chemical space of functionalized fluorinated molecules. Proving an array of original CF2FG and SCF2FG sources would be useful for the organic and medicinal chemist, providing tools for exploring new chemical spaces as well as offering new perspectives in drug discovery programs. We believe that innovation and future developments related to the residues of the non-persistent fluorinated moieties CF2R and SCF2R will constitute an interesting and promising lead to follow for developing new products for enhanced societal benefits.
3.1. New tools for forging C–SCF2PO(OEt)2 bonds [35, 36]
In recent years, there has been considerable interest in the synthesis of molecules substituted with SCF2H and SCF2FG (FG = functional group). Conventional methods involve introducing the CF2H motif onto sulfur derivatives. However, since 2015, new tools have enabled the direct introduction of SCF2H and SCF2FG moieties, which is a considerable advance. In this context, we chose to focus on the SCF2PO(OEt)2 moiety, a novel fluorinated moiety (Hansch–Leo parameter (π) of 0.76). Indeed, benefiting from an efficient route to prepare anilines N-substituted with an SCN group [37], we synthesized a new electrophilic reagent III enabling the introduction of the SCF2PO(OEt)2 group starting from N-SCN-mesityl aniline via the in situ generation of a CuCF2PO(OEt)2 species (Scheme 4) [35].
Synthesis of an electrophilic SCF2PO(OEt)2-containing reagent and the study of its applications. Reaction conditions: (i) CH3COCl (3.0 equiv.), reagent III (1.8 equiv.), NMP, 25 °C, 18 h, air. (ii) TsOH (2.5 equiv.), reagent III (1.2 equiv.), CH2Cl2, 25 °C, 2 h, air. (iii) MsOH (1.2 equiv.), reagent III (1.0 equiv.), CH2Cl2, 25 °C, 14 h, air. (iv) TFA (1.2 equiv.), reagent III (1.2 equiv.), CH2Cl2, 40 °C, 24 h, air. (a) BiCl3 (2 equiv.), reagent III (1.2 equiv.), H2O (2 equiv.), DCE, 45 °C, 40 h, Ar. (b) BiCl3 (1.8 equiv.), reagent III (1.2 equiv.), H2O (1.8 equiv.), DCE, 60 °C, 6 h, Ar.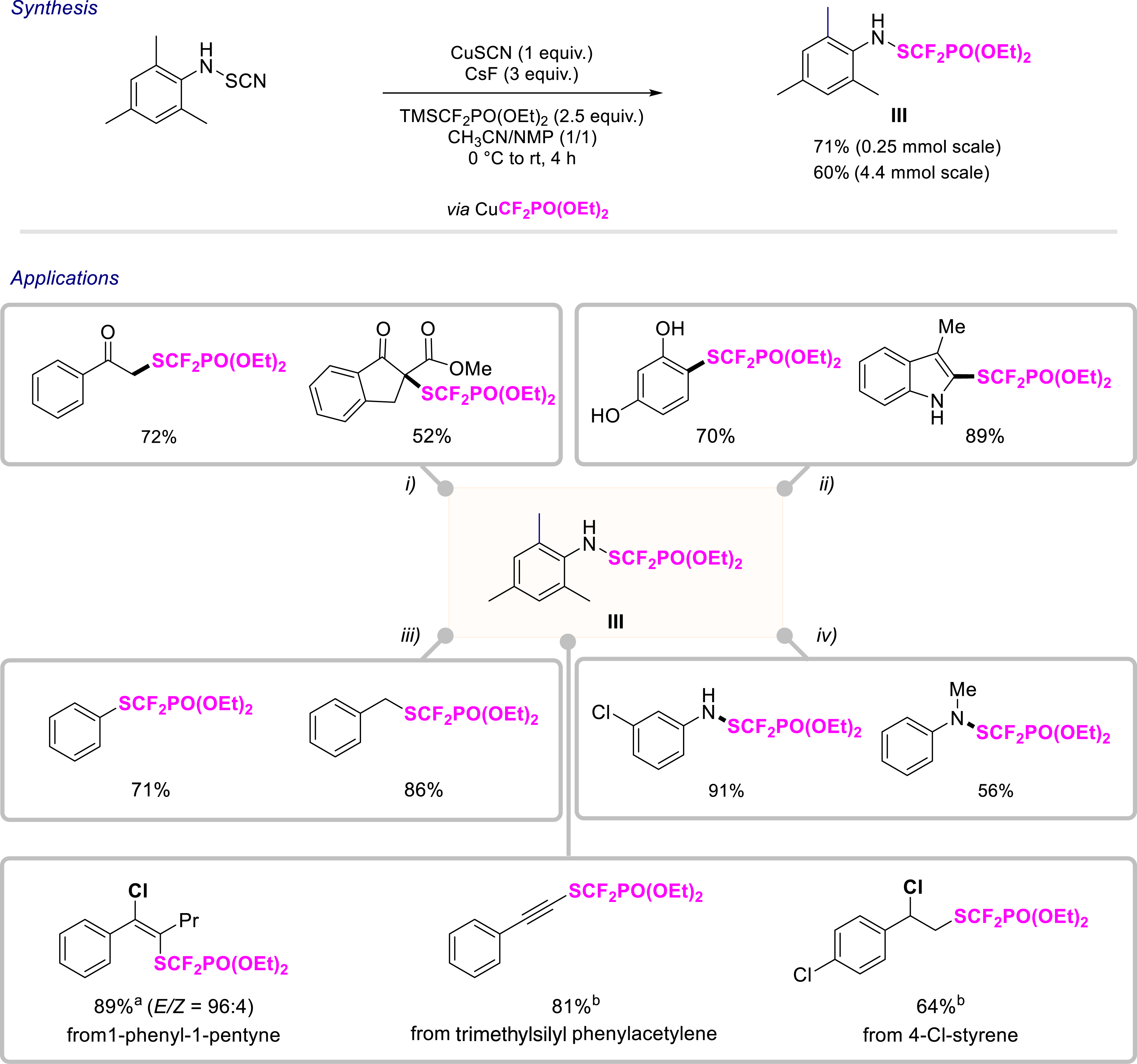
This reagent was shown to introduce the SCF2PO(OEt)2 group onto a number of nucleophiles. First, the electrophilic character of this reagent was evaluated in Friedel–Crafts reactions. In this way, several electron-rich indole or aromatic derivatives could be functionalized. This reaction was extended to the functionalization of acetophenone derivatives or a β-ketoester. Remarkably, the transfer of this motif from the reagent to other primary and secondary anilines, was also achieved. Finally, the synthesis of non-symmetrical disulfides was carried out and aromatic, heteroaromatic, and benzylic derivatives were obtained. Furthermore, to demonstrate the synthetic utility of our approach, we synthesized diflumidone in three steps from 3-aminobenzophenone in 74% overall yield, hitherto synthesized in 28% yield from the same aniline. With this new electrophilic source III in hand, we turned our attention to the functionalization of other classes of compounds, in particular unsaturated alkene and alkyne derivatives enabling the formation of C(sp)–, C(sp2)–, and C(sp3)–SCF2PO(OEt)2 bonds. A BiCl3-mediated difunctionalization reaction of alkynes and alkenes enabled the synthesis of many novel aliphatic and vinyl molecules substituted with the SCF2PO(OEt)2 moiety (40 examples, up to 89% yield). In addition, an original and selective synthetic route for obtaining alkynes featuring the SCF2PO(OEt)2 motif has been developed [36].
Although reagent III is effective in many reactions, some synthetic limitations remain. As a result, a novel source of SCF2PO(OEt)2 enabled to be used in radical processes [24] was developed [38]. Thus S-(diethyl phosphonodifluoromethyl)benzenesulfonothioate, reagent IV, was synthesized in one step from reagent III in the presence of sodium para-toluenesulfinate in acetic acid for 16 h at room temperature (Scheme 5). The process is robust as IV was isolated in 81% yield on a 1.13 mmol scale and 89% yield on a 3.25 mmol scale. We studied the stability of reagent IV. Although its decomposition was observed in the presence of various bases in dichloromethane as a solvent, IV was quite stable under acidic conditions. The functionalization of a wide variety of aldehydes with NaN3 and PIFA in EtOAc (26 examples, up to 87% yield) and the synthesis of unsymmetrical (hetero)aromatic, benzylic, and aliphatic disulfides (12 examples, up to 91% yield) were smoothly achieved in the presence of LiBr in HFIP.
Synthesis of S-(diethyl phosphonodifluoromethyl)benzenesulfonothioate and its applications.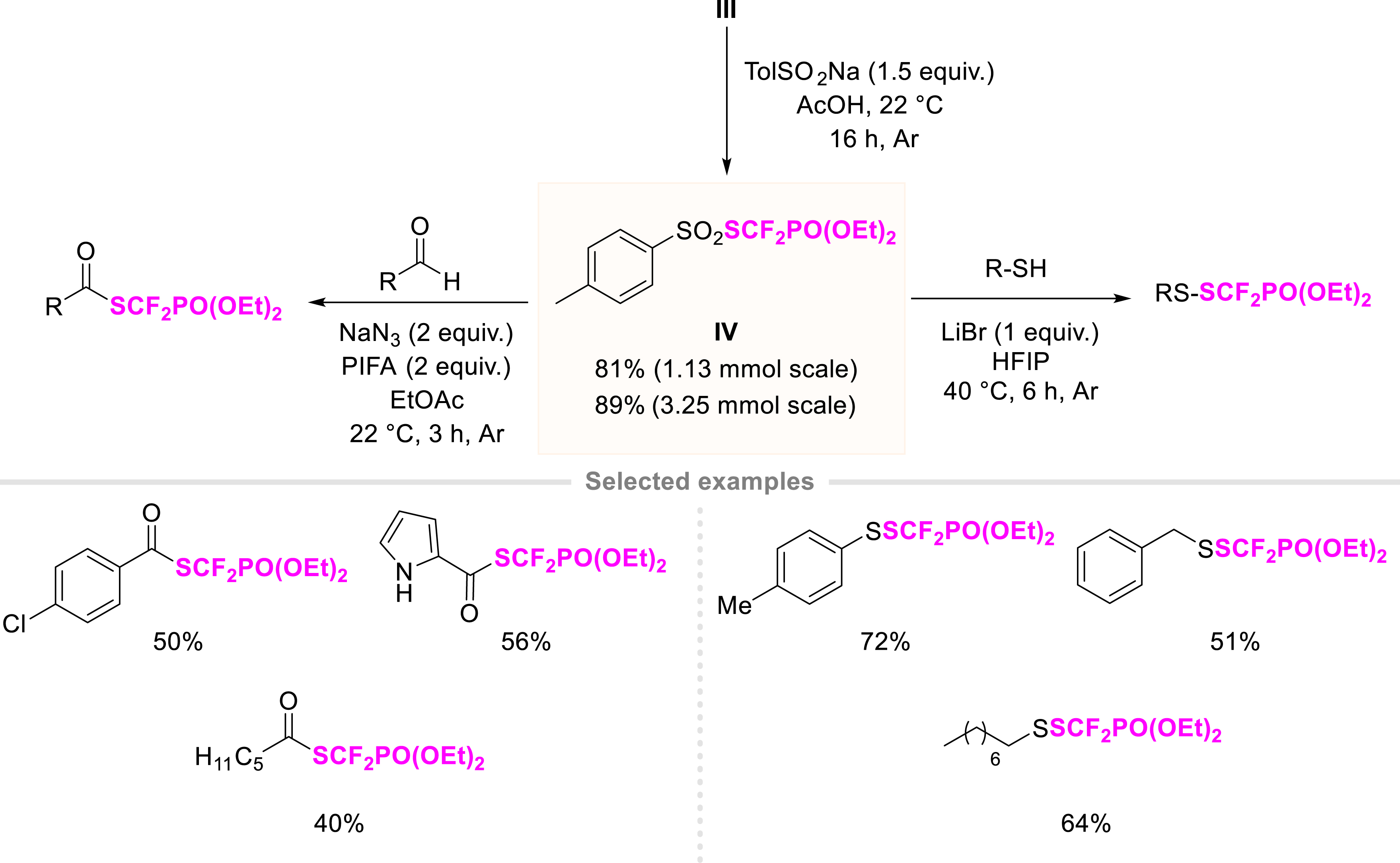
3.2. Extension to SCF2CO2Et residues
Intrigued about the potential of these ArSO2SCF2FG reagents, we investigated the SCF2CO2Et series (Scheme 6) [39]. A library of [(ethoxycarbonyl)difluoromethyl]arylsulfonothioate reagents was synthesized efficiently regardless of substitution patterns and the nature of substituents (11 examples, up to 98% yield) using an in-house synthesized PhNHCF2CO2Et reagent [40]. To valorize this library of reagents, (ethoxycarbonyl)difluoromethylthiolation of benzaldehyde was achieved in the presence of PIFA, sodium azide, and the designed reagents. It turned out that the nature of substituents on these reagents strongly impacted the efficiency of the transformation with aldehydes. Thus, an efficient synthetic route to (ethoxycarbonyl)difluoromethylthioesters was developed using reagent V (11 examples, up to 93% yield), broadening the chemical space of SCF2CO2Et-containing molecules.
Synthesis of a library of [(ethoxycarbonyl)difluoromethyl]arylsulfonothioate reagents and the synthesis route to (ethoxycarbonyl)difluoromethylthioesters.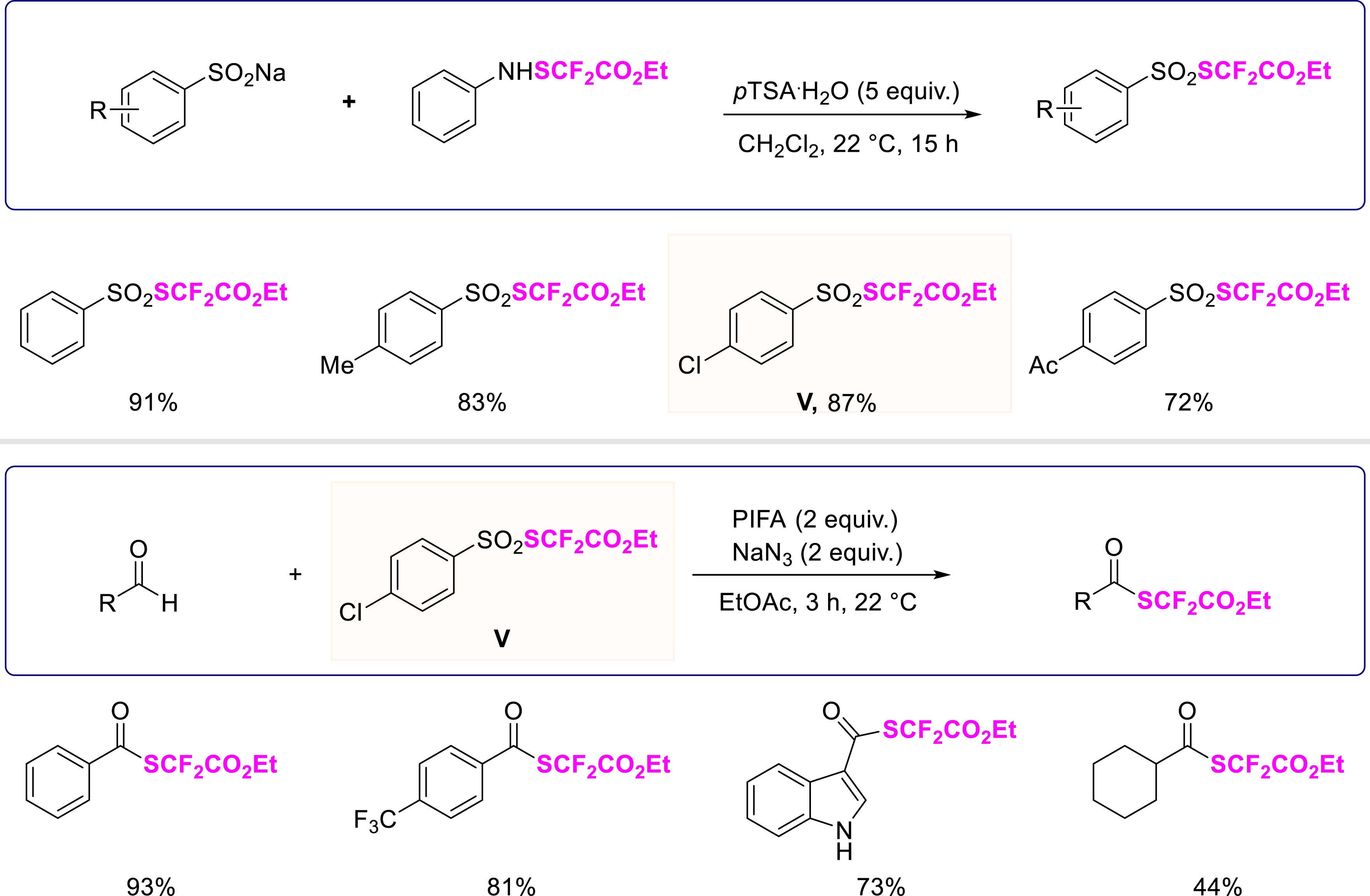
3.3. Application of designed reagents to distal functionalization of unactivated C(sp3)–H bond
The newly designed reagents IV and V were also successfully applied to the challenging distal C(sp3)–H bond functionalization by means of a HAT process (Scheme 7). Indeed, primary, secondary, and tertiary alcohols were selectively functionalized at the δ-position by various fluorinated groups (SCF3, SCF2H, SCF2PO(OEt)2, and SCF2CO2Et) [41, 42]. This metal-free process was possible thanks to the astute use of phenyliodine(III) diacetate (PIDA) under blue light irradiation [43].
Construction of a carbon–SCF2R bond on aliphatic alcohols at a distal position via a 1,5-hydrogen atom transfer.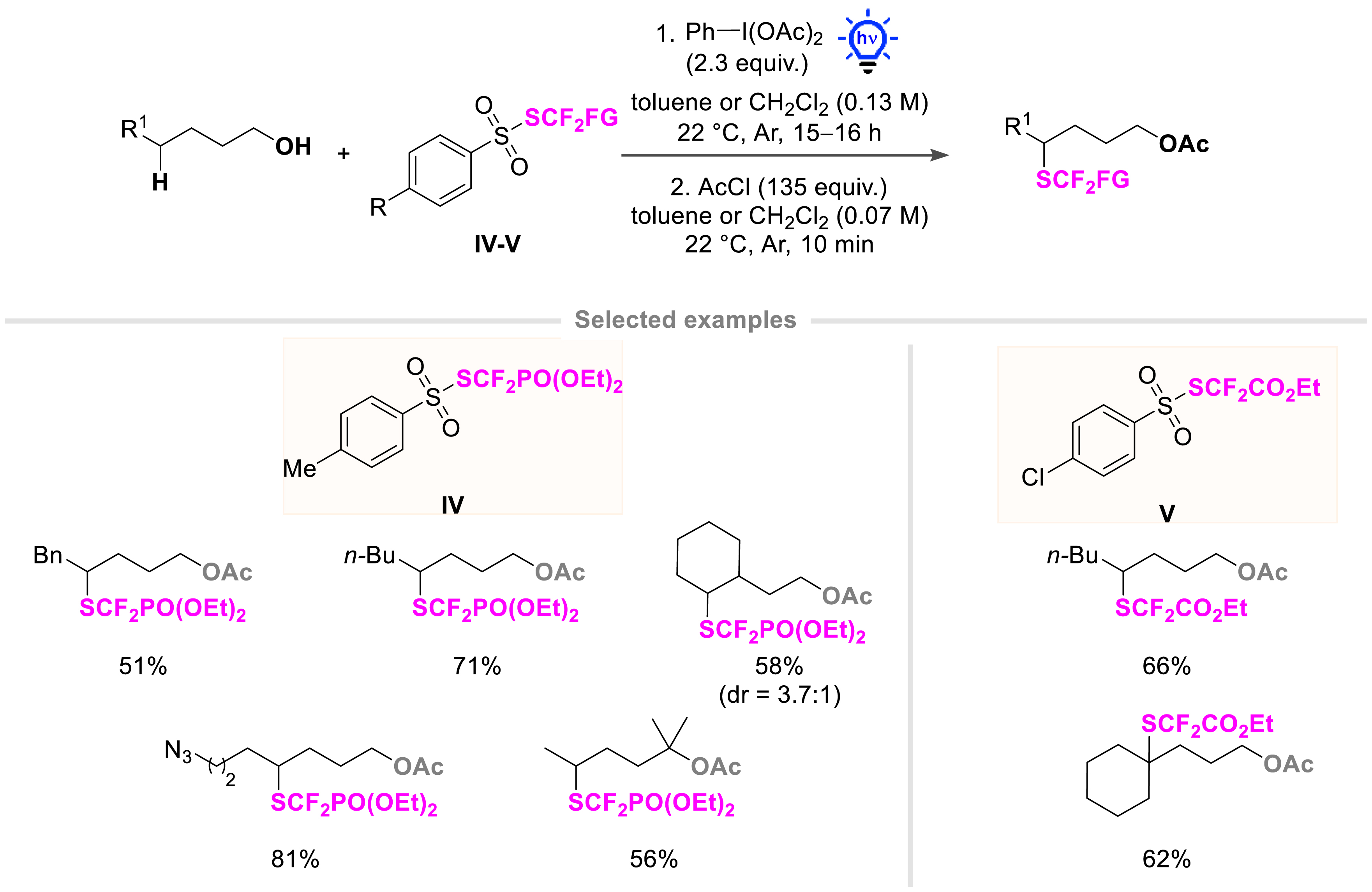
4. Conclusion
Over the years, synthetic developments have been made to push forward the boundaries of organofluorine chemistry, especially a new trend based on functionalized fluorinated residues. In this account, an overview of our recent contributions to expand the toolbox for emerging fluorinated groups is provided. By breaking away from the traditional fluorine atom and trifluoromethyl group, these newly designed fluorinated groups provide new opportunities by bringing about a high degree of modularity. Although in its infancy, the design of tools and synthetic transformations to produce functionalized fluorinated residues will offer interesting perspectives for drug discovery together with more sustainable fluorinated molecules and key solutions to face contemporary societal challenges.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Acknowledgments
This work has been partially supported by University of Rouen Normandy, INSA Rouen Normandy, Centre National de la Recherche Scientifique (CNRS), European Regional Development Fund (ERDF), Labex SynOrg (ANR-11-LABX-0029), Carnot Institute I2C, Graduate School of Research XL-Chem (ANR-18-EURE-0020 XL CHEM), and Region Normandie. TB thanks the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 758710). JR thanks the French National Research Agency (ANR-21-CE07-0035) for a doctoral fellowship. ZC thanks the CSC for a doctoral fellowship. The French National Research Agency (ANR-21-CE07-0035-02 and ANR-22-CE92-0083) is gratefully acknowledged for generous financial support.





 CC-BY 4.0
CC-BY 4.0