Version française abrégée
1 Introduction
Le germanium intervient dans la fabrication de nombreux matériaux de haute technologie : composants électroniques, fibres optiques, optique infrarouge [11]. Les principaux types de gisements exploités ont été répertoriés [3]. Des teneurs anomales en Ge ont été jadis détectées dans des concentrés de pyrite du gisement épithermal à AuAg de Roşia Montană (monts Apuseni, Roumanie [1]). Des études microscopiques, au MEB et à la microsonde électronique ont été réalisées sur plusieurs échantillons de la veine Cârnicel, afin d'identifier les phases porteuses ainsi que leur distribution et d'évaluer le potentiel de récupération de Ge.
2 Géologie et minéralogie
Le gisement de Roşia Montană (Fig. 1) est lié au volcanisme d'âge Néogène présent tout au long de la chaîne Carpatho-Balkanique [15]. Dans cette région, le socle cristallin est présent sous forme de fragments dans différentes colonnes de brèches [27]. Des flyschs d'âge Crétacé supérieur et une formation volcano-sédimentaire d'âge Néogène (Badenien–Sarmatien) couvrent l'essentiel de la zone étudiée [5,6]. Les roches volcaniques néogènes sont représentées par la dacite de Cetate (dômes de Cetate et de Cârnic) et des pyroclastites avant la mise en place d'andésites stériles [4,6,8,14].
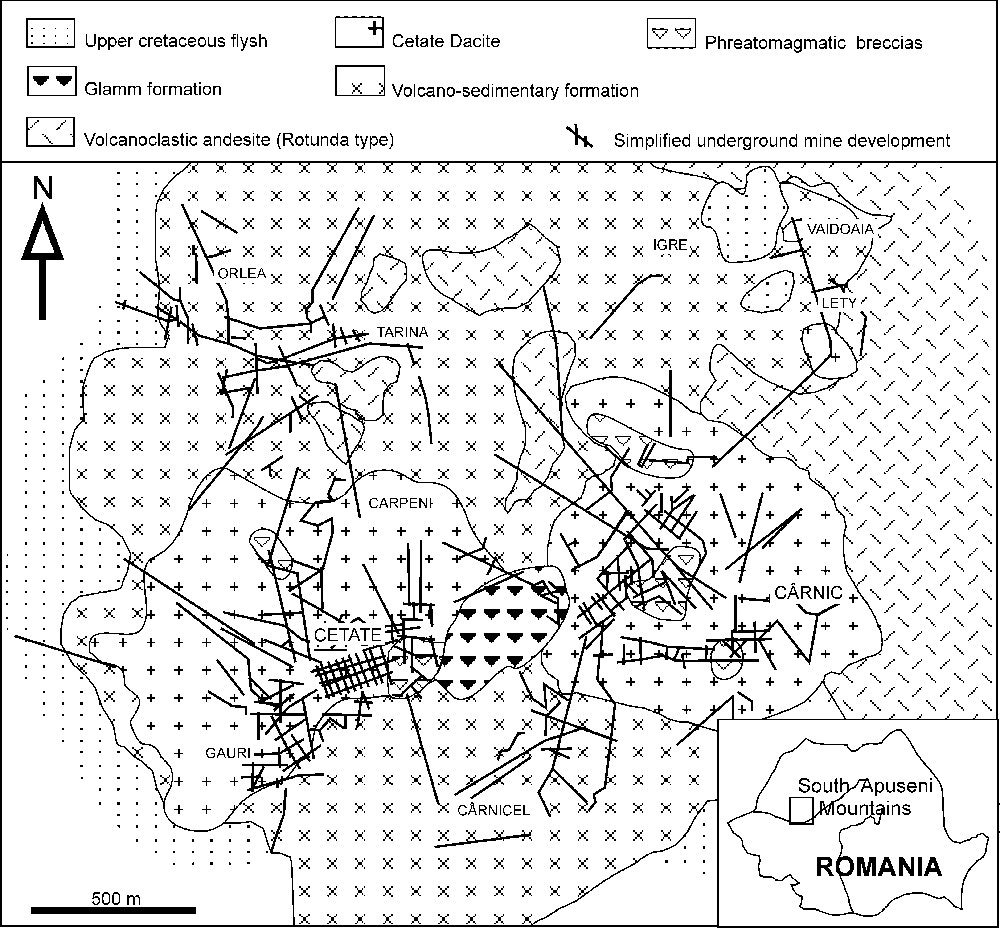
Simplified geological map of the Roşia Montană ore deposit (modified from [6]).
Carte géologique simplifiée du gisement de Roşia Montană (modifiée d'après [6]).
Les échantillons ont été prélevés dans la veine Cârnicel, riche en Ag (environ 1 kg/t Ag et 5 g/t Au). Cette veine est caractérisée par une alternance de rubans de minéraux de gangue (rhodochrosite–rhodonite–quartz) et de rubans minéralisés. La minéralisation se compose de galène, de sphalérite, de chalcopyrite, de tétraédrite, de pyrite et d'alabandite. Cet assemblage contient également d'abondants tellurures (hessite ± altaïte, argyrodite, sylvanite) et de rares grains d'électrum. La roche encaissante est une brèche phréatomagmatique extracratérielle composée de fragments de dacite, de roches sédimentaires et cristallines dans une matrice silto-argileuse.
3 Argyrodite
En section polie, l'argyrodite montre une association fréquente avec la hessite (Fig. 2). Sa couleur est grise, avec une arrière-teinte bleutée, qui contraste avec l'argyrodite de référence. Les analyses réalisées à la microsonde électronique permettent de calculer une formule chimique moyenne proche de Ag8,04Ge0,9S3,77Te2,07 (Tableau 1). Une solution solide entre argyrodite et canfieldite existe [30] et de nombreuses occurrences de canfieldites riches en Te ont été décrites [10,13,16–19,25,31] (Tableau 2). Dans le diagramme de la Fig. 3, les points d'analyses de l'argyrodite sont groupés autour d'une composition théorique proche de Ag8GeTe2S4 (Fig. 3), mais plusieurs analyses montrent des rapports S/Te supérieurs à 2 et suggèrent la possibilité d'une solution solide entre Ag8GeS6 et Ag8GeTe6, équivalent synthétique de l'argyrodite [7].
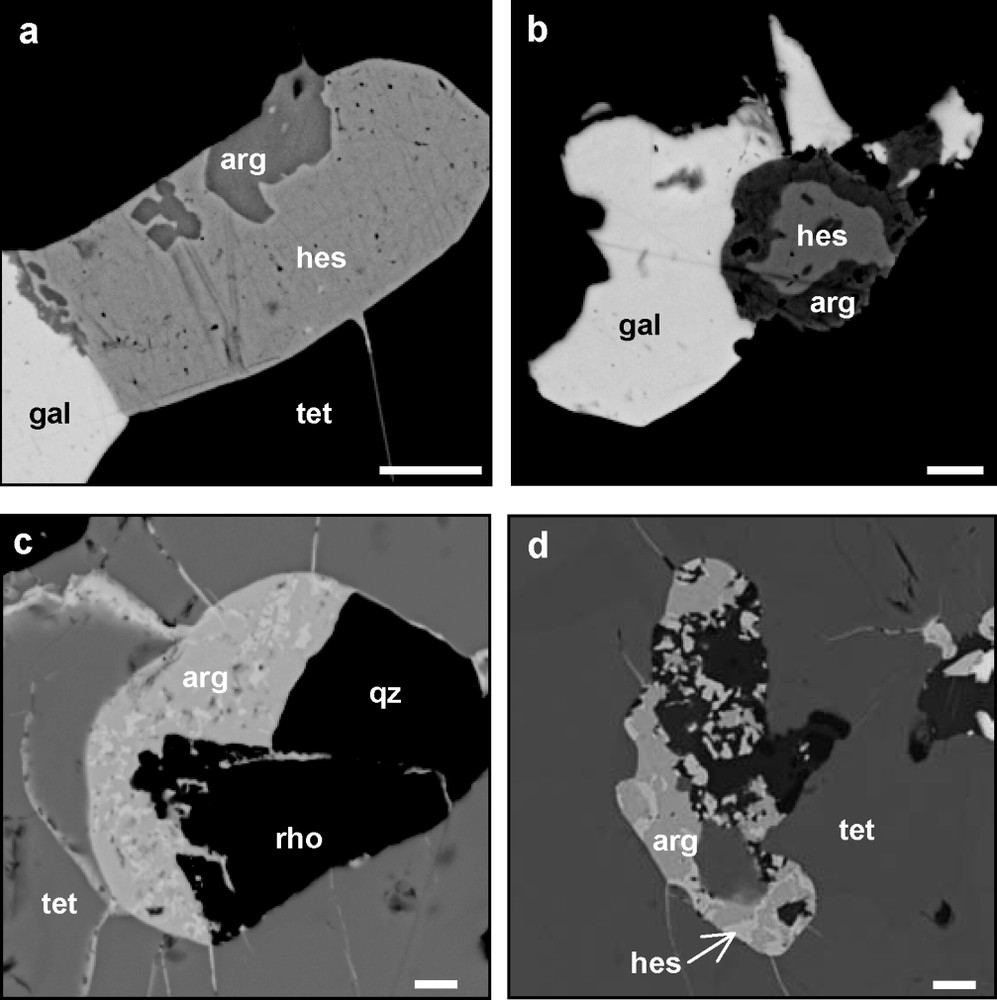
SEM microphotographs of argyrodite bearing assemblages of the Cârnicel vein. (a) Subhedral argyrodite crystals included in hessite (sample RM 611B). (b) Hessite (light grey) included in argyrodite (sample LPC 42 122. (c) Argyrodite rich in hessite wires in tetrahedrite (sample LPC 42 122). (d) Argyrodite, hessite associated with chalcopyrite (in black) in tetrahedrite (sample RM 611B). gal: galena; hes: hessite; arg: argyrodite; tet: tetrahedrite; qz: quartz; rho: rhodochrosite. Scale bar: 5 μm.
Photographies MEB des assemblages à argyrodite de la veine Cârnicel. (a) Cristaux subautomorphes d'argyrodite en inclusion dans la hessite (éch. : RM 611B). (b) Hessite en inclusion dans l'argyrodite (éch. : LPC 42 122). (c) Argyrodite riche en inclusions de hessite dans la tétraédrite (éch. : LPC 42 122). (d) Argyrodite, hessite et chalcopyrite (en noir) dans la tétraédrite (éch. : RM 611B). gal : galène ; hes : hessite ; arg : argyrodite ; tet : tétraédrite ; qz : quartz ; rho : rhodochrosite. Barre d'échelle : 5 μm. Masquer
Photographies MEB des assemblages à argyrodite de la veine Cârnicel. (a) Cristaux subautomorphes d'argyrodite en inclusion dans la hessite (éch. : RM 611B). (b) Hessite en inclusion dans l'argyrodite (éch. : LPC 42 122). (c) Argyrodite riche en inclusions de ... Lire la suite
Electron probe microanalyses of Te-bearing argyrodite. L: sample LPC 42 112, R: sample RM611A. ∗: assuming the total number of atoms to be 15. nd: not determined
Composition chimique de l'argyrodite riche en tellure (analyses à la microsonde électronique). L : échantillon LPC 42 112, R : échantillon RM611A. ∗ : Nombre total d'atomes estimé de 15. nd : non déterminé
| N° | 94 | 104 | 110 | 15 | 19 | 4 | 5 | 105 | 14 | 16 | 32 | 42 | 11 | 6 | 43 | 9 | 8 |
| Sample | R | R | R | L | L | R | R | R | L | L | L | L | R | R | L | R | R |
| Weight percent | |||||||||||||||||
| Ag | 66.70 | 66.85 | 65.19 | 65.78 | 65.24 | 65.03 | 65.15 | 66.95 | 65.69 | 65.18 | 65.36 | 66.22 | 65.95 | 63.62 | 63.65 | 63.97 | 64.62 |
| Cu | 0.00 | 0.00 | 0.43 | 0.54 | 0.00 | 0.35 | 0.63 | 0.00 | 0.43 | 0.00 | 0.76 | 0.00 | 0.32 | 0.49 | 0.00 | 0.87 | 0.22 |
| Fe | 0.25 | 0.00 | 0.00 | 0.00 | 0.00 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.00 | 0.61 | 0.00 | 0.20 | 0.50 |
| Zn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.35 | 0.00 | 0.26 | 0.00 |
| Mn | 0.27 | 0.00 | 0.00 | 0.00 | 0.38 | nd | nd | 0.00 | 0.00 | 0.28 | 0.00 | 0.00 | nd | nd | 0.00 | nd | nd |
| Ge | 4.66 | 4.93 | 4.94 | 4.89 | 5.18 | 5.37 | 5.05 | 5.12 | 5.13 | 4.96 | 4.57 | 5.16 | 5.38 | 4.89 | 4.58 | 4.62 | 4.47 |
| Pb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.26 | 0.20 | 0.47 | 0.00 | 0.19 |
| S | 9.76 | 9.87 | 9.73 | 9.39 | 9.52 | 9.14 | 8.89 | 9.74 | 9.69 | 9.50 | 9.14 | 9.67 | 8.46 | 8.52 | 9.06 | 7.72 | 7.50 |
| Te | 18.38 | 18.89 | 18.92 | 19.03 | 19.09 | 19.12 | 19.22 | 19.36 | 19.43 | 19.63 | 20.09 | 20.13 | 20.29 | 20.68 | 20.69 | 22.21 | 23.33 |
| T | 100.02 | 100.55 | 99.21 | 99.62 | 99.42 | 99.26 | 98.94 | 101.16 | 100.35 | 99.54 | 100.17 | 101.18 | 100.65 | 99.37 | 98.46 | 99.84 | 100.82 |
| Chemical formula∗ | |||||||||||||||||
| Ag | 8.07 | 8.06 | 7.95 | 8.04 | 7.96 | 8.05 | 8.13 | 8.05 | 7.94 | 7.99 | 7.98 | 7.96 | 8.20 | 7.96 | 7.95 | 8.14 | 8.23 |
| Cu | 0.00 | 0.00 | 0.09 | 0.11 | 0.00 | 0.07 | 0.13 | 0.00 | 0.09 | 0.00 | 0.16 | 0.00 | 0.07 | 0.10 | 0.00 | 0.19 | 0.05 |
| Fe | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.15 | 0.00 | 0.05 | 0.12 |
| Zn | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.05 | 0.00 |
| Mn | 0.06 | 0.00 | 0.00 | 0.00 | 0.09 | − | − | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | − | − | − | − | − |
| Ge | 0.84 | 0.88 | 0.89 | 0.89 | 0.94 | 0.99 | 0.94 | 0.91 | 0.92 | 0.90 | 0.83 | 0.92 | 0.99 | 0.91 | 0.85 | 0.87 | 0.85 |
| Pb | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.03 | 0.00 | 0.01 |
| S | 3.97 | 4.00 | 3.99 | 3.86 | 3.91 | 3.80 | 3.73 | 3.94 | 3.94 | 3.91 | 3.76 | 3.91 | 3.54 | 3.59 | 3.81 | 3.30 | 3.21 |
| Te | 1.88 | 1.92 | 1.95 | 1.97 | 1.97 | 2.00 | 2.03 | 1.97 | 1.99 | 2.03 | 2.07 | 2.04 | 2.13 | 2.19 | 2.19 | 2.39 | 2.51 |
Electron-probe microanalyses of Te-bearing canfieldites. ∗: Assuming the total number of atoms to be 15
Analyses à la microsonde électronique de canfieldites riches en tellure. ∗ : Nombre total d'atomes estimé de 15
| Ref. | [12] | [12] | [13] | [13] | [14] | [14] | [15] | [16] | [17] | [17] | [18] | [18] |
| Weight percent | ||||||||||||
| Ag | 56.44 | 58.33 | 52.52 | 63.51 | 63.30 | 60.90 | 65.12 | 61.86 | 62.78 | 62.94 | 67.60 | 71.22 |
| Cu | 3.13 | 1.74 | – | – | – | – | – | – | – | – | – | – |
| Fe | 2.91 | 1.00 | – | – | – | – | – | 0.88 | – | – | – | – |
| Zn | 0.12 | 0.04 | – | – | – | – | – | – | – | – | – | – |
| Bi | – | – | – | – | 2.00 | – | – | – | – | – | – | – |
| Sn | 9.41 | 10.19 | 8.86 | 9.27 | 9.10 | 7.80 | 10.57 | 9.08 | 8.62 | 8.70 | 7.17 | 6.36 |
| S | 10.74 | 8.99 | 8.99 | 9.34 | 9.60 | 8.60 | 13.95 | 8.04 | 9.14 | 9.25 | 11.45 | 11.70 |
| Te | 17.10 | 21.09 | 19.07 | 20.04 | 19.70 | 17.50 | 8.69 | 18.60 | 20.32 | 20.13 | 13.78 | 10.72 |
| Se | – | – | – | – | 0.20 | 0.50 | – | 3.48 | – | – | – | – |
| T | 99.85 | 101.38 | 89.44 | 102.16 | 103.90 | 95.30 | 98.33 | 101.94 | 100.86 | 101.02 | 100.00 | 100.00 |
| Chemical formula∗ | ||||||||||||
| Ag | 6.68 | 7.26 | 7.33 | 7.92 | 7.86 | 8.13 | 7.57 | 7.78 | 7.94 | 7.94 | 8.17 | 8.61 |
| Cu | 0.63 | 0.37 | – | – | – | – | – | – | – | – | – | – |
| Fe | 0.67 | 0.24 | – | – | – | – | – | 0.21 | – | – | – | – |
| Zn | 0.02 | 0.01 | – | – | – | – | – | – | – | – | – | – |
| Bi | – | – | – | – | 0.13 | – | – | – | – | – | – | – |
| Sn | 1.01 | 1.15 | 1.12 | 1.05 | 1.03 | 0.95 | 1.12 | 1.04 | 0.99 | 1.00 | 0.79 | 0.70 |
| S | 4.28 | 3.76 | 4.22 | 3.92 | 4.01 | 3.86 | 5.46 | 3.40 | 3.89 | 3.92 | 4.66 | 4.76 |
| Te | 1.71 | 2.22 | 2.33 | 2.11 | 2.07 | 1.97 | 0.85 | 1.98 | 2.17 | 2.15 | 1.41 | 1.10 |
| Se | – | – | – | – | 0.03 | 0.09 | – | 0.60 | – | – | – | – |
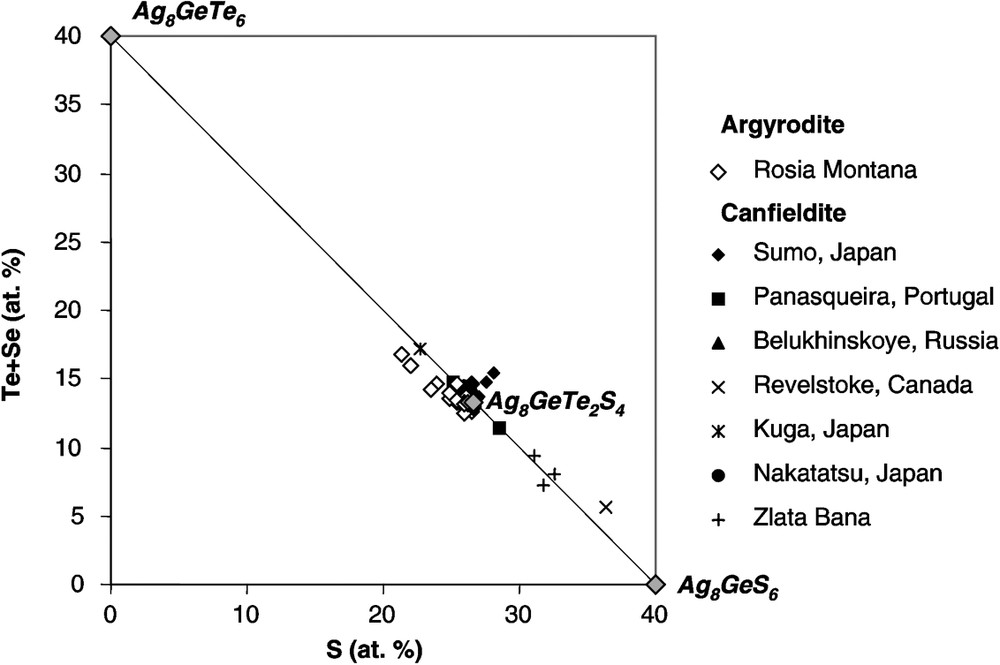
Te + Se versus S (in atomic %) of the Te-rich argyrodite from Roşia Montană and analyses of Te-bearing canfieldite from the literature (see Table 2 for references). Synthetic Ag8GeTe6 and theoretical Ag8GeTe2S4 compositions are also reported.
Te + Se versus S (en % atomique) des argyrodites à tellure de Roşia Montană et analyses des canfieldites à tellure de la littérature (Tableau 2 pour références). Les composés synthétique Ag8GeTe6 et théorique Ag8GeTe2S4 ont également été reportés.
4 Discussion
Longtemps suspectée [1,27], la présence et la nature chimique du porteur de Ge à Roşia Montană sont clairement établies. Il semble qu'il s'agisse de la première description d'argyrodite riche en Te, alors que l'existence de canfieldite à tellure est établie. Les analyses à la microsonde sur sphalérite et tétraédrite, porteurs potentiels de Ge [2,12], ne montrent pas de teneurs significatives en Ge. De nombreux gisements situés dans la chaîne Carpatho-Balkanique contiennent des minéraux germanifères, en Bulgarie [22,28] et en Serbie [9]. Il s'agit de dépôts épithermaux de type acide. L'argyrodite a été identifiée dans un seul épithermal neutre, Wolyu, en Corée [32]. La présence d'alabandite, de sphalérite dépourvue de fer et de minéraux de germanium dans la veine Cârnicel suggère des conditions de fugacité en soufre intermédiaires entre celles rencontrées dans les gisements épithermaux acides et neutres [3,23]. En Roumanie, des minéraux germanifères sont également décrits dans d'autres gisements des monts Apuseni [1,24,29] et dans la région de Baia Mare [1,20,21,26]. Les dépôts épithermaux apparaissent comme des hôtes potentiellement intéressants pour le germanium et doivent être évalués en termes de récupération.
1 Introduction
Germanium (Ge) is a high-technology metal largely used in electronic applications, infrared instruments, as well as optic fibres [11]. The more currently cited sources of Ge include sulfide ore deposits, iron oxide deposits, oxidized sulfide ore deposits, pegmatites, greisens and skarns, coal and lignified wood [3]. The epithermal ore deposits in which various Ge-bearing minerals have been frequently reported have only a minor commercial importance.
In 1962, metallurgical tests conducted on ore concentrates from the Roşia Montană AuAg epithermal ore deposit (South Apuseni Mountains, Romania) have revealed the presence of anomalous Ge contents in pyrite concentrates [1]. Recent mineralogical tests combined with SEM microscopy and EPMA analyses performed on samples collected from the Ag-rich Cârnicel vein allowed us to study the distribution, the mineralogical expression and consequently the potential of recovery of Ge.
2 Geology and ore mineralogy
The Roşia Montană (RM) world-class epithermal AuAg deposit (reserves: 217 Mt of ore @ 1.5 g/t Au and 7.5 g/t Ag), situated in the northern part of the southern Apuseni Mountains (Fig. 1), is related to Neogene volcanism that outcrops all along the Carpatho-Balkan belt [15]. In the RM area (Fig. 1) the crystalline basement occurs only as fragments within various breccia pipe structures [27]. The sedimentary rocks in the area are represented by Upper Cretaceous flysch and Neogene rocks (Badenian and Sarmatian) [5,6]. The Neogene sedimentary rocks occur as narrow sequences within a volcano-sedimentary formation. The volcanic rocks are represented by Cetate dacite (Cetate and Cârnic domes), to which is related the RM ore genesis and dacite pyroclastics – the so-called volcano-sedimentary formation. The volcanic activity continues with barren andesitic lava flows and pyroclastics (Rotunda type) [4,6,8,14].
The studied samples were collected in the Ag-rich, north–south Cârnicel vein (around 1 kg/t Ag and 5 g/t Au) characterised by a well marked banded structure with alternating bands of ore minerals and gangue minerals (rhodonite/rhodochrosite–quartz). The ore assemblage is mainly composed of dominant galena, Fe-free and Mn-rich sphalerite (3.4 to 6.3 wt% Mn), argentiferous tetrahedrite – mean composition: (Cu9.47Ag0.39Zn1.58Mn0.3)(As0.63Sb3.6)S12.92 – associated with minor amounts of chalcopyrite, pyrite, marcasite, and rare alabandite. Besides common base metal sulfides, the ore assemblage is completed by abundant hessite, argyrodite, rare altaite and electrum grains (Ag0.23Au0.76), and sylvanite (at the interface between hessite and electrum). The host rock of Cârnicel vein is an extracraterial phreatomagmatic breccia (vent breccia) composed of sub-angular to sub-rounded rock fragments (dacite, sedimentary and crystalline rocks) held together in a coarse-to-silty–clayey matrix. The dominant rock fragments are adularised dacite and dacitic ground mass.
3 Argyrodite
Argyrodite mainly occurs as anhedral to subhedral grains up to 100 μm in size included in hessite (Fig. 2a), but reverse assemblages are also observed (Fig. 2b). In most cases, argyrodite is intimately associated with hessite wires (Fig. 2c). Its reflection colour is grey, with a strong bluish tint that differs from the violet tint of Te-free argyrodite. No reflection pleochroism or internal reflection has been observed. A weak anisotropism is detected in air with slightly uncrossed nicols.
Representative EPMA analyses of argyrodite are listed in Table 1. Beside Ag, Ge, Te and S, traces of Fe, Cu, Zn, Pb and Mn are locally detected, but are probably due to the host sulfides and gangue minerals. Sn and Se are systematically below detection limit. The average chemical formula, assuming the total number of atoms to be 15, is Ag8.04Ge0.9S3.77Te2.07. A solid solution exists between argyrodite and canfieldite [30], and several studies have reported the existence of Te-bearing canfieldite [10,13,16–19,25,31] (Table 2). In Fig. 3, Te + Se versus S of Te-bearing argyrodite and Te-(Se)-bearing canfieldite is plotted. The argyrodite analyses are grouped around a hypothetical compound Ag8GeTe2S4, but several points extend toward more Te-rich compositions (S/Te atomic ratio ranges between 1.28 and 2.11), suggesting a possible solid solution between Ag8GeS6 and Ag8GeTe6, a synthetic equivalent of argyrodite not known in nature [7]. Compared to argyrodite, Te-rich canfieldite is characterized by highly variable S/Te ratio (1.7 to 6.4), whereas significant Se contents are reported. However, the high Te contents of several analyses of argyrodite may be also due to the presence of undetected hessite wires (Fig. 2c).
4 Discussion
After many years of uncertainties [1,27], the existence and the chemical composition of a Ge carrier mineral within Roşia Montană ore deposit are now established. This phase, identified as argyrodite, shows a substitution of S by Te. To our knowledge, this is the first reported occurrence of Te-bearing argyrodite, whereas the existence of Te-bearing canfieldite was reported in several studies [10,13,16–19,25,31]. Sphalerite with Ge contents exceeding 1000 ppm in the Saint-Salvy deposit (Tarn, France [2]) and tetrahedrite are potential hosts for Ge [12]. The Ge content of these two phases, investigated by EPMA, is systematically null or below the detection limit (around 400 ppm in the used analytical conditions). In the absence of whole-rock analyses for Ge, it is not yet possible to conclude about the presence of other Ge-carriers in Roşia Montană.
Germaniferous minerals (germanite, briartite, renierite, Ge-rich colusite) are reported in several deposits of the Carpatho-Balkan belt in Radka, Chelopech, Krassen (Panagyurishte district, Bulgaria [22,28]) as well as in Tilva Ros (Bor district, Serbia [9]). All these deposits are mainly considered as high-sulfidation epithermal type deposits. To our knowledge, argyrodite was only reported in the Korean Wolyu deposit, clearly identified as a low-sulfidation epithermal AuAg deposit [32]. The presence of alabandite, Fe-free sphalerite, germaniferous minerals in the Cârnicel vein strongly suggests intermediate sulfidation conditions [3,23].
The occurrences of germanite, and members of the canfieldite–argyrodite series are also reported in other deposits of the southern Apuseni Mountains: Bucureşci-Rovina and at Băiţa-Crăciuneşti [1]; Bucium1 [29]; Pârâul lui Avram [24]. In the three former deposits, enargite is also reported. In the Baia Mare region, the occurrence of germanite was briefly reported at Ilba-Handal [1], Baia Sprie [21], Toroiaga [26], and Cavnic [20]. The presence of germanium may be considered as a common signature of several ore deposits of the Carpatho-Balkan belt. Epithermal ore deposits of high- and low-sulfidation type are potentially interesting hosts for Ge and have to be evaluated for its recovery and refining.
Acknowledgments
The authors would like to highlight the support given by Gabriel Resources and Roşia Montană Gold Corporation during the field researches as well as for the permission to present this paper. Gary O'Connor (Vice-president Exploration, Gabriel Resources) is thanked for his help and useful discussions. Y. Moëlo and E. Marcoux are thanked for their constructive reviews of the manuscript.
1 Within Bucium area occur several epithermal and one porphyry copper ore deposits (Bucium-Frasin, Bucium-Rodu, Bucium-Arama, Bucium-Tarniţa, etc.). Taking into account the description given by [28], the occurrence of germanite corresponds to Bucium-Arama epithermal deposit.


