1 Introduction
The accumulation of trace metals and metalloids in soils is of increasing concern due to the negative effects on soil ecosystems (Brown and Calas, 2011) and potential health risks. Soil-to-plant transfer of trace metals, especially consumption of vegetables, is one of the major pathway of human exposure to soil contamination, as soils contaminated with low levels of trace metals and other trace elements are frequently used for vegetable growing (Moreno et al., 2003; Spiter et al., 2008; Wang et al., 2004). Vegetable materials are often used as bio-indicators for establishing the degree of pollution related to chemicals in the environment (Nascentes et al., 2001).
The prediction of phytoavailability is then of crucial importance for the assessment of environmental quality and health risks. Phytoavailability is regulated by a complex system of chemical and biological processes and their interactions. The phytoavailability varies with plant species and soils properties (metals source, loading rate, soil pH, redox potential, texture, organic matter, mineral composition, …) as well as external factors such as climate or human agricultural practice (Bose et al., 2008; Kabata-Pendias, 2004; Li et al., 2006; Wang et al., 2004; Zhao et al., 2004).
The total concentration of metals in soils is not a good indicator of availability, due to the different and complex distribution patterns of metals among various chemical species or solid phases. Indeed, sorbed elements onto solid particles are potentially available, as they may be extracted due to changes in the physico-chemical properties of the environment such as salinity, pH, redox potential, and concentration of chelators (Abollino et al., 2002; Bourg, 1988, 1995; Calmano et al., 1993; Chuan et al., 1996; Ernst, 1996; Forstner, 1993; Forstner and Kersten, 1988; Forstner et al., 1986; Gambrell and Patrick, 1988). There is no consistent opinion of which forms of the species are easily available to plants.
The “labile forms”, defined in terms of elements that exist in solid phases in equilibrium with the pore water (Charlet et al., 2011), are often considered as the most easily bioavailable forms (Shan et al., 2003). So, the phytoavailable part of metallic elements is often considered to be mainly through the soil solution (Li et al., 2006). However, it is well known that elements can be directly taken up from the solid-phase, i.e. by a diffusion process (Li et al., 2006), or thanks to some reactions resulting from biological activities related to microorganisms or plants roots, that are able to release compounds that can complex with metals (Laurie and Manthey, 1994; Salt et al., 1998), and/or induce local redox or pH variations, which finally modify the fixation and mobility of the metals in soils (Torri and Lavado, 2009; Wang et al., 2009).
Thus, the importance of solid-phase metal fractions in phytoavailability is now widely recognised as being of crucial importance (Obrador et al., 2007).
For this purpose, many chemical extraction procedures have been proposed and their use to predict bio- or phytoavailability has become a conventional approach (Kabata-Pendias, 2004; Kubova et al., 2008; Li et al., 2006). The most widely used methods are single and/or sequential extraction procedures, correlated to element accumulation in organisms.
Numerous studies have then been realised over the past decades, that have tried to estimate metal phytoavailability by comparison and correlation of the quantities of metals accumulated in plants with chemical extraction results; that is to say, the quantities of the metals within the different labile soil fractions (Bose et al., 2008; Fang et al., 2007; Kubova et al., 2008; Meers et al., 2005, 2007; Torri and Lavado, 2009; Wang et al., 2009).
Single extractions procedures essentially fall into three categories depending on the nature of the reactant used, that can be:
- • salts as CaCl2 or Ca(NO3)2 (An and Kampbell, 2003; Fang et al., 2007) in order to leach cations that are adsorbed onto solid materials due to permanent structural charges (phyllosilicates, phyllomanganates and sometimes organic matter);
- • dilute solutions of acids. Dilute HCl is one of the most widely used reagents (Da Silva et al., 2002; Kubova et al., 2008; McCready et al., 2003; Négrel et al., 2000; Sutherland, 2002), because low pH favors the dissociation of the existing complexes;
- • complexing or reducing agents, such as EDTA, a strong chelator, which is able to complex with most metals contained in the soils (Fang et al., 2007; McCready et al., 2003).
Acids are supposed to leach elements associated to carbonates and oxides, whereas EDTA would leach elements from oxides and organic matter (Baize, 1997; Juste, 1989; Ure et al., 1995). Other considered that salts are the best to predict the phytoavailablility of trace metals (Houba et al., 1996; Kabata-Pendias, 2004; Meers et al., 2007).
Sequential extractions, compared to single leaches, are more expensive and time-consuming, but have the advantage of characterizing the different labile fractions. They remain one of the most useful tools for solid speciation of particulate elements, to study the origin, the fate, the biological and physicochemical availability and transport of sorbed elements (Da Silva et al., 2002; Lebourg et al., 1996; Leleyter and Probst, 1999; Ma and Uren, 1998; Tessier et al., 1979; Ure et al., 1995). Several sequential extraction procedures are commonly used in the literature and differ by the number of steps and reactants used. The most commonly sequential extraction procedures used (Quevauviller et al., 1994; Rauret et al., 1999; Tessier et al., 1979; Ure et al., 1993) have been performed in a more efficient and selective extraction procedure described by Leleyter and Probst (1999). This method was chosen from several procedures because it was checked for selectivity, reproducibility, and repeatability of the different steps (for details, see Leleyter, 1999 and Leleyter and Probst, 1999), because this procedure can be used for different solid materials as riverine sediments, marine sediments or soils (Leleyter et al., 2012) and it was commonly reported in the literature (Aubert et al., 2004; Bur et al., 2009; Cecchi et al., 2008; N’guessan et al., 2009; Salvarredy-Aranguren et al., 2008).
To date, no method has been considered as universally applicable for the assessment of metal fractions in soils and plant bioavailability and the conclusions still remain very contradictory (Fang et al., 2007; Obrador et al., 2007; Sauvé et al., 1996; Wang et al., 2003).
The aim of this work was to compare the phytoavailable concentrations of metals (Cd, Cr, Fe, Mn, Ni, Pb, Zn) predicted by chemical extractions (two single leaches (HCl and EDTA) and one sequential extraction procedure) with the concentrations analyzed in radish plants grown on 3 different soils, in order to gain information about the plant availability, mobility and soil distribution.
2 Materials and methods
2.1 Field sites and general soil parameters
Three soils from Normandie (France), designed as S1, S2 and S3, were selected for this experiment. S1 is a soil from a private garden in a rural area. S2 is an agricultural soil, which has been periodically amended and/or treated with various health plant products. S3 is an industrial soil, sampled on an ancient steel factory in an urban area. Surface soils samples were collected from the top 10 cm, air-dried (7–8 days) and passed through a 2 mm sieve before further use, analysis and treatment.
Soil pH was measured in water (pHw) and KCl (pHKCl), according to NF ISO 10390. Carbonate content was evaluated according to NF ISO 10693 by measuring the CO2 released by addition of HCl.
2.2 Mineralisation procedure
The total concentration of each element was obtained after microwave assisted acid digestion. About 0.2 g of a soil sample was digested with 10 mL of aqua regia (HNO3/HCl = 1:3; all reagents were Prolabo Normapur grade) in a Berghof speedwave MWS-2 microwave oven, during 50 min (3 steps: temperatures: 100 to 175 °C; power 40 to 80%). The digests were diluted with ultrapure water to 50 mL and stored, in polyprolpylene tubes, at 4 °C. The solutions were filtrated at 0.45 μm (HVLP, Millipore) prior to analysis by ICP-AES.
2.3 Extraction procedures
To get a rapid evaluation of the mobile and/or bioavailable metals fraction, representative aliquots of each soil sample were leached by different chemical reagents (0.25 mol.L−1 HCl or 0.05 mol.L−1 EDTA). This involved mixing 10 mL of the extractant with 1 g of sediment and shaking at room temperature for 1 h. The residue was then filtered on 0.45 μm pore size Millipore filter (polyvinylidene of fluoride (CHR-CFF-)n) and the leachate is stored in a polypropylene bottle at 4 °C until chemical analyses.
Moreover, all the soil samples were leached by an optimized sequential chemical extraction procedure (Leleyter and Baraud, 2006; Leleyter and Probst, 1999). This procedure dissolves selectively and efficiently all the chemical constituents of the soils which can be affected by changes in physico-chemical conditions. The leaching procedure (Table 1) was performed in a watertight container to prevent evaporation, with continuous agitation to increase the interaction surface between the reagent and the sediment. The indicated quantities refer to 1 g dry soil. After each reaction, the residue was filtered and washed with 20 mL of distilled water. At each step of the procedure, the leachate volume was measured in order to prevent the loss of distilled water in the filtration apparatus. The ‘labile fraction’ (F6) of the sample is the sum of the previous leached fractions.
Procedure d’extraction séquentielle.
| Reagents | Duration (hour) | Temperature (T °C) | Fractions |
| 10 ml H2O | 0.5 | 20 | F1: elements dissolved by water |
| 10 ml of 1 M Mg(NO3)2, pH = 5.0 | 2 | 20 | F2: really exchangeable elements |
| 10 ml of 1 M NaOAc/HOAc, pH = 4.5 | 5 | 20 | F3: elements bound to acido-soluble fraction |
| 10 ml of 0.1 M NH2OH HCl, pH = 3.5 | 0.5 | 20 | F4.a. elements bound to reducible fraction (manganese oxides) |
| 10 ml of [0.2 M (NH4)2C2O4 + 0.2 M H2C2O4] | 4 | 20 | F4.b. elements bound to reducible fraction (amorphous iron oxides) |
| 10 ml of [0.2 M (NH4)2C2O4 + 0.2 M H2C2O4, + 0,1 M C6H8O6] | 0.5 | 80 | F4.c. elements bound to reducible fraction (crystalline iron oxides) |
| 3 ml of 0.02 M HNO3 + 8 ml of 35% H2O2 Then 5 ml of 3,2 M CH3COONH4 |
5 0.5 |
85 85 |
F5. elements bound to oxidable fraction |
2.4 Plants growth experiment
The radish was selected for various reasons. Radish is an important, common dicotyledonous vegetable whose storage root-stem portion is in direct contact with soil (Souza and Rauser, 2003). It is a useful indicator of trace metal bioavailability in soil, as a moderate accumulator (Davies and Hougton, 1984), and has been used in various experiments that focused on metals bioavailability or even bioaccessability (Gaw et al., 2008; Intawongse and Dean, 2008; Li et al., 2006; Sauvé et al., 1996). Moreover, the radish has an only 3 week growth period and maturity.
The soils were placed in plastic pots, holding about 2 kg each. Fifteen seeds were sown on each soil and the pots were placed under natural field conditions. Plants were watered every alternate day, or more frequently as required. No fertilizer was added.
After 3 weeks, the plants were harvested, washed with tap water, then rinsed thoroughly with deionised water and separated into shoots and roots. Such a washing process should have no significant influence on the root metal content (Wang et al., 2003). Only the edible part of roots (referred to hereafter as the roots) was kept, as it was not possible to remove the adhered soil particles from the very thin fibrous roots, that were then eliminated.
The tissues were oven-dried at 80 °C for 48 h before weighing and analysis. The root and shoot tissues were then ground in an agate mill and about 0.2 g of tissue was digested with 10 mL of 70% HNO3 in a microwave oven (Berghof speedwave MWS-2).
In order to compare the chemical composition of our studied radishes, we bought a bunch of radishes from an important supermarket and we analyzed it in the same way.
2.5 Chemical analysis
Elemental analyses of the tissues digests and of the various leachates were performed using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) (Varian Vista-MPX). Appropriate calibration standards were run periodically interspacing the samples, to monitor any loss of efficiency. The quality control of ICP-AES data was assessed by the analysis of blank reagents and calibration standards prepared with commercially available solutions (Varian solution standards). Accuracy of ICP-AES measurements was determined using various certified reference materials. These data and the limits of detection are reported in Table 2. All the concentrations determined in this study presented relative standard deviation inferior to 5% for the tissue contents, 10% for the soils total concentrations and to 15% for the leachates obtained by the various chemical extractions.
Analyse du matériau certifié (HR-1).
| HR-1 | ||
| CV | MV | |
| Al | 59.3 ± 22.8 | 50.4 ± 3.7 |
| Ca | 67.7 ± 3.9 | 61.3 ± 1.9 |
| Cd | 3.9 ± 1.2 | 5.0 ± 1.0 |
| Cr | 126 ± 45 | 96 ± 3 |
| Cu | 79.9 ± 11.4 | 79.0 ± 4.0 |
| Fe | 30.6 ± 7.4 | 22.0 ± 1.8 |
| Mn | 549 ± 83 | 394 ± 22 |
| Ni | 39 ± 15 | 28 ± 3 |
| Pb | 139 ± 37 | 122 ± 4 |
| Zn | 1105 ± 173 | 1130 ± 22 |
| Units | mg/kg or g/kg |
3 Results and discussion
3.1 Soils
3.1.1 Soils general characteristics
Table 2 reports the total concentration values analyzed for Al, Ca, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn, as well as the pH and carbonate content values determined in the 3 soils. pH values around 7.5 were observed for S1 and S2, whereas a slightly higher value of 8.5 was found for S3, which also presented a higher carbonate content (21%) compared to S1 and S2 (< 1%). Non-essential and toxic metals such as Cr, Cd and Pb were detected in the 3 soils.
The quantification of metal contamination is estimated by the calculation of an enrichment factor (EF) (Table 3) obtained according to:
Concentrations totales en métaux (mg/kg de sol sec), pH du sol, teneurs en carbonates et facteur d’enrichissement (EF donné en gras entre parenthèses) pour les 3 sols.
| Al | Ca | Fe | Mn | Cd | Cr | Cu | Ni | Pb | Zn | pH w/KCl | %CaCO 3 | |
| S1 | 48 643 | 10 214 (0.5) | 25 544 (0.9) | 618 (1.1) | 2 (14) | 36 (0.6) | 32 (1.0) | 14 (0.3) | 41 (5) | 96 (2) | 7.7/7.4 | 0.5 |
| S2 | 35 234 | 6850 (0.4) | 20 492 (0.9) | 558 (1.4) | 37 (0.8) | 12 (0.5) | 17 (0.5) | 16 (3) | 49 (2) | 7.5/7.3 | 0.8 | |
| S3 | 29 448 | 124 585 (9.5) | 60 862 (3.4) | 2891 (8.4) | 5 (68) | 131 (3.6) | 24 (1.2) | 24 (0.9) | 58 (12) | 117 (5) | 8.6/8.3 | 21.3 |
Aluminium is commonly used as a geochemical normaliser in EF calculations, as it presents the advantages of being naturally concentrated in soils and sediments (strongly associated with the aluminosilicate matrix), generally with minimal anthropogenic contamination and a metal to Al ratio relatively constant in the crust. Ideally, the background values would be estimated from the corresponding area, but in the absence of these data, earth crust values are sometimes substituted (Feng et al., 2011; Leleyter et al., 2012; Zhu et al., 2011). In our case, as no local background data were available, a general world average concentration values (reported for the continental crust by Allègre and Michard, 1973) were used as reference values, mainly in order to compare the 3 soils of interest. For all the metals analysed, the highest EF values were observed for the industrial soil S3. Enrichments factors superior to 2 were noticed for Pb and Cd within the 3 soils, and for Cr, Fe, Mn and Zn only in the industrial soil S3.
3.1.2 Sequential extraction
The detailed distribution of trace metals in the different geochemical fractions was determined thanks to the studied sequential extraction procedure. The quantities (μg/g) of elements leached by each step (F1 to F5), as well as the total labile fraction (F6) are reported in Table 4. For each element, the ratio of the extracted concentration to the total content in soil can be expressed as percentage values and are reported in Fig. 1.
Concentrations élémentaires dans les différents lessivats (en μg/g de sols secs) HCl, EDTA et dans les fractions de F1 à F6.
| Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |
| LD | 0.01 | 0.04 | 0.05 | 0.08 | 0.01 | 0.03 | 0.05 | 0.04 |
| HCl | ||||||||
| S1 | 0.06 | 0.71 | 9 | 623 | 192 | 0.8 | 19 | 24 |
| S2 | 0.15 | 0.61 | 3 | 705 | 123 | 1.4 | 4 | 5 |
| S3 | 0.01 | 0.22 | 0.05 | 7 | 232 | 0.2 | < | 3 |
| E DTA | ||||||||
| S1 | 0.03 | 0.14 | 11 | 398 | 72 | 0.6 | 16 | 18 |
| S2 | 0.15 | 0.17 | 4 | 981 | 337 | 2.1 | 8 | 4 |
| S3 | 0.07 | 0.05 | 3 | 210 | 152 | 0.3 | 15 | 21 |
| F6 | ||||||||
| S1 | 0.9 | 12 | 29 | 12 773 | 394 | 6 | 31 | 85 |
| S2 | 0.7 | 10 | 10 | 9660 | 376 | 9 | 9 | 32 |
| S3 | 4 | 106 | 88 | 33 181 | 2103 | 13 | 64 | 153 |
| F1 | ||||||||
| S1 | < | < | 0.2 | 28 | < | < | < | 0.23 |
| S2 | < | < | 0.1 | 24 | < | < | < | 0.23 |
| S3 | < | < | 0.1 | 6 | < | < | < | 0.27 |
| F2 | ||||||||
| S1 | < | < | 0.1 | 1 | < | < | < | 0.12 |
| S2 | < | < | < | < | < | 0.06 | < | 0.05 |
| S3 | < | < | < | < | < | < | < | < |
| F3 | ||||||||
| S1 | < | 0.3 | 0.71 | 28 | 45 | 0.1 | 2 | 11 |
| S2 | 0.04 | 0.3 | 0.4 | 76 | 42 | 0.4 | 0.4 | 2 |
| S3 | 0.3 | 3 | 0.9 | 2149 | 524 | 1 | 2 | 39 |
| F4 | ||||||||
| S1 | 0.7 | 6 | 24 | 9212 | 305 | 3 | 11 | 50 |
| S2 | 0.5 | 7 | 8 | 7041 | 188 | 6 | 5 | 23 |
| S3 | 3 | 66 | 47 | 25 127 | 1142 | 8 | 3 | 93 |
| F5 | ||||||||
| S1 | 0.2 | 5 | 5 | 3504 | 44 | 2 | 18 | 24 |
| S2 | 0.2 | 3 | 2 | 2499 | 26 | 2 | 4 | 6 |
| S3 | 0.6 | 37 | 41 | 5899 | 437 | 3 | 59 | 20 |
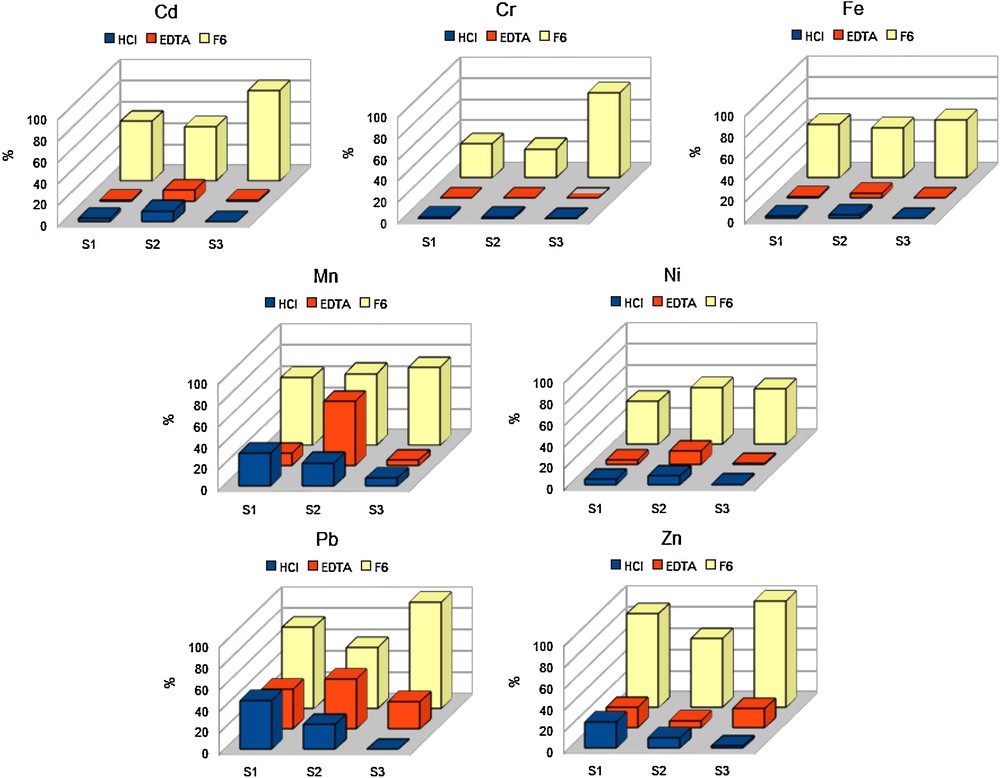
Percentages of metals leached by HCl, EDTA or F6.
Pourcentage de métaux lessivés par HCl, EDTA ou F6.
The metals studied (Cd, Cr, Mn, Ni, Fe, Zn, Pb) appeared mainly associated with reducible and oxidable fractions (F4 and F5 fractions). Significant acido-soluble fractions were additionally observed for all the studied metals, particularly for Zn and Mn, and particularly for S3 soil (which presents the higher carbonate content, see Table 3) compared to the initial total content. These results are consistent with many other studies that report the scavenging effect of iron oxides and organic matter (Balistrieri and Chao, 1990; Bryn and Adnan, 1997; Gaiero et al., 2002, 2003; Levy et al., 1992; Manceau et al., 1992; Trolard et al., 1995).
The elements present in the exchangeable fraction are really weakly bound and are in equilibrium with the composition of the dissolved phase. Elements present in the ‘acido-soluble’ fraction are logically very sensitive to pH variations, whereas elements scavenged in the reducible fraction are very sensitive to redox conditions and to microbiological activity during diagenesis microbially redox reactions, which will result in dissolution of the reducible phase, consisting of Fe and Mn oxides, and a possible release of associated elements (Bryn and Adnan, 1997). In the same way, the oxidizable fraction, which consists largely of organic and sulphide (Tessier et al., 1979), is also very sensitive to redox conditions and to microbiological activity. The residual fraction, composed of detrital silicate minerals, resistant sulphides, and refractory organics (Tessier et al., 1979) is unlikely to be reactive during sedimentation and diagenesis and thus induces little environmental nuisance (Bryn and Adnan, 1997). The lability, then the phytoavailability is supposed to decrease from the first to the last step of the sequential extraction procedure. Thus, the soluble plus exchangeable fractions are often considered as the most available to plants (Obrador et al., 2007; Torri and Lavado, 2009). It can also be estimated that the entire labile fraction (here F6) might be phytoavailable to some extent.
3.1.3 Single extractions
According to the HCl and EDTA single extractions, Pb, Mn were the most mobile metals whereas Fe and Cr were the less extracted elements for the three different soils. Zn and Ni then constituted an intermediate group, with an intermediate mobility, that varied depending on the soil considered and/or the extraction procedure applied. These trends were confirmed by the sequential (F6) extraction even if the order of some elements changed from one situation to another. Finally, the order of the metals mobility (then potential phytoavailability) for the three different soils could be resumed by:
(Mn, Pb) > (Cd, Ni, Zn) >(Fe, Cr).
A quantitative comparison was realised thanks to the concentrations analyzed (and/or the % values calculated relatively to the total level in soil, for each metal) in the various leachates (HCl, EDTA and F6) for the studied elements. EDTA and HCl extractions gave similar trends (Fig. 1), except for Mn and Pb in S2 and Zn in S3. Whatever the soil was, HCl seemed more efficient than EDTA to remove Cr. For the other elements, the EDTA efficiency, compared to HCl, varied from one soil to another, i.e., Pb was better extracted by EDTA on S2 and S3, but by HCl on S1 (Fig. 1).
3.1.4 Comparison of the labile fractions estimated by the various extractions.
The quantity of an element leached either by the entire sequential procedure applied (represented then by the F6 fraction) or by the HCl or the EDTA single leach, can be considered as a potential prediction of the phytoavailability of this particular element. The results (Fig. 1) obtained from the different extraction single and sequential procedures were compared. As already reported by Leleyter and Baraud (2005), the applied sequential extraction was far more aggressive than the two single extractions used, for each of the 3 soils, as the F6 concentrations values were the highest (Fig. 1). This is particularly true for Cr and Fe that were poorly extracted by HCl or EDTA.
3.2 Plant data
3.2.1 Biomass production (dry weight: DW)
Visually, toxic symptoms were developed by plants grown on S2 and S3 soils. This included reduction of the root growth and leaves expansion as well as some kind of chlorosis. A substantial decrease of the total root and shoot dry weight (DW) (Table 5) was observed, compared to plants grown on S1 soil.
Concentrations élémentaires dans les radis et production de bio-masse (DW en g). (< : non détecté). (LD : limites de détection).
| DW(g) | Al | Ca | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |
| LD | 0.6 | 6 | 0.1 | 0.3 | 0.4 | 0.7 | 0.1 | 0.2 | 0.4 | 0.3 | |
| S1_R | 0.6451 | 74 | 4334 | < | 46 | 7 | 75 | 16 | 0.4 | < | 30 |
| S1_S | 1.2096 | 276 | 26 550 | 1.2 | 39 | 5 | 292 | 53 | 0.3 | < | 31 |
| S1_Cwp | 1.8547 | 206 | 18 823 | 0.8 | 42 | 6 | 216 | 40 | 0.3 | < | 31 |
| S2_R | 0.2081 | 160 | 6246 | 5 | 37 | 4 | 66 | 10 | 0.3 | < | 18 |
| S2_S | 0.4843 | 457 | 29 998 | 12 | 82 | 5 | 705 | 45 | 0.5 | < | 19 |
| S2_Cwp | 0.6924 | 368 | 22 860 | 10 | 68 | 5 | 513 | 35 | 0.4 | < | 19 |
| S3_R | 0.1897 | 155 | 7374 | < | 69 | 4 | 21 | 16 | 0.2 | < | 15 |
| S3_S | 0.5917 | 425 | 35 571 | 1.5 | 178 | 4 | 1026 | 86 | 0.5 | < | 13 |
| S3_Cwp | 0.7814 | 360 | 28 726 | 1.1 | 151 | 4 | 782 | 69 | 0.4 | < | 14 |
| Ref_R | 6 | 572 | < | < | 2 | 7 | < | < | 0.5 | 8 | |
| Ref_S | 5 | 156 | < | < | 1 | < | < | < | < | 2 |
The total (15 plants) root dry weight was 0.65 g on the S1 soil, against about 0.20 g on S2 or S3. The total shoot dry weight was more important for S1 (1.21 g) than for S3 or S2 (about 0.5 g). This suggests that the S2 and S3 soils were less adapted to radish growth than S1, and/or contained compounds (studied metals or additional inorganic/organic compounds, not studied in the present work) that were available at toxic levels. Marchiol et al. (2004) also noticed that, the concomitant presence of metals had a severe influence on plant growth but also on the phytoextraction efficiency, probably due to the additive effects on plant metabolism.
3.2.2 Tissue element profiles
Since roots and shoots were not desorbed, the concentrations of elements reflected element adsorbed to cell walls and actually present within the cells. Table 5 reports the various concentrations (μg/g of dry weight) found in the tissues (as well as metal elements concentrations determined in radishes which were bought in a shop). General trends can be drawn from these results, despite the different nature of the soils.
3.2.2.1 Root contents
Fe, Ni, Mn and Zn were observed at similar levels in the roots. The concentrations detected increased (maximum × 2) from S3 to S1 soil, except for Mn.
Non-essential elements (Cr, Cd) were detected in the analyzed roots, except for Pb, although this metal had been observed at significant levels in the labile fractions, for any of the 3 soils. Cr accumulated in the roots, the highest content being observed for the industrial soil S3. Cd was only detected in the roots of plants grown on the agricultural soil S2.
Kubova et al. (2008) also observed that Pb was poorly taken up by various plants despite the presence of this metal at very high concentrations in the soils studied. Our result suggests that Pb was poorly phytoavailable for the radish. In the 3 soils, Pb was mainly present in the reducible and oxidable fractions, with the F4 + F5 fractions containing about 90% or more of the labile Pb for the 3 soils (Baraud and Leleyter, 2005, 2006). It might then be assumed that this Pb-fraction was not available to the radish uptake. This result is in full agreement with Wang et al. (2003). They explained the low phytoavailability of Pb as a result of its fractionation, with the main part of Pb strongly bound to the soil particles. However, they noticed a positive correlation between plants shoots contents and the exchangeable and water soluble Pb fractions, suggesting that these forms were actually available to the plants. No Pb was detected in the F1 (water soluble), nor the F2 (exchangeable) fractions for the 3 soils we studied, which might explain that Pb was not or very poorly taken up and was finally not detected in the plants in our case. However, it can be noticed that the analyses of radishes bought from an important supermarket reveal non-negligible quantities of Pb. Moreover, it appeared that these commercial radishes might have been produced from hydroponic solution (soilless) as the ground-markers (as Al, Ca, Fe, Mn) were poorly present. Then, supposing that nutrient solution were not constituted of, nor polluted by Pb, this Pb contamination was probably due to later contamination (treatment, transport, …) and does not contradict the results we obtained in this study, as the Pb detected on these radishes was probably mainly adsorbed onto the tissues.
The reason why the root level of Cd remained under the analytical detection limit for S1 and S3 grown plants could be a low Cd uptake and/or a significant translocation towards the shoots. Such different patterns in radish root and shoot Cd concentrations have been reported by various authors (Gaw et al., 2008; Li et al., 2006; Marchiol et al., 2004; Yang et al., 2009). Li et al. (2006) observed much higher Cd concentrations in the shoots than in the roots, by factors from 1.5 to 4.1 depending on the soil tested. Marchiol et al. (2004) also report the appreciable efficiency of radish in translocating Cd from root to shoots. The enhanced availability to plants on S2 might be explained by the different treatment (amendments, fertilizers, …) this soil underwent. Indeed, some evidence has been reported on fertilizers increasing Cd uptake in plants, as a result of potassium or accompanying anions such as chloride or sulfate anions influence (Zhao et al., 2004).
3.2.2.2 Shoot contents
Similar levels of Ni were detected for all the plants over the 3 soils, as well as for Mn in S1 and S2. The values observed for Zn in shoots were quite identical to their values in the roots. Cd, Fe and Cr were detected in all the shoots at higher levels than in the roots for the plants grown on S2 (Cr) and S3 (Cd, Fe). The presence of Cd, actually detected in the shoots for the 3 soils, supports the idea that Cd had been actually taken up by the plants, even those grown on S1 and S3, despite it not being detected in their roots. Fe levels between 20 and 75 μg/g were determined in the roots against 300 to 1000 (S3) μg/g in the shoots, suggesting that Fe was highly translocated to the shoots.
So, in the shoots, the metals levels observed were generally higher than in the roots, despite that they remained within the same range. Such a trend had been previously observed, suggesting that radish leaves concentrate metals (Davies and Houghton, 1984; Marchiol et al., 2004).
3.2.2.3 Whole plant contents
The whole plant concentration (Cwp) was also calculated for each element according to:
For essential and non-essential metals, Cwp values presented more or less the same trends than the shoots concentration values. This was due to the higher values of shoots DW combined with the similar range of concentrations observed in shoots and roots.
Overall, this total metal accumulation in the whole plant was in the order: Fe>Cr>Mn>Zn>Cd>Ni>Pb for the plants grown on the 3 different soils. This is in full agreement with Bose et al. (2008) for different experimental conditions (Canna indica L. grown on industrial amended soils). Gaw et al. (2008) also reported the order of Fe>Cd>Pb for radish leaves and roots. It is interesting to note that the less mobile elements Cr and Fe, according to the chemical extractions performed, were the more accumulated in the plants. Such a high uptake and accumulation of Fe, despite its low availability, might be a result of the very high total level of this element in the soils. However, this was not the case for Cr, which was present at low concentration especially on S1 and S2 and was poorly mobilised by any extraction. This suggests that the Cr chemical form was highly phytoavailable.
3.3 Comparison of soils extractions and plants data
A simple regression (Pearson correlation) was used for the phytoavailability correlation analysis of the total concentrations in the plants (Cwp) with the various soil fractions obtained by single extractions (with HCl or EDTA) and by the sequential procedure. The correlation coefficients are reported on Fig. 2. Only the Cwp concentrations were used in the correlation analysis to represent the phytoavailable elements, considering that the total amount of element in the whole plant was a better indicator of the element availability to plant.
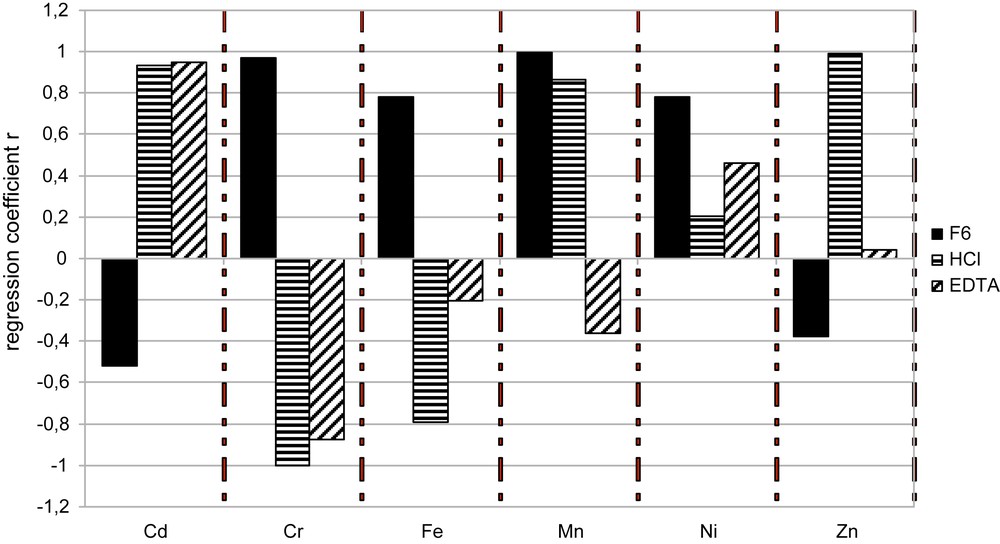
Correlation (n = 6) coefficient between element concentration in the tissue and in HCl, EDTA or F6 fractions.
Coefficients de corrélation (n = 6) entre les concentrations élémentaires dans les radis et dans les fractions HCl, EDTA ou F6.
It seems evident (Fig. 2) that none of the proposed chemical extraction was correlated to phytoavailable elements (for radish), for all the studied elements. The HCl extraction was correctly related to the phytoavailable part of Cd, Mn and Zn whereas F6 sequential extraction seemed correlated with the phytoavailable part of Cr, Fe, Mn and Ni. Finally, it seems that the HCl and sequential chemical extractions brought different and thus complementary/additional information for the study of the phytoavailable metals in soils. For all the studied elements, EDTA extraction seemed less adapted than the two other extractions used.
Bioconcentrations factors (BCF: equal to the ratio between metals concentrations in tissues and soils) are sometimes used to estimate phyotavailability of metals on contaminated soils (Kubova et al., 2008; Li et al., 2006; Spiter et al., 2008). BCF are considered as an indicator of the tendency for an element to accumulate in plants, which highly depends on the metals, soils characteristics and plant genotype. The higher the value is, the easier the plant absorbs and accumulates the metal from the soil (Yang et al., 2009). BCF values, calculated from the Cwp values and the soil total contents, were inferior to 1 and similar for Fe and Ni (0.01–0.04), then for Mn and Zn (0.2–0.7). For Cd, similar BCF values were obtained for S1 (0.6) and S3 (0.4), but much higher for S2 (12.1). Such values and differences for Cd have already been observed (Gaw et al., 2008; Li et al., 2006). It suggests that Cd was far more phytoavailable in S2, in agreement with the HCl and EDTA predictions.
4 Conclusions
In this work, the relationship between the extractable contents of various essential and non-essential elements in 3 individual soils of different nature and the content in radishes was investigated.
Toxic metals (Cr, Cd, Pb) were detected in the 3 studied soils and were also detected in the radish tissues (roots and shoots), except for Pb, which was obviously poorly phytoavailable. According to the sequential procedure used, Pb was mainly present on the reducible fraction in the 3 soils, suggesting that this Pb-fraction was not available to plant uptake. Cd appeared to be more phytoavailable to the radish on the agricultural soil. Cd levels were higher in the shoots than in the roots for the 3 soils. Cr accumulated in the roots, the highest content being observed for the industrial soil. The radish leaves concentrated essential metals such as Fe, Ni, Mn and Zn.
The applied sequential extraction was far more aggressive than the two single extractions used, for each of the 3 soils. The labile trace metals (Mn, Ni, Fe, Zn, Pb, Cr, Cd) were mainly associated with reducible and oxidable fractions, which explains that the sequential extraction appeared far more efficient, compared to the single leaches used.
Possible relationships between the whole plant metals concentrations and the metal extractability by chemical procedures were investigated. We showed that HCl extraction seems to correctly predict the phytoavailability of Cd, Mn and Zn, whereas EDTA could only be used for Cd phytoavailability evaluation.
Whatever the soil was, the sequential extraction procedure could be considered as a better indicator of phytoavailabilty, than the two single extractions studied, for Cr, Fe, Mn and Ni. Indeed, the radish was able to mobilise the non-essential toxic metal Cr, to a quite large extent, from the reducible and oxidable fractions that could not be estimated by any of the single leaches applied. In such a case, the phytoavailabilty can then only be predicted by an adapted sequential procedure.
The chemical forms of the species actually available to plants (i.e. phytoavailable) are not yet elucidated. The chemical speciation of the elements in soils and in plants has to be further investigated. In our opinion, it can only be assumed that the residual fraction determined according the more aggressive chemical extraction (i.e. sequential procedure) contains elements that are never phytoavailable, whereas the non-residual fraction is composed by elements which could be phytoavailable. It remains difficult, regarding the results of the various studies existing in the literature, to find a unique chemical extraction which would be the best suited for the prediction of metals phytoavailability. At least, the method/chemical reactant would probably have to be adapted for each metal of interest, each soil concentration range, and might also vary depending on the simultaneous presence of other metallic element. The main advantage of using single extraction is that rapid results are obtained. However, when several elements have to be simultaneously considered, there is no reactant able to correctly predict the behaviour of all the elements of interest. Then, for a better risk assessment, various procedures have to be applied, using the best reactant for each element. Finally, if a single protocol should be applied for each element, it would be time consuming and sequential extraction would appear as more rapid.


