1 Introduction
Besides contributing greatly to the principles of chemical weathering, Jacques Ébelmen was also the founder of earth system science as it considers the evolution of atmospheric CO2 and O2 over a long geologic time scale (millions of years). The work summarized in his classic paper (Ébelmen, 1845) discusses in detail all of the major processes that affect the levels of atmospheric CO2 and O2, and his analysis, based on present-day knowledge, is complete and contains no omissions or reasoning errors. This was over a 100 years before the independent deduction of the major CO2 and O2-controlling processes by Urey (1952), Garrels and Perry (1974) and Holland (1978). In the present article, attention will be focused on Ébelmen's work on the global cycles of carbon and sulfur and how they have affected past levels of CO2 and O2.
In addition to discussing the carbon and sulfur cycles in relation to the work of Ébelmen, some recent results of modeling by the author (Berner, 2004, 2006, 2008) are presented in terms of calculated variations of CO2 and O2 over the Phanerozoic Era (past 542 million years). This is an example of how one can quantify the ideas originally expressed by Ébelmen.
2 CO2 and the silicate–carbonate subcycle
Carbon dioxide is controlled on a geological or multimillion year time scale by the “long-term” carbon cycle (Berner, 2004; Berner et al., 1983; Walker et al., 1981). This cycle involves transfer of carbon between rocks, the atmosphere and the oceans. One aspect of the cycle is the plant-assisted weathering of Ca and Mg-containing minerals resulting in the uptake of atmospheric CO2 and the subsequent removal of the carbon as carbonates in oceanic bottom sediments. Carbon dioxide is restored to the atmosphere by volcanic and metamorphic degassing, and this process is designated as the silicate–carbonate subcycle.
The uptake of atmospheric CO2 via the plant-assisted weathering of Ca (and Mg) silicates is the result of a series of reactions. First, there is photosynthetic uptake of atmospheric CO2 by land plants. The plant roots and their associated mycorrrhizae then produce organic acids whose carbon is derived from the plants. These acids react with calcium and magnesium silicate minerals to form dissolved Ca++ and Mg++ plus dissolved organic acid anions with the organic anions rapidly oxidized in most soils to HCO3−. The dissolved Ca++ Mg++ and HCO3−are then transported to the oceans by rivers. There the Mg++ is taken up and Ca++ is released during basalt seawater reaction and all Ca++ is ultimately precipitated as CaCO3. Most of these reactions were foreseen by Ébelmen (1845), (see Berner and Maasch, 1996):
« L’action des matières organiques, soit pendant la croissance des végétaux, soit pendant leur décomposition, contribue vraisemblablement à la décomposition des silicates. On sait que certains éléments minéraux, la silice, les bases alcalines et terreuses, le fer et le manganèse sont essentiels à la constitution des végétaux ».
« On peut bien admettre que les racines de ceux-ci puissent produire ou accélérer la décomposition des silicates avec lesquels elles sont en contact… il est probable que des acides organiques autres que l’acide carbonique concourent a cette réaction ».
« Les carbonates dissous dérivés de l’altération sur le continent finiront par se déposer, ou seront absorbés par les animaux marins, mollusques ou zoophytes. Les carbonates alcalins, réagissant sur les sels calcaires contenus dans l’eau de la mer, en précipiteront une quantité proportionnelle au carbonate de chaux ».
(The action of organic matter, either during the growth of vegetation or accompanying its decomposition, contributes probably to the weathering of silicates. One knows that certain elements from minerals, silica, alkalies, alkaline earths, iron and manganese are essential for the constitution of vegetation.
One can admit that the roots (of vegetation) can produce or accelerate the weathering of silicates with which they are in contact. On the other hand, the decomposition of organic materials in the soil exercises, as we have already seen, a dissolving action on many of the materials, which enter into its decomposition, particularly on the ferruginous constituents, and it, is probable that some organic acids other than carbonic acid contribute to this reaction.
The terrestrially derived (dissolved) carbonates end up by being deposited or they are taken up by marine animals, molluscs, and zoophytes. The alkali carbonates react with the calcareous salts contained in seawater, precipitating a proportional quantity of calcium carbonate).
The overall process was stated by Urey (1952) in terms of the reaction:
| (1) |
The restoration of CO2 buried in carbonates is accomplished by the thermal decomposition of the carbonates via volcanism, metamorphism and deep diagenesis. This was also foreseen by Ébelmen (1845):
« Je vois dans les phénomènes volcaniques la principale cause qui restitue à l’atmosphère l’acide carbonique que la décomposition des roches a évacué ».
(I see in volcanic phenomena the principal cause that restores carbon dioxide to the atmosphere that is removed by the decomposition of rocks).
This is summarized by the Urey reaction (1) reading from right to left.
3 The organic carbon subcycle
The other major part of the long-term carbon cycle is that involving the burial of organic matter in sediments and the surficial weathering, or the thermal decomposition at depth, of the organic matter. Organic matter burial affects atmospheric CO2 by taking the remains of both terrestrial and marine organisms and burying them in sediments before they can be oxidized back to CO2. In this way, burial represents a net gain of photosynthesis over respiration. Also as a result of burial, the oxygen produced by photosynthesis is not all consumed by respiration and this results in production of O2 with release to the atmosphere. For every mole of organic carbon buried a mole of O2 is left in the atmosphere. The overall reaction is:
| (2) |
The organic subcycle is balanced by the oxidation of organic matter which is reaction (2) written from right to left. This is accomplished either by the oxidative weathering of organic matter in exposed sedimentary rocks or by the atmospheric oxidation of reduced carbon gases released by volcanism, metamorphism or deep diagenesis.
The above reaction was also foreseen by Ébelmen (1845) as evidenced by his table showing the processes that affect atmospheric CO2 and O2. This is shown, in English translation, in Box 1.
4 O2 and the long-term sulfur cycle
The sulfur cycle over geologic time involves the transfer of oxidized sulfur (sulfate) to and from the oceans, the reduction of sulfate to sulfide in marine sediments, the oxidation of sulfide to sulfate by weathering on land and by the oxidation of volcanically-derived reduced sulfur gases in the atmosphere. This process exerts a major control of atmospheric O2, but a minor control on atmospheric CO2. Ébelmen was one of the first scientists to use modern-style chemical notation and his reactions summarizing the conversion of sulfate to sulfide and vice versa are:
For pyrite formation:
| (3) |
For pyrite weathering:
| (4) |
Note that the compositions and superscripts are archaic, having subsequently been replaced by non-oxide compositions and by subscripts, and atmospheric oxygen (denoted as O) is not expressed as O2 because the oxygen molecule had not yet been discovered. He considered that the formation of 15 moles of organic carbon (denoted as C) was accompanied by the formation of 30 moles of atmospheric oxygen (O). This can be written as:
| (5) |
The summation of reactions (3) and (4) are equivalent to reaction (5) written backwards. When reaction (5) for organic C burial is included, the combination of (3), (4) and (5) is basically correct and truly represents the role of the long-term sulfur cycle as it affects the variation of atmospheric O2 (and to a much lesser extent CO2).
5 A mathematical model for Phanerozoic CO2 and O2
A computer model called GEOCARBSULF for the combined long-term (multimillion year) cycles of carbon and sulfur has been constructed (Berner, 2006, 2008) which enables the calculation of the levels of atmospheric CO2 and O2 over geologic time. Basic assumptions (see Berner, 2004 for further discussion) of the model are:
- • volcanic and metamorphic release of CO2 to the earth surface (combined atmosphere + biosphere + hydrosphere) is balanced by the uptake of CO2 by the chemical weathering of Ca and Mg silicates;
- • addition of carbon, sulfur, calcium and magnesium to the oceans by weathering is balanced by their rate of removal to bottom sediments and reaction with marine volcanic;
- • the rate of global degassing is affected by changes in seafloor spreading rate and the evolution of calcareous marine plankton;
- • the rate of silicate weathering is affected by changes in: global mean land temperature, global water runoff from the continents, the rate of continental physical erosion (that exposes fresh rock to the atmosphere), land area underlain by silicates, basalt vs. granite weathering, and the evolution of land plants;
- • the rate of carbonate weathering is affected by changes in: global mean land temperature, global water runoff from the continents, land area underlain by carbonates, and the evolution of land plants;
- • the rates of organic carbon and pyrite sulfur weathering (oxidation) are affected by the mass of each on the continents and rates of global physical erosion;
- • the rate of calcium sulfate weathering is affected by its mass on the continents and changes in global water runoff;
- • global mean land temperature is affected by the CO2 greenhouse effect and changes in solar radiation and continental paleogeography;
- • the rock record of 13C/12C and 34S/32S are used to calculate rates of organic carbon and pyrite sulfur burial (O2 production) and organic carbon and pyrite sulfur oxidative weathering (O2 consumption);
- • the fractionation of carbon isotopes (during photosynthesis) and sulfur isotopes (during bacterial sulfate reduction) is a function of the O2 content of the atmosphere.
Results of the modeling for CO2 and O2 over Phanerozoic time (past 542 million years) are shown in Figs. 1 and 2. The large variations in CO2 and O2 shown by the figures were already predicted by Ébelmen:
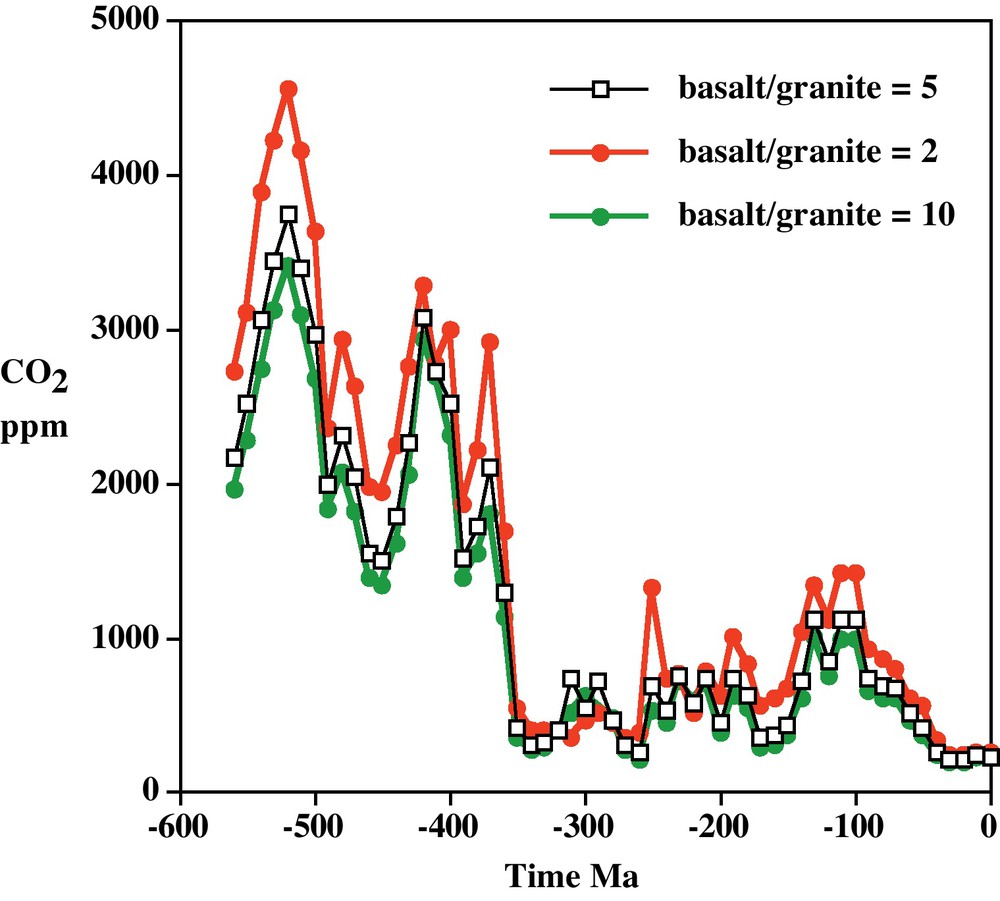
Plot of CO2 vs. time based on the GEOCARBSULF model (Berner, 2006, 2008) showing sensitivity to the ratio of basalt/granite intrinsic weatherability.
Diagramme de CO2 en fonction du temps, basé sur le modèle GEOCARBSULF (Berner, 2006, 2008) montrant la sensibilité au rapport d’altérabilité intrinsèque basalte/granite.
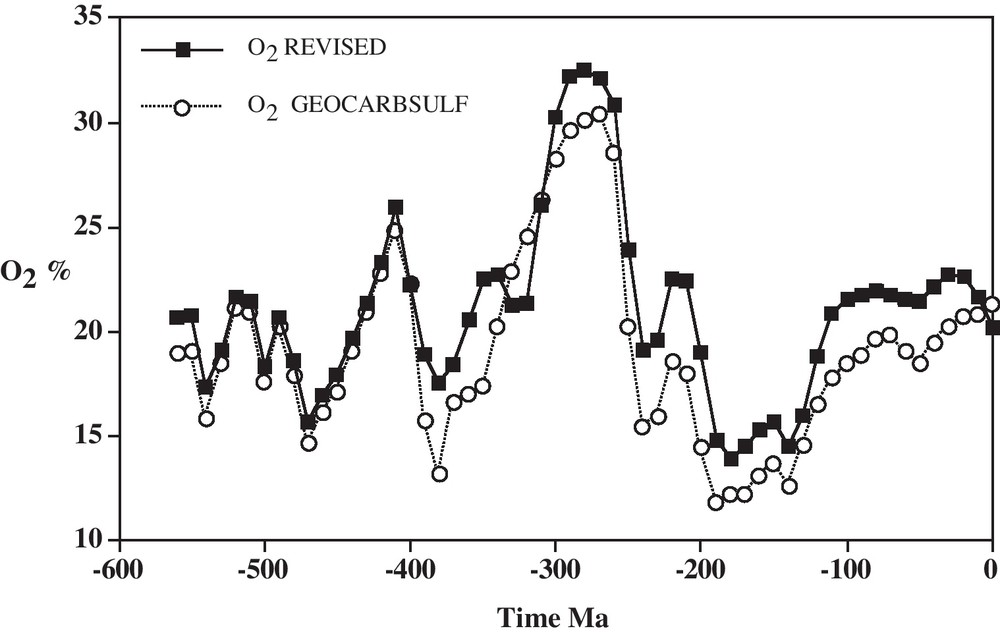
Plot of O2 vs. time based on the GEOCARBSULF model (Berner, 2006) with revisions based on updated carbon isotopic data (Berner, 2009).
Diagramme de O2 en fonction du temps, basé sur le modèle GEOCARBSULF (Berner, 2006), avec des révisions sur la base de données sur le carbone isotopique mise à jour (Berner, 2009).
« Plusieurs circonstances tendent néanmoins à prouver qu’aux anciennes époques géologiques, l’atmosphère était plus dense et plus riche en acide carbonique et peut-être en oxygène, qu’à l’époque actuelle. Les variations dans la nature de l’air ont été sans doute constamment en rapport avec les êtres organisés qui vivaient à chacune de ces époques ».
(Many circumstances, nonetheless, tend to prove that in ancient epochs the atmosphere was denser and richer in CO2, and perhaps O2, than at present. The variations in the nature of the air without doubt have always been in keeping with the organisms that were living at each of these epochs).
6 Conclusions
Jacques Ébelmen stated correctly the important fundamentals of how atmospheric CO2 and O2 are affected by long-term natural processes. This work was far ahead of its time relative to the work being performed by nineteenth century earth scientists, but, unfortunately, it was basically lost to history. Over a 100 years later his ideas were independently repeated by Urey (1952), Holland (1978) and Garrels and Perry (1974). As shown in the present article, the foundations by Ébelmen can be used to construct a quantitative model for the actual levels for CO2 and O2 over geologic time. For comparison, the reader is referred to other similar models such as those of Tajika (1998), Gibbs et al., 1999; Wallmann (2001), Kashiwagi and Shikazono (2002), Hansen and Wallmann (2003), Bergman et al., 2003, Donnadieu et al., 2004, Arvidson et al., 2006 and Goddéris et al., 2008. Goddéris et al., 2008 also cite the work of Ébelmen. What is needed in the future are better and more comprehensive models but they will all rest eventually on the foundation created long ago by Jacques-Joseph Ébelmen.


