1 Introduction and background
A critical aspect of the global cycles of the elements is the transfer of material from the continents to the oceans. Recent estimates of the total mass transferred from the continents to the oceans via various processes are shown in Fig. 1. This mass transfer is dominated by particulate material transported by rivers as bedload and suspended material; these estimates suggest that this particulate flux exceeds by a factor of ∼20 the combined mass fluxes of the dissolved riverine load and atmospheric dust (Gaillardet et al., 2003; Jickells et al., 2005; Walling, 2006). The role of particulate fluxes to the oceans has received increased recent interest because these fluxes are strongly sensitive to climate changes (Gislason et al., 2009) and anthropogenic influences (Syvitski et al., 2005; Walling, 2006). If only 5% of the riverine transported particulate material dissolves after its arrival in the oceans, it will dominate the chemical evolution of seawater as well as the feedback between continental weathering and climate (Gislason et al., 2006).
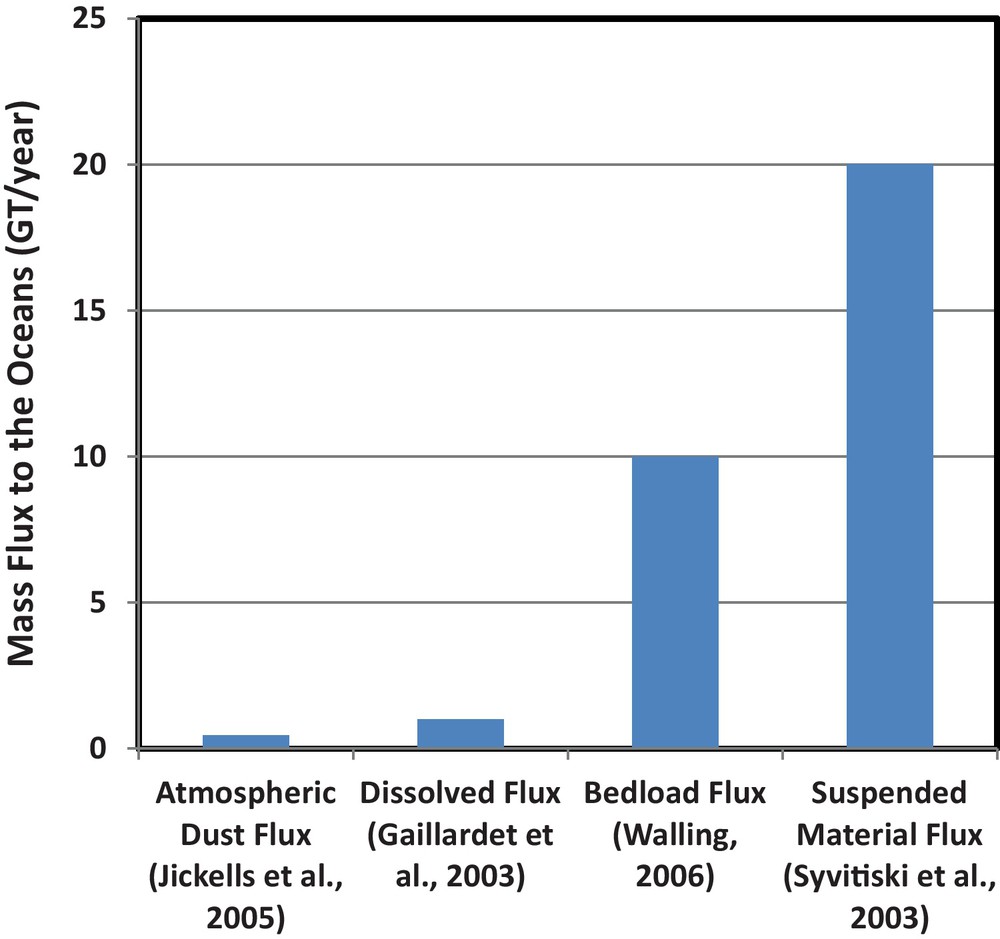
Estimates of total mass of material transported from the continents to the oceans by selected processes.
Estimation de la masse totale de matériel transporté des continents aux océans par des processus sélectionnés.
The degree to which particulate material dissolution will influence the composition of each element in the ocean varies strongly among the elements. The ratio of the concentration of selected elements in riverine transported suspended material to their concentration in seawater is shown in Fig. 2. The relative concentration of the elements varies by over nine orders of magnitude. Soluble elements like Br and Na are more concentrated in seawater than in river-transported suspended material. In contrast, insoluble elements, including Al, Fe, and the Rare Earths are more than seven orders of magnitude more concentrated in suspended particulate material than in seawater. It follows that just a small quantity of seawater-particulate material interaction could lead to large changes in the isotopic composition of the insoluble elements such as Fe and Nd, but would negligibly alter the corresponding compositions of soluble elements such as Br.

Ratio of global suspended river-transported particulates to seawater concentration of selected elements. The suspended material data composition was taken from Viers et al. (2009) whereas the composition of seawater was taken from Turekian (1968).
Rapport entre le volume global de particules transportées en suspension par les rivières et la concentration de l’eau de mer en éléments sélectionnés. La composition du matériel en suspension est issue de Viers et al. (2009), tandis que la composition de l’eau de mer est issue de Turekian (1968).
As riverine particulate material is comprised of a number of different phases, each having distinct dissolution rates and saturation states (e.g. Schott and Oelkers, 1995; Schott et al., 2009), the release rates from particulate material may vary widely from element to element. This manuscript assesses this possibility by comparing recent results of two extreme examples: Sr and Nd. Strontium is a soluble element whose average concentration in riverine transported suspended material exceeds that of seawater by only a factor of 23. Moreover, the total flux of Sr to the oceans by dissolved riverine transport is approximately equal to the flux brought to the oceans within particulate material (e.g. Oelkers et al., 2011). Strontium transport to the oceans is of high current interest due to the observation that the present-day isotopic composition of the ocean is inconsistent with the relative contributions of the global dissolved riverine Sr flux and that inputted to the oceans at mid-ocean ridges (e.g. Allègre et al., 2010; Davis et al., 2003; Jeandel et al., 2011; Jones et al., 2012b; Palmer and Edmond, 1989; Vance et al., 2009). In contrast, Nd is an insoluble element; its average concentration in riverine transported suspended material exceeds that of seawater by nearly seven orders of magnitude. Because of its relatively short residence time in the oceans, Nd isotopic ratios are commonly used as a tracer of paleocirculation and weathering (Burton et al., 1997; Frank, 2002).
2 Review of experimental results and discussion
The role that riverine transported material plays on the global cycles of the elements depends greatly on its dissolution rate in seawater. Quantifying particulate material/seawater interaction is challenging because the chemical composition of many elements in seawater is buffered by the saturation or supersaturation of a large number of potentially precipitating secondary phases. Because the release of elements into seawater by particulate material dissolution likely provokes secondary phase precipitation, elemental concentrations may be relatively unchanged in seawater by particulate material dissolution. This coupling of particulate dissolution and secondary mineral precipitation is commonly observed in ocean shelf sediments (e.g. Aller et al., 1996; McKee et al., 2004). One way to quantify the degree of particulate dissolution in seawater independent of the effects of secondary phase precipitation is through the use of stable isotope compositions as a tracer of particulate seawater interaction (e.g. Arsouze et al., 2009; Jeandel et al., 1998; Jones et al., 2012a; Lacan and Jeandel, 2001, 2005; Wimpenny et al., 2010).
This approach has been adopted to determine elemental release rates from riverine transported particulate material via batch experiments. In each experiment, known quantities of particulate material and seawater (with known compositions) were placed in closed-system reactors (e.g. Gherbi et al., 2010). The fluid phase was regularly sampled and analyzed for both total element concentration and the isotopic ratio of selected elements. As mentioned above, secondary minerals likely precipitated during these experiments, as seawater is saturated or supersaturated with a large number of phases. As such, it is impossible to determine unambiguously the degree to which each element was released from the particulate material from total element concentrations. If, however, it can be assumed that there is no isotopic fractionation of an element during dissolution and/or precipitation (as is the case for the radiogenic Sr and Nd isotope systems), the mass of an element transferred from the particulate phase to seawater during dissolution in a closed-system reactor can be calculated from:
The rate of Nd release during three laboratory batch experiments, to be reported by Pearce et al. (in preparation), are shown in Fig. 3. In each of these batch experiments, 30 g of particulate material was added to ∼30 kg of seawater. This large volume of seawater is required for Nd isotope ratio measurements. The three particulate materials considered were:
- • a bedload sand collected from the base of the Hvítá river (West Iceland);
- • an estuarine sand collected from the Borgarfjörður estuary (West Iceland);
- • a marine sand collected near the Kerguelen Islands (southern Indian Ocean).
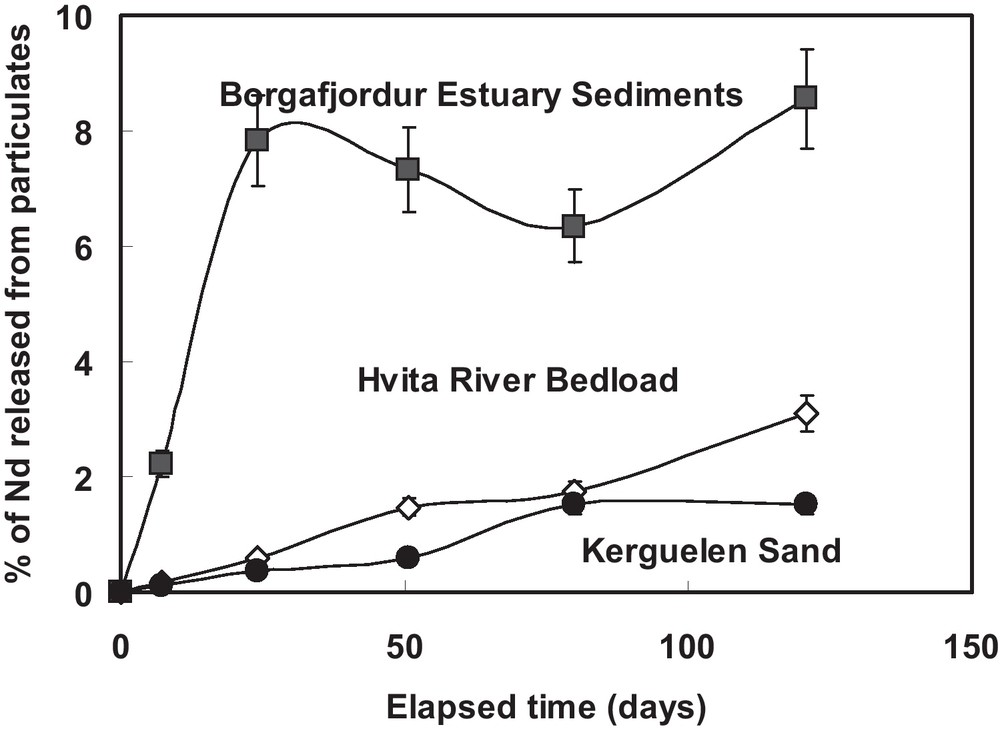
Percent of Nd released during laboratory batch particulate dissolution experiments in seawater as a function of time (after Pearce et al., in preparation).
Pourcentage de Nd libéré dans l’eau de mer pendant les expériences « batch » de dissolution particulaire en laboratoire, en fonction du temps (selon Pearce et al., en préparation).
Each of these sands is basaltic in composition. These sands contain 72.6, 87.0, and 331.3 ppb Nd, and have surface areas of 6.35, 7.35, and 2.21 m2/g, respectively. Results shown in Fig. 3 indicate that from 1.5 to 8.5% of the Nd originally present in the solid phase dissolved into the seawater during the 125-day laboratory batch experiments. This overall Nd release rate corresponds to 10−17 to 10−19 mol Nd/m2/s, which is significantly lower than the dissolution rates of the common Nd bearing minerals plagioclase, apatite, and monazite at pH 8 and 25 °C (Blum and Stillings, 1995; Chairat et al., 2007; Kohler et al., 2005; Oelkers and Poitrasson, 2002).
Similar closed-system experiments, performed to assess the degree of Sr release from both continental and volcanic particulate material have been reported by Jones et al. (2012a, b). In these experiments, the mass ratio of particulate material to seawater was 1:3. Eight particulate materials including riverine bedload and river-transported suspended material were studied, as well as, two volcanic ashes. The surface areas of these particulate materials ranged from 1.5 to 22.3 m2/g. A summary of the Sr released into seawater during these experiments is illustrated in Fig. 4. Between 0.15 and 28% of the Sr contained in the particulate material were released during the 180-day batch experiments. The degree to which Sr is released depends strongly on the identity of the particulate material. The most reactive material is the riverine suspended load collected from the Jökulsá í Fljótsdal River in NE Iceland. This river drains a glacier and the suspended material is dominated by reactive basaltic glass. This particulate material also has the highest surface area of the particulates studied. The second most reactive studied particulate material was bedload material collected from Sveinsgil tributary of the Tungnaá River in S Iceland, which drains the rhyolitic caldera associated with the Torfajökull volcano. The least reactive particulate material was that of the bedload collected from the Mississippi river near its mouth in New Orleans. Less than 1% of the Sr contained in the Mississippi bedload material was released to seawater during the 180-day batch experiment. Although it is difficult to draw definitive conclusions based on the reactivity of just eight distinct particulate material-seawater experiments, results suggest that:
- • suspended riverine material is more reactive that its corresponding bedload material, perhaps due to its higher surface area;
- • fresh volcanic material is more reactive than older continental material.

Percent of Sr released during laboratory batch particulate dissolution experiments in seawater as a function of time (after Jones et al., 2012b).
Pourcentage de Sr libéré dans l’eau de mer pendant les expériences « batch » de dissolution particulaire en laboratoire, en fonction du temps (selon Jones et al., 2012b).
These observations are consistent with the results of laboratory investigations (as summarized by Wolff-Boenisch et al., 2006), which show that dissolution rates are proportional to their surface area and increase substantially with the decreasing SiO2 content of the mineral or glass. The observation that volcanic particulate material more readily releases Sr suggests that one effect of particulate material dissolution on the global scale is that this process will release to the ocean Sr that is on average more unradiogenic than that of the average particulate material. As such, this process may help close the currently perceived imbalance in the ocean Sr fluxes (Allègre et al., 2010; Davis et al., 2003; Jones et al., 2012a, b; Peucker-Ehrenbrink et al., 2010; Vance et al., 2009).
The exact process by which elements are released from particulate material to seawater is currently unclear. Several processes, including dissolution, ion exchange, and desorption may each play a role. Dissolution most likely occurs as numerous primary minerals and glasses, including pyroxene, basaltic glass, and plagioclase, are undersaturated in seawater, and the Si concentrations in the fluid phase increase during the experiments (Brady and Gíslason, 1997; Jones et al., 2012a, b; Stefánsdóttir and Gislason, 2005). In addition, the dissolved Si isotope ratios measured in the Kerguelen plateau surface waters suggest that a lithogenic source releases Si to these waters (Fripiat et al., 2011). Ion exchange is favored by the elevated concentration of sodium and other aqueous cations in seawater, available for exchange reactions (Oelkers et al., 2009). Metal desorption may, however, be unfavored as particulate material arrives in seawater. This is because the pH of seawater is, in general, higher than that of continental rivers, and an increase of pH tends to favor the adsorption of positively charged ions rather than desorption (e.g. Hatje et al., 2003; Parks, 1990).
A significant observation is that to a first approximation, the rate of release from riverine transported particulate material of Nd is similar to that of Sr. Neodynium and strontium are representative of two extremes: Nd is an insoluble element that is more than 107 times more concentrated in particulate material than in seawater whereas Sr is a soluble element that is only 23 times more concentrated in particulate material as in seawater. As such, it seems likely that most elements will be significantly released from riverine transported particulate material upon its arrival to the oceans.
3 Conclusions
The results summarized above have a number of consequences for our understanding of the global cycles of the elements:
- • riverine transported particulate material (i.e. suspended and bedload) is chemically reactive in the ocean; from 0.25 to 28% of Sr and 1.5 to 8% of Nd contained in reacted particulate material was released to seawater during 3–6 month batch experiments;
- • the mass of riverine transported particulate material fluxes to the ocean is ∼20 times greater than the sum of riverine dissolved flux and the atmospheric dust flux, thus it is likely that the dissolution of particulate material dominates the chemistry of numerous elements in seawater;
- • dissolution of particulate material has been demonstrated to release significant quantities of Sr and Nd to seawater. These fluxes may help account for the current perceived imbalances in marine Sr isotope mass balances, and confirm the “Nd boundary exchange” hypothesis of Lacan and Jeandel (2005) and Arsouze et al. (2009).
Acknowledgements
We thank Jérôme Gaillardet, Derek Vance, Jacques Schott, and Oleg Pokrovsky for insightful discussions and encouragement. M.T. Jones and C.R. Pearce were supported by the EC Marie Curie “MIN-GRO” Research and Training Network (MRTN-CT-2006-035488). M.T. Jones is currently supported by a Marie Curie Intra-European Fellowship (PIEF-GA-2009-254495).


