Prologue
The United Nations has proclaimed 2022 as the International Year of Glass, highlighting the role of glass in the scientific, economic, artistic and cultural fields. Glass is essential to many vital technologies, as it facilitates the transition to a more sustainable world and beautifies our lives.
This United Nations resolution is a unique and historic event for glass. It is the result of a long journey initiated in 2018 which has been made possible thanks to the support of 1500 universities and research centers, learned societies and associations, museums, artists, manufacturers and by companies in 79 countries and across 5 continents. The formal resolution was adopted at the United Nations General Assembly on 18 May 2021.
Glass is obviously the material of our daily lives that we find in tableware, food packaging, bottles for beverage, cosmetic perfumery and pharmacy. We are surrounded by glass in our homes, we use it for transport (float glass, glass wool, rockwool, or textile glassfiber) and technological applications in optics and energy (photonics, battery). Glass is also an asset for prostheses (bioglasses for bone implants, for example) and we must not forget that glass is also an important repository for memories of humanity and the history of our planet and the universe. This special issue of C. R. Geosciences brings together several papers on these various aspects after a short introduction on this material, glass. Cormier et al. [2022] describe the glass structure and focus on cationic mixing based on X-ray and neutron diffraction at high temperature. Their paper shows also the link between glass and liquid. Next, the article edited by Bauchy team details challenges and opportunities of the computational techniques needed to understand the structure and properties of glasses and liquids [Liu et al. 2022]. Glass is not only our daily glass as shown by the paper by Pradel and Piarristeguy [2022] who show that glass is not only composed of oxide, but that the world of glasses is more complex because it is possible to obtain glasses with all the elements [Pradel and Piarristeguy 2022] and with all the chemical bonds; a complementary article on metal glasses will be published in the CR Physique [Champion 2022]. Glass has always accompanied humanity and it allows us to transcend our beliefs and fears, as shown by symbols, stories and myths around decorated and stained glasses. The paper by Hunault et al. [2022] presents an emblematic stained glass, and how its restoration has allowed to learn more about its origin and history, the rose of the Sainte-Chapelle. McCloy and Schuller [2022] discuss waste vitrification. Glass has multiple uses in our daily lives and it will become more and more present tomorrow, as shown in the article by Blanc et al. on optical fibers and sensor development [Blanc et al. 2022].
Brauer and Hupa [2022] demonstrate that glass can also use to rebuild parts of the human body. They explain how since the first work made by Hench et al. [1971], it is possible to use glass for regenerating bone tissues. Actually, several glass compositions are used in medicine and dentistry [Brauer and Hupa 2022] and it is easy to imagine that in the coming years, the proportion of bioglasses will further increase.
Glass is also a witness to our past, because the investigation of glasses found either in meteorites or in crystals in the form of glass inclusions enable us to trace the history of our planet. These glasses may have different water content which is the main volatile component in magmas and plays an important role in eruptive mechanisms. The paper by Le Losq et al. focuses on how water affects the polymerization of glasses and liquids [Le Losq et al. 2022]. Another key to understanding eruptive processes is the evolution of magma crystallization [Vetere et al. 2022]. Finally, Moretti [2022] focuses on the volatile-melt interaction, showing how these affect the redox state, hence the speciation state of gases released by active volcanic systems.
1. Introduction
Glass is a homogeneous material that can form from almost all elements of the periodic table (Figure 1) and all types of chemical bonds. This material differs from other states of matter (crystalline solid, liquid, gas and plasma) firstly by its solid state which is characterized by an absence of order at a great distance and, secondly, by the presence of a second-order transition, called glass temperature transition. The reader will find detailed and recent publications on all families of glasses and their properties in the publication “Handbook of Glass [Musgraves et al. 2019]”. In this introduction, we will present some of the general concepts accepted by the entire scientific community and taught in universities and engineering schools.
Periodic table with possible role of elements in glass or melt. Elements in white can be in glass without any specific role. Elements in white are almost never investigated in glass structure, but they can be present in a glass probably as a gas or network modifier.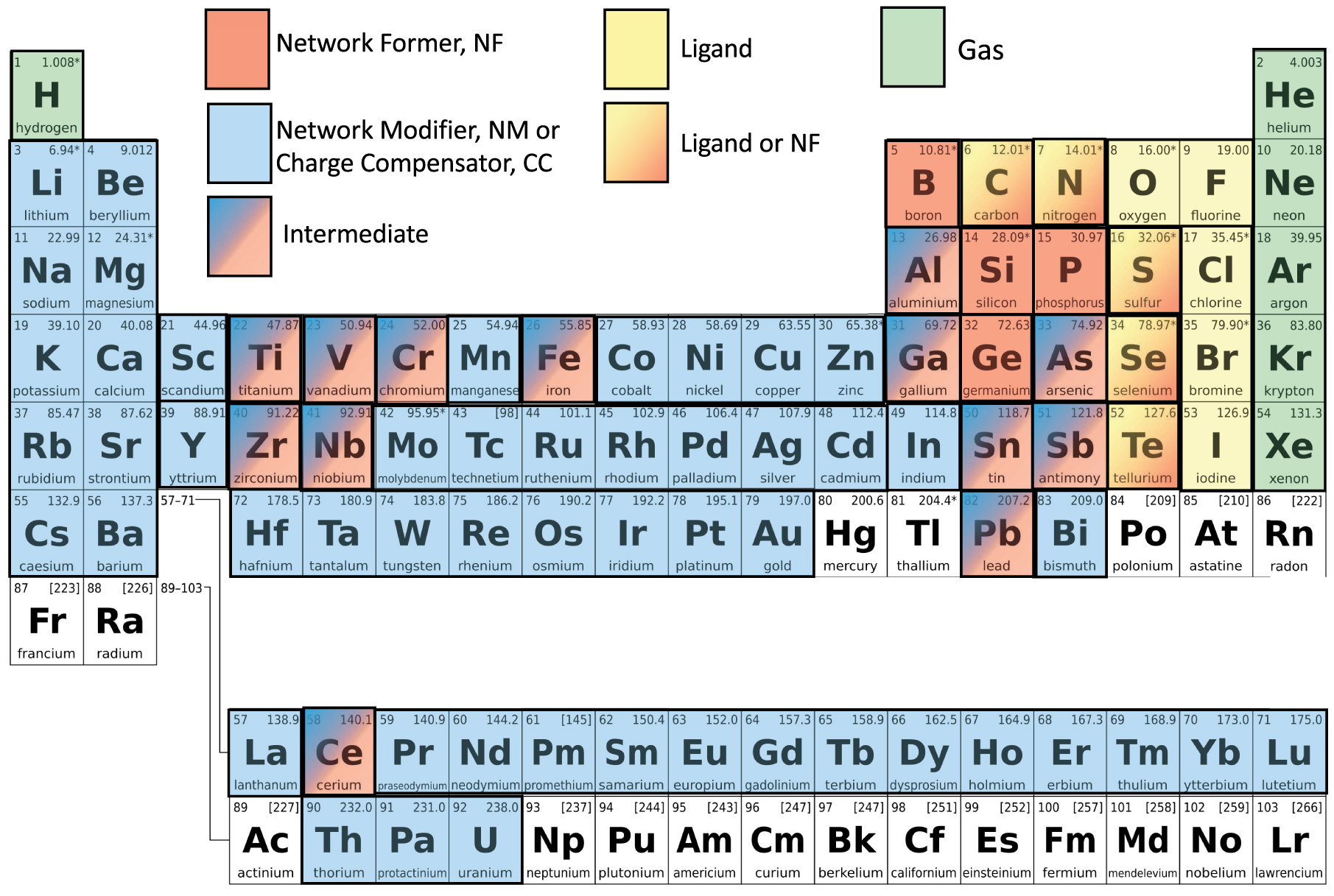
A glass is an amorphous solid. The term solid implies high viscosity, usually greater than 1011 Pa⋅s. This viscosity limits the flow of the material. The term amorphous implies the absence of long-range order which reveals an analogy with the liquid state. A glass is therefore a solid whose certain properties are close to those of liquids. Parks and Huffman even speak of “a fourth state of matter” [Parks and Huffman 1926].
The glass state can occur for many chemical compositions as long as the crystallization phenomenon is avoided. There are mineral glasses and other common glasses based on silicate compounds, oxides, salts, metal, organic glasses such as gels, caramels, candies. In fact, it is possible to obtain glasses regardless of the type of chemical bond: covalent, ionic, metallic, Van der Waals or hydrogen. The state of glass therefore is characteristic of condensed matter and there can be an infinite palette of chemical composition of glasses, each with its own structure. As we will see, each of them, individually, can be defined as a material or substance in its own right with its own physical and chemical characteristics such as glass transition temperature (Tg), X-ray pattern or Raman spectrum, elemental chemical analysis, density, refractive index, etc.
Many methods of preparation make it possible to obtain amorphous materials. They are generally developed by rapid cooling of a liquid phase, but it is also possible to obtain them from a gas phase, by amorphization of a crystalline phase or by sol-gel methods [Descamps 2017]. The most common method of glass forming is by freezing of a liquid during rapid cooling. During the cooling of a liquid, there is a continuous increase in viscosity to such a value that the material can be considered a solid. We can therefore imagine the structure of the glass as being similar to that of a liquid, whose entire atomic movements are blocked. Glasses have, like liquids, a disordered structure with only a short-range order. For example, silica, a vitreous phase, consists of SiO4 tetrahedra, associated with each other by an oxygen atom but without long-range periodicity of the lattice, seen in crystals. The oxygen atom connecting two silicon atoms is said to bridge. Figure 2 shows a 2D schematic representation of SiO4 tetrahedra for crystal and glass.
2D representation of: (a) a crystal structure consisting of Si atom (black circle) and oxygen (white circle), there is a geometric pattern (hexagon) and a periodicity while in (b) atomic arrangements are present (rings with 3, 4, 5, 6 tetrahedra) but these arrangements are not regular or periodic [according to Zachariasen 1932]. Note that both structures have the same number of Si atoms, but do not occupy the same surface area due to a lower density for glass than for crystal [modified from Neuville and Cormier 2022].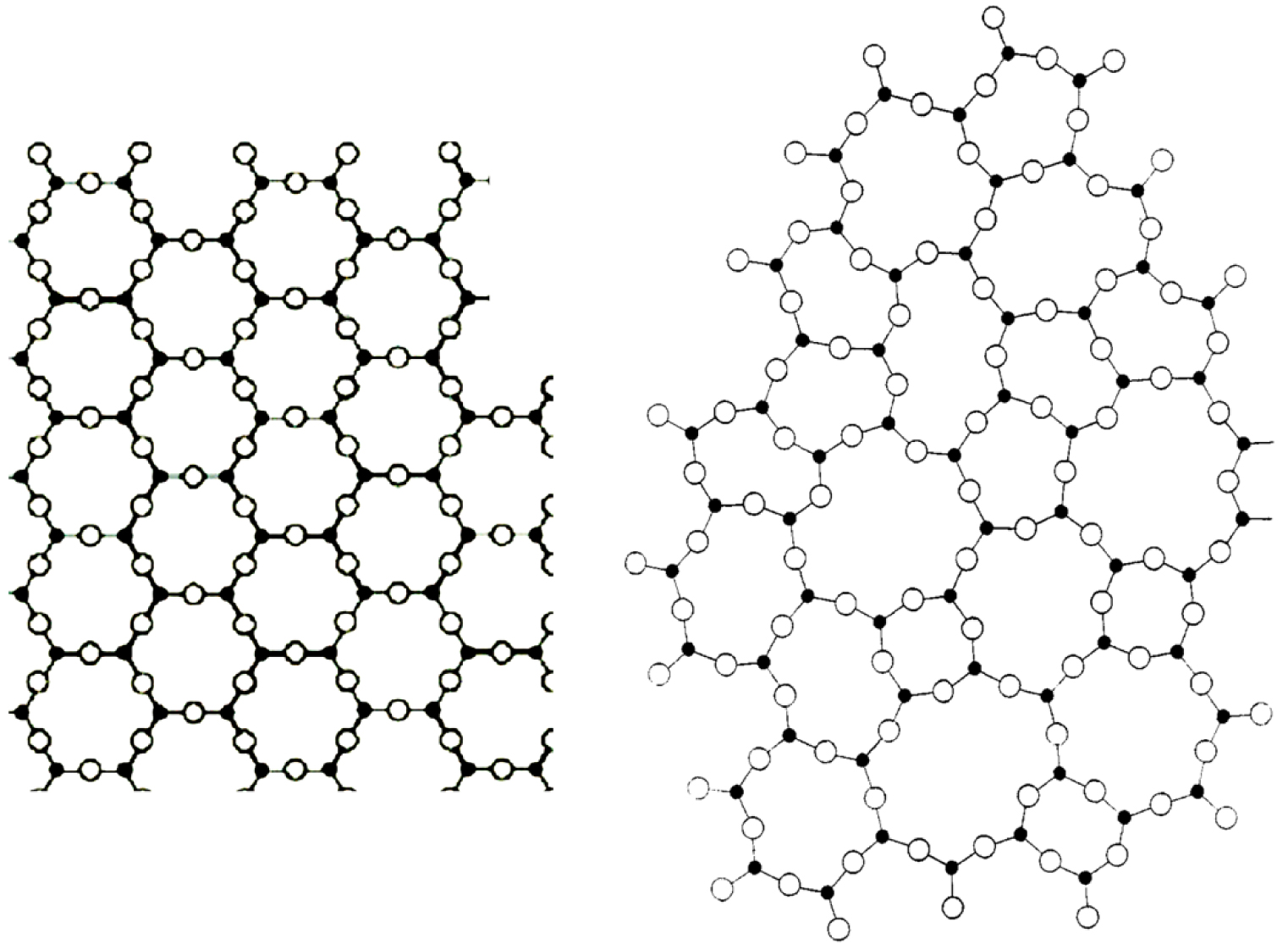
2. A disordered structure?
In Section 1, we mentioned a glass composed only of silicon and oxygen whose respective roles are that of network former and ligand, but how to differentiate the structure of silica from that of a quartz crystal? Figure 3 clearly shows for SiO2, amorphous or crystalline (quartz or low-cristobalite), the spectral differences obtained by two different structural probes (X-ray diffraction, Raman spectrometry).
(a) X-ray diffraction, (b) Raman spectrometry in blue (quartz or low-cristobalite) and SiO2 amorphous in red. For practical reasons, spectra are reported in arbitrary units. The conditions of acquisition of the spectra of the crystalline and amorphous phases are different for the same technique and from one technique to another [redrafted from Cormier and Neuville 2022].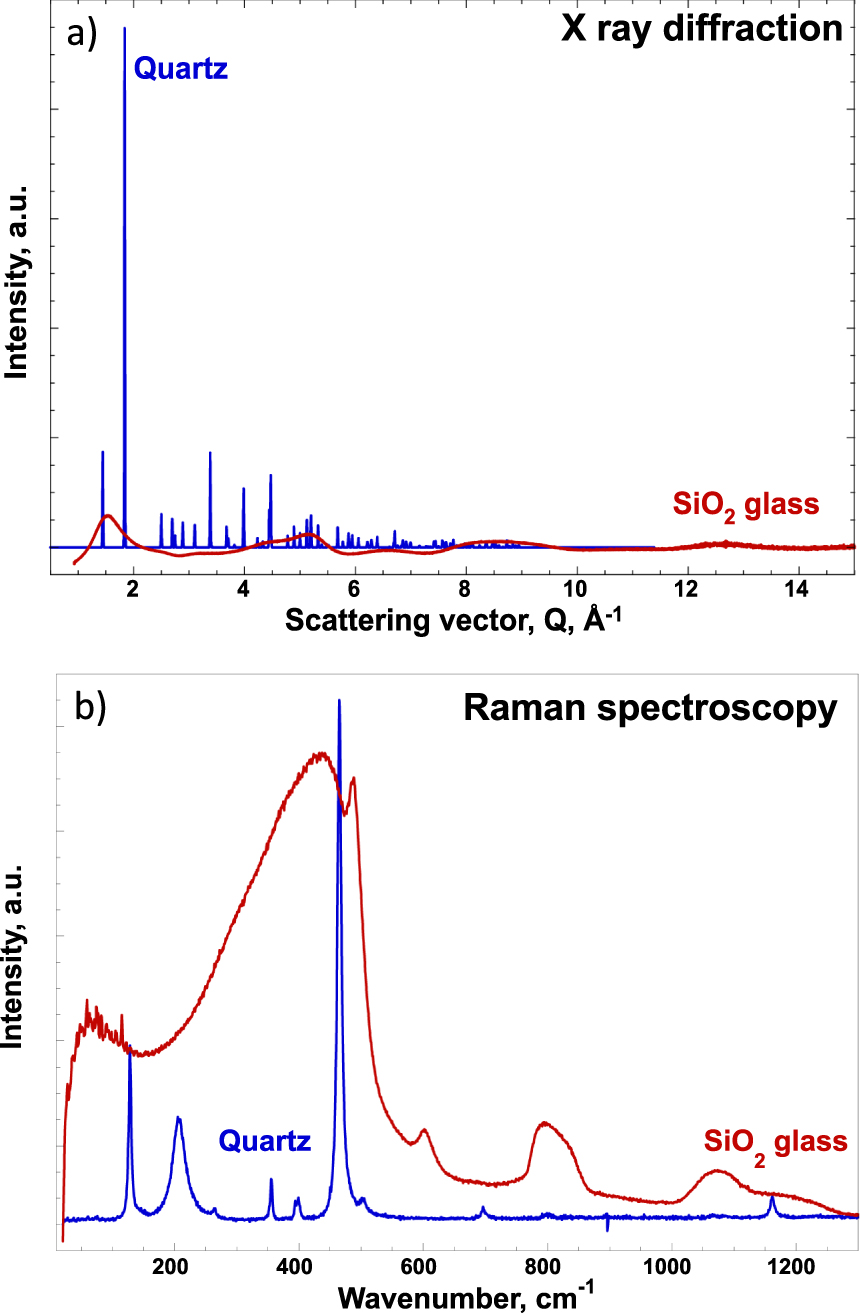
Regardless of the type of glass the experimental technique used, the crystalline phase data show fine peaks while those of glass show broad peaks. More precisely, X-ray diffraction shows very intense Bragg peaks up to a distance of 8 Å−1 for quartz crystal and very low intensity wide strips for glass. The short-range order for distances less than 3 Å characterizes the SiO4 coordination polyhedron, interatomic distances and bond angles, as well as the possibility of having heteropolar bonds (Si–O). To summarize, at a short distance, there is a local order that concerns the first layer of coordination. This is governed by the chemical bond that imposes the number of neighbors (coordinance), the interatomic distances, and the symmetry of the site, for example a tetrahedron SiO4 in silicates.
The region at the longest distance between 3–20 Å corresponds to the sequences and arrangements between polyhedra. It is on this scale that the distinction is made between crystalline and amorphous phase. A detailed analysis of this region provides important information on the knowledge of the angles between polyhedra, the type of connectivity (corner-, edges- or faces-sharing), the 2D or 3D dimensionality of the network and, potentially, the existence of structural/compositional heterogeneities. For X-ray diffraction, long-range or high-energy signal loss materializes the difference between an ordered and disordered structural state [Cormier and Neuville 2022]. Raman spectrometry also highlights the differences between the spectrum of quartz and that of silica glass. In particular, the Raman spectrum of silica reveals glass-specific structures, which do not exist in the crystalline phases: these are rings with three or four SiO4 tetrahedra characterized by peaks at 490 and 605 cm−1, called D1 and D2, respectively. In addition, it should be noted that the Raman spectral efficiency of a quartz crystal and amorphous silica is strongly different. Indeed, the Raman spectrum of quartz is acquired in 10 s against 100 s for that of silica glass.
To summarize, at medium distance, the order is defined by the relations between the different sites, for example, the angle between two tetrahedra SiO4 in silicates. This order in glasses is difficult to characterize experimentally and is the subject of numerous experimental studies and numerical simulations [Liu et al. 2022]. Its characterization is essential because this scale of organization is directly related to the origin of most of the properties of glasses, such as density, ion conduction or chemical durability. Finally, at a great distance, there is no periodicity of structures, unlike crystals.
This short paragraph shows that, whatever the structural technique used, it is particularly difficult to grasp the nature and extent of the medium-distance order characteristic of the structural disorder of glass. In addition, this deficit of information is one of the major current challenges in the structural knowledge of glasses.
3. The glass transition temperature
Unlike the case of a crystallized solid, glass does not have a melting temperature. Conversely, the solidification of a glass corresponds to a continuous and gradual increase in viscosity during the cooling of a liquid, without the appearance of a crystalline structure. In Figure 4, we reported the variations in viscosity as a function of reciprocal temperature, for several molten silicates. There is no break in the viscosity curves between the values obtained at high temperature in the liquid state and those at low temperature in the glass state. This shows the continuous transition from glass to liquid for a property such as viscosity. The glass transition is a kinetic phenomenon that characterizes the loss of internal thermodynamic equilibrium. Indeed, the properties of a glass no longer depend only on pressure and temperature, but also on the speed, |q|, at which the glass transition temperature occurs (for a detailed discussion refer to Neuville and Le Losq [2022]).
Viscosity as a function of reciprocal temperature for a silica glass, SiO2, sodium silicate glasses, NS3 and NS2, containing respectively 75 and 66 mol% of SiO2 and the difference to 100 being Na2O, the compositions NS1.5 and CS1.5 correspond respectively to 60 SiO2–40 Na2O and 60 SiO2–40 CaO (mol%) from Neuville [2006] for CS1.5, NS1.5, NS2. The compositions NA75.6 and NA75.12 contain 75 mol% SiO2 each and 6 or 12 mol% Al2O3, the difference to 100 being Na2O [data of Le Losq et al. 2014] for NS3 and NAX. Y compositions, Napolitano et al. [1965] for B2O3; from Sipp et al. [1997] for NB82.10 = Pyrex.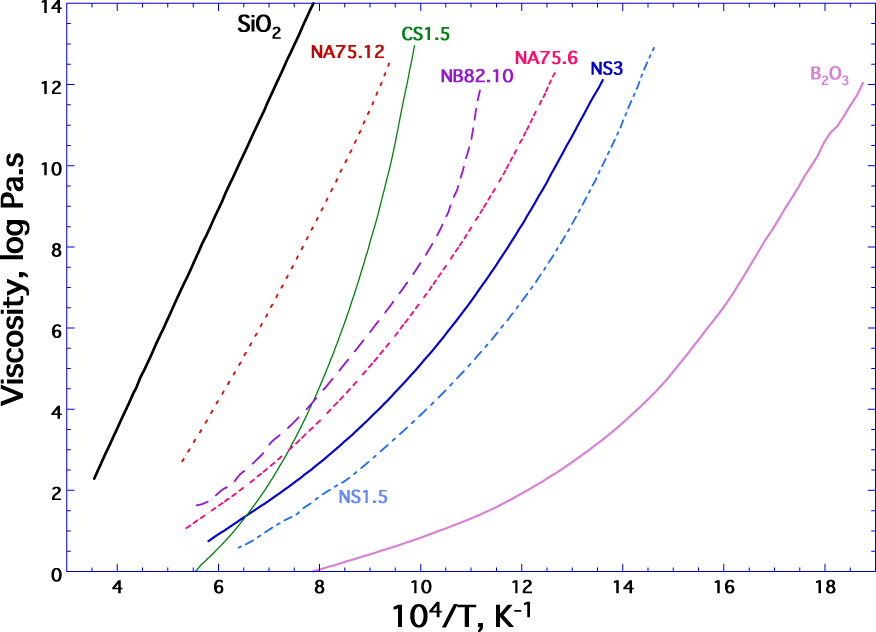
The simplest and oldest characterization of this transition has been described by Parks and Huffman [1927]. They note that by heating a glass, “it continuously passes from the amorphous state to the liquid state, its properties changing regularly with temperature, without any discontinuity”. In fact, as they write, “while there is no precise temperature comparable to the melting point of a crystal, there is nevertheless a temperature interval, net and reproducible, in which many properties change with a speed that approaches that is observed in the fusion of a crystal. In short, there is a softening interval instead of a melting point. Glass as it exists below this softening interval differs so markedly from the liquid that exists above that it could well be considered a different state from the substance.”
The glass transition corresponds to a zone of temperature and pressure in which properties such as specific heat undergo a second-order transition [Moynihan et al. 1976a, b]. Therefore, the physicochemical properties of a glass depend on the speed at which the temperature varies and therefore on its thermal history.
It is theoretically possible to obtain identical temporal variations for all properties, such as thermal expansion or viscosity. It is therefore clear that there is a response time to the temperature variation. We call this time 𝜏, relaxation time. We define it as follows: a liquid has a large number of different configurational states [Goldstein 1976, 1969, 2011]. Each state corresponds to a minimum of potential energy. When the temperature decreases, the time of structural relaxation of a liquid, 𝜏, increases. When 𝜏 is large enough, the liquid can no longer carry out cooperative structural rearrangements [Goldstein 1976, 1969]. Thus, the number of configurations that can be reached by a system decreases with temperature and, at sufficiently low temperature (T < Tg), the liquid freezes: the system can no longer change its configurational state (a more detailed discussion can be found in Neuville and Le Losq [2022]). In addition, the configuration state of the glass does not change on cooling from Tg to 0 K. It can be noted that Raman and infrared vibrational spectroscopy suggest that the structure of a glass is an image of the instantaneous configuration of the liquid [Kashio et al. 1980; Kusabiraki and Shiraishi 1981a, b; Neuville and Mysen 1996].
Viscosity is an important and widely used property by the glass industry. It has therefore been, for the sake of uniformity, standardized. The temperature of the glass transition is industrially defined as the temperature at which the viscosity is 1012 Pa⋅s, for a cooling rate of the order of a few °C⋅min−1, which corresponds to the cooling rates of the glass in industrial processes. This viscosity value corresponds to relaxation times of about 15 min.
From a thermodynamic point of view, we can state that temperature and pressure are not enough to characterize the glass state. Tool and Eichlin [1931] introduced the concept of fictive temperature, Tfic, to characterize glass at constant pressure. We can define it as the temperature at which the glass would be in a steady state if it could be heated instantly. The fictive temperature can be defined during cooling in dilatometry or calorimetry, as being the glass transition temperature.
It is possible to give a formal thermodynamic definition of the fictive temperature, considering it as an order parameter. It is recalled that the glass transition resembles a second-order transition for which V, H, 𝜂 are continuous functions but not Cp, 𝛼, K [see the reviews Neuville and Le Losq 2022].
4. Chemical role of elements in glass
4.1. Cations
The structure of glasses involves a large family of elements divided into several groups, network formers, network modifiers or charge compensators, intermediate elements, coloring elements, refining.
(i) “Network formers” are the oxides that can form a glass alone These elements are, mainly, in their cationic forms: Si4+, B3+, Ge4+…. These network former cations have very covalent, and therefore, directional cation–oxygen bonds that ensure the three-dimensional framework of the glass. These elements form small size polyhedral with a ligand-like oxygen (for example, tetrahedral for Si and Ge or triangular for B). In the case of silicate glasses, two cations are connected to each other by so-called bridging oxygens but in thio-silicate glasses, they are connected by a bridging sulfur [Pradel and Piarristeguy 2022]. These connected polyhedra form a three-dimensional disordered lattice. So, it is possible to build a glass, with several ligands, O, S, Se, Te. For this short introduction, we will only talk about ligand O in oxide glasses.
Note that silicon oxide, SiO2, or silica, is the majority oxide of commercial glasses, and is the main former of the vitreous network. In the case of silica, the tetrahedral entities are denoted Qn where n represents the number of bridging oxygens carried by the central atom Q (typically Si). The silica glass thus consists of Si surrounded by 4 bridging oxygens, all connected to other tetrahedra to form the three-dimensional network of species Q4 with Si–O–Si bridges (Figure 2b). This structure is characterized by very high viscosity on the one hand and an Arrhenian dependence of viscosity with temperature on the other hand (Figure 3). Boron oxide B2O3 also acts as a lattice former, but it forms BO3 plane triangles. Its structure therefore has a strongly two-dimensional character, which explains why the viscosity of B2O3 is significantly lower than that of the three-dimensional network of silica (about 14 orders of magnitude near 1000 °C, Figure 3). In addition to reducing viscosity, boron oxide is added to glasses because it greatly improves the thermal and mechanical properties of glass as in the case of Pyrex® glass (composition NB82.10, Figure 4).
(ii) “Network modifiers” are oxides that do not form glass by themselves. These are mainly alkaline and alkaline-earth elements. The introduction of Rn+On∕2 (n = 1 or 2) changes the continuity of the three-dimensional network constituted by the network former. The network is broken with formation of non-bridging oxygens, which are no longer bound only to network formers. Alkaline and alkaline-earth elements play a similar role with regard to the structure and main properties of glasses and melts, but have some different characteristics. Thus, sodium oxide, Na2O, transforms silica into silicate by chemical attack and is mainly used to reduce the temperature necessary for the fusion of SiO2 compound. On the contrary, calcium oxide, because it forms slightly stronger Ca–O bonds than Na–O bonds, increases the melting temperature of the mixture and also improves several properties such as the durability of the glass. These 3 oxides, SiO2, Na2O and CaO represent more than 95% of the weight composition of industrial glasses and are, therefore, generally called sodalime silicate glasses. From a practical point of view, a large majority of commercial glasses also contain potassium oxide, K2O, and magnesium oxide, MgO. Magnesium oxide is usually added because it improves the mechanical properties and the hydrolytic strength of glasses like aluminum oxide. However, these two oxides also tend to increase the temperature of the silicate bath and the working temperatures of the glass. By adding Rn+On∕2 to silica, non-bridging oxygens are created, which establish R⋯O ionic bonds with the Rn+ cation. The R⋯O bond is weaker than that involved in Si–O–Si bridges, resulting in a strong decrease in viscosity. This is clearly visible by comparing the viscosity curve of SiO2 and NS3 glass compositions (Figure 4). The formation of non-bridging oxygens can be easily investigated by several techniques like Raman spectroscopy or nuclear magnetic resonance (NMR). Their proportions increase with sodium content which increases the weakly connected entities, Q3, Q2…, contributing to the decrease in viscosity. In Figure 4, NS1.5 has a lower viscosity than NS3, which results from an increase of the sodium content from 25 to 40 mol%. As we have seen with NS3, non-bridging oxygens are formed when lattice modifying elements (Li+, Na+, K+, Mg2+, Ca2+, Fe2+…) are added to depolymerize the 3D network made by the network former. In Figure 4, comparing the viscosity curve of NS1.5 which is lower than that of CS1.5, it is clear that sodium participates more in the breaking of the network than calcium.
(iii) “Charge compensators”, alkaline and/or alkaline earth elements can change their roles and become charge compensators in presence of (AlO4)− and (BO4)− which are tetrahedra with a deficit charge with respect to SiO4 tetrahedra. The aluminum fits into the network in a tetrahedral site, similar to Si4+. But, being an Al3+ cation, the AlO4 tetrahedron has a charge deficit that requires compensation to stabilize it energetically, which is accomplished by the proximity of an alkaline or alkaline earth element. The case of boron, B3+, is different because it is the result of a change in boron coordination. B2O3 glass is a network of BO3 triangles, fully stabilized with a low viscosity curve, which can be transformed into a network (BO4)− tetrahedra by the addition of an alkali or alkaline-earth oxides, which requires a charge compensation around (BO4)− similar to (AlO4)−. The formation of these (BO4)− tetrahedra increases the connectivity of the network, which is evident from the fact that the viscosity curve of the NB82.10 glass is more viscous than that is observed for B2O3 melt (Figure 4).
(iv) “Intermediate elements”, display a behavior as network formers but in general cannot form glass alone. It should be noted that Al, can form glasses, called inverted glasses [Neuville et al. 2008a, b, 2010; Licheron et al. 2011; Drewitt et al. 2012, 2017]. The main intermediate elements are Al3+, Pb4+, Ti4+ or Fe3+. Aluminum is mainly in coordination 4 [Stebbins and Xu 1997; Neuville et al. 2004a, b, c; Neuville 2006; Neuville et al. 2008a, b; Stebbins 2008; Le Losq et al. 2014; Drewitt et al. 2022; Neuville and Le Losq 2022]. By adding Al2O3 to the NS3 glass composition, viscosity increases strongly and becomes quasi-Arrhenian again, as illustrated in Figure 3 for the viscosity of NA75.6 and NA75.12 (albite glass composition) which contain 6 and 12 mol% of Al2O3 respectively. A similar behavior is observed when considering the viscosity of molten wollastonite, CaSiO3, and anorthite, CaAl2Si2O8. Both albite and anorthite glass compositions are fully polymerized systems and form a three-dimensional network of and SiO4 tetrahedra with Na+ or Ca2+ in the holes of the three-dimensional network, playing the role charge compensator of aluminum. Al3+ in four-fold coordination then plays a role of network former. Al3+ can also be in five-fold coordination [Neuville et al. 2004a, b, c; Neuville 2006; Neuville et al. 2008a, b; Le Losq et al. 2014; Drewitt et al. 2022; Neuville and Le Losq 2022]; in this condition, it is even possible to define a role of reticator (i.e. an element that ensures a strong connection between network former elements, according to Patrick Royall et al. [2008]).
Intermediate elements can change their role as a function of the chemical composition of the glass and their proportions in the glass [i.e. Neuville 2006; Neuville et al. 2008a], temperature [i.e. Stebbins et al. 1992; Stebbins and Farnan 1992; Stebbins 2008; Neuville et al. 2008b] and pressure [i.e. Allwardt et al. 2005, 2007] but also according to oxygen fugacity [i.e. Moretti 2005].
(v) Transition elements, can play the role of coloring or decoloring agents [Hunault et al. 2022]. A more detailed discussion about colored glasses can be found in Cormier et al. [1999], Galoisy et al. [2005], Hunault et al. [2014, 2016], and in the review of de Ligny and Möncke [2019].
4.2. Anions
Cations make up at most 50% of a glass, the rest being anions. In the anion subnetwork, the most important element is usually oxygen which occupies different positions. We distinguish in fact (Figure 5):
Structural sketch of a calcium alumino-silicate glass.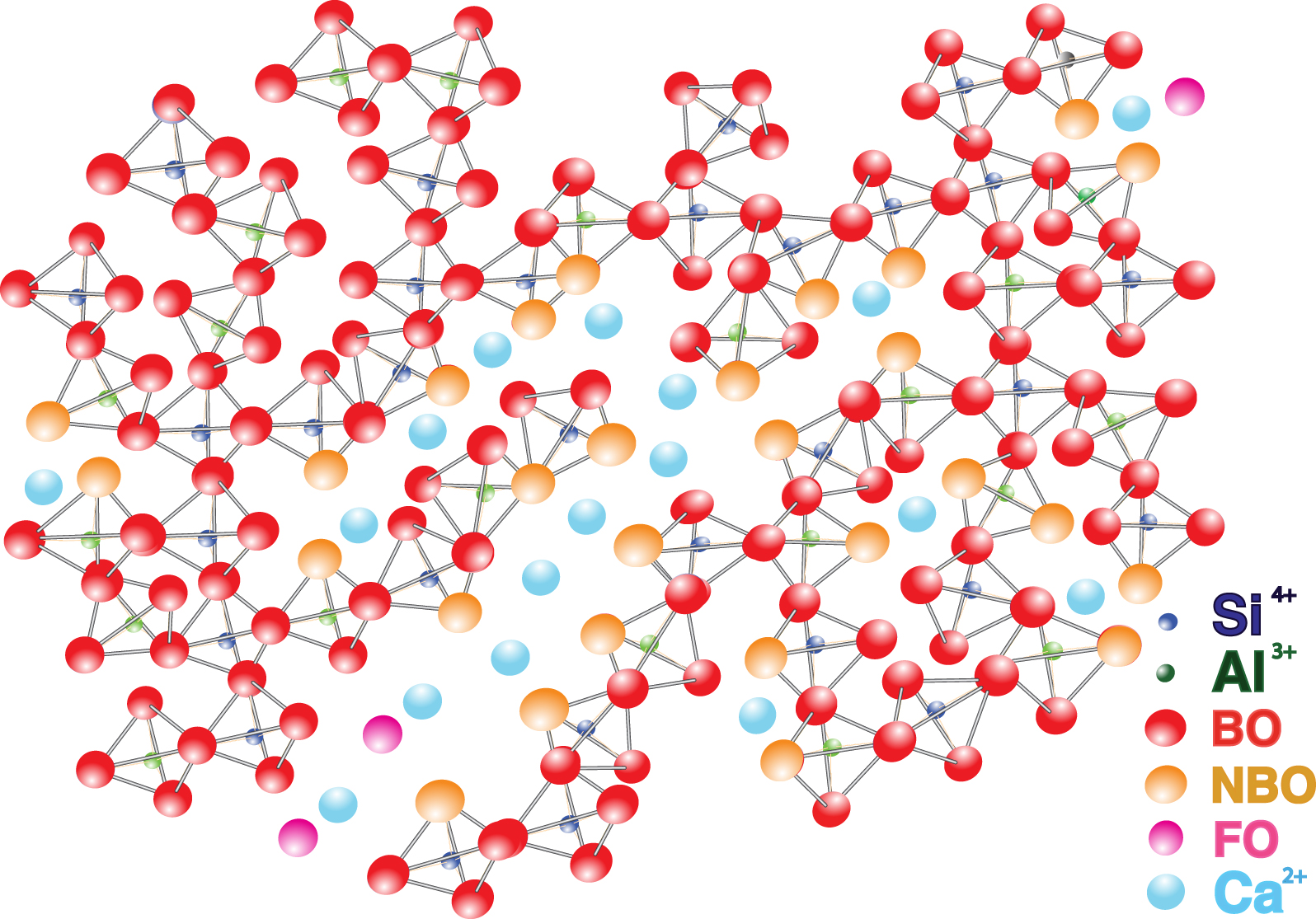
- bridging oxygens, BO, which link tetrahedra together and which have no effective charge because they are polarized by two covalent bonds;
- non-bridging oxygens, NBO, associated with a single tetrahedron. They therefore have a residual charge because they are partially polarized by a single covalent bond;
- free oxygens, FO, associated with no tetrahedron and which are therefore less polarized;
- tricluster oxygens, connected to three polyhedra, usually one or two tetrahedra and one pentahedron. These tricluster oxygens represent only a small percentage and play a minor role in the structure and properties of glasses and liquids [Iuga et al. 2005].
This vision of a network made by oxygens with different roles is complementary to that described by the cations. Some experimental techniques such as 17O NMR, make it possible to visualize this anionic network and highlight the connectivity of the network [Eden 2012; Stebbins et al. 2001].
This anionic network is also observed in chalcogenide glasses in which sulfur or selenium play a role of ligand identical to that of oxygen. In these glasses, it is possible to show, as already mentioned, the existence of bridging or non-bridging sulfur [Pradel and Piarristeguy 2022].
5. A universal idea of the glass structure?
The ideas developed in the previous paragraphs show that a glass, seen by the cationic or anionic network, has a disordered character. However, Greaves et al. [1981] showed using X-ray absorption near edge structure (XANES) at the Na K-edge that the short-range order around Na+ in silicate glasses was relatively well established. Moreover, these cations have a non-random distribution within the silicate network [Greaves 1985]. Following these pioneering studies, spectroscopic (Extended X-ray absorption fine structure, NMR, neutron diffraction …) or rheological (viscosity and calorimetry) studies have shown that cation–oxygen distances and cation coordinance are well defined [Taylor and Brown 1979a, b; Calas and et Petiau 1983; Cormier et al. 2001a, b, 1998, 2022; Eckersley et al. 1988; Le Losq et al. 2017; Neuville et al. 2007; Neuville 2006; Neuville et al. 2004a, b]. Other studies have confirmed the existence of medium range orders around modifying cations in the structuring of the silicate or aluminosilicate network [Gaskell et al. 1991; Greaves 1985; Cormier et al. 2001a, b; Meyer et al. 2004; Greaves and Sen 2007; Kargl and Meyer 2008; Caurant et al. 2010; Le Losq et al. 2017]. According to these studies, cations are not only randomly distributed within the structure of glass, which implies a modification of the classical model proposed by Warren et al. [Warren 1941; Warren and Biscoe 1938]. Greaves [1985] proposed the Modified Random Network (MRN) distributed into domains enriched in modifying cations, separated from domains enriched in network former. It is possible to distinguish channels or clusters, more or less connected where network modifiers, non-bridging and free oxygens, and also volatile elements are concentered. Molecular dynamic simulations on sodium and potassium silicates also highlight the existence of such structures in the liquid state [Kargl and Meyer 2008; Meyer et al. 2004]. The existence of percolation channels can explain the rapid ion diffusion decoupled from the network in glasses and liquids near Tg [Greaves and Sen 2007] which can also explain nucleation and growth as proposed by Neuville and Le Losq [2022]. In addition, it is in agreement with the observation of unmixing zones along the MO–SiO2 binary joints (with M = Mg, Ca, Na), which can be explained by the immiscibility between M–Q3 pairs and Q4 species [Neuville 2006].
Greaves and Ngai proposed a “modified” version of the MRN model for vitreous tectosilicates [Greaves and Ngai 1995]: the Compensated Continuous Random Network (CCRN)) model predicts that charge compensating cations are also present in channels but at the limits and close to elements or entities such as (AlO4)− or (BO4)− that require a charge compensation. The existence of subnetworks, one enriched in network former and one in the network modifier is in agreement with the simulations of Shintani and Tanaka [2006], which suggest that structures showing a quasi-crystalline order exist in the supercooled liquid close to Tg and strongly influence the dynamics of the system. There is therefore probably a link between structural and compositional heterogeneities [Moesgaard et al. 2010]. These percolation channels enriched in network modifier elements can also be the starting points of unmixing and/or nucleation zones [Dargaud et al. 2011, 2012]. This picture of a glass and melt organized in percolation channels is now recognized as a universal approach to the structure of oxide glasses and also chalcogenide glasses.
6. Conclusion
Whether by its structure or properties, a glass with a given chemical composition is a unique material. The oldest glasses in our solar system are several billion years old and could be observed on the Moon surface (4.53 billion years). On Earth, the oldest natural glasses are younger, because glass is a highly alterable material in the presence of water, which causes its alteration in a few million years: this makes it difficult on Earth to find glasses older than a few hundred million years.
Regardless of the place and time of manufacture, whether natural or man-made, if the chemical composition and formation conditions are identical, the glass manufactured will be identical in terms of structure and properties. The chemical composition and the conditions of formation are therefore the criteria that will determine the king of glass to be obtained. It is important to emphasize that glass is not the addition of several oxides but a new material resulting from the reaction of the mixture of raw materials.
In this short introduction, we have shown that glass is a material with a disordered structure, characterized by the absence of order at a long distance, and presenting a phenomenon of glass transition. The structural role of chemical elements in a glass is relatively well constrained between network former, network modifier, charge compensator, intermediate and transition elements. The role of the elements can vary depending on their concentration, and the conditions of formation (pressure, temperature, oxygen fugacity etc.). All these features make glass a unique and ubiquitous material.
Conflicts of interest
The author has no conflict of interest to declare.
Acknowledgments
A big thank you to François Chabaud who gave me the opportunity to make this issue on glass within the framework of the International Year of Glass and thanks also to all the authors who made very beautiful papers which allow to show the universality of glass. Ongoing friendly discussions with Laurent Cormier, Annie Pradel, Delia Brauer and Roberto Moretti are very much appreciated.




 CC-BY 4.0
CC-BY 4.0