1. Introduction
Deep-sea foraminifera (Eukaryota, Rhizaria) are an important ecological group of benthic meiofauna (see the review by [Gooday 2003; Zeppilli et al.2015]). In natural settings, their faunal patterns (standing stocks, diversity, microhabitat) are controlled by many physico-chemical parameters. Organic-matter flux reaching the sea floor is one of their most important ecological constraints [Gooday 2003]. The organic-matter flux acts indirectly as an ecological limiting factor when it induces either temporary or long-term hypoxia either in the sediment or in the bottom water Fontanier et al. [2014], Gooday et al. [2000], Kurbjeweit et al. [2000], Schumacher et al. [2007]. Sediment gravity flows passing down active submarine canyons can supply organic detritus and inorganic particles to the deep ocean. Foraminiferal faunas living in these naturally disturbed habitats are characterized either by various stages of colonization occurring after physical disturbance (e.g. turbidity flows), or by equilibrium phases related to the gradual accumulation of organic-matter (e.g. eutrophication) [Duros et al.2011, 2013; Fontanier et al.2008a,b; Hess and Jorissen 2009; Hess et al.2005; Koho et al.2007, 2008]. Due to their relatively short life cycle, environmental change can be quickly recorded via foraminiferal assemblage change. Therefore, foraminifera make ideal candidates for the monitoring of environmental stress related to human activities Schönfeld et al. [2012], Zeppilli et al. [2015].
Between 1967 and 2015, bauxite residues (namely red mud) were dumped into the Cassidaigne Canyon by the Gardanne alumina refinery (South-East of France) (see review by Dauvin [2010]). Bauxite red mud (a combination of liquid effluent and residual solid) was drained away by a submarine pipe and discharged at a water depth of 320 m, about 8 km offshore the coast. This sedimentary material spread along the axis of the Cassidaigne Canyon and on its lateral flanks to great depths (>2000 m) [Dauvin 2010; Fabri et al.2013; Fontanier et al.2012, 2015]. The total coverage of discharged red mud was estimated to be more than 900 km2. In January 2016, red mud dispersal ceased and industrial wastes are now sieved and the solid fraction stored on land (see https://alteo-environnement-gardanne.fr/-Fabrication-et-stockage for further information). Since then, only residual liquid effluent has been released from the pipeline outlet into the Cassidaigne Canyon. This liquid, characterized by a density lower than the ambient sea water, is gradually diluted as it rises up the water column.
Environmental studies have been carried out for the last five decades in order to elucidate the impact of red mud on the deep-sea metazoan benthos [Bourcier 1969; Bourcier and Zibrowius 1973; Bourcier et al.1993; Fabri et al.2013; Vitiello and Vivier 1974; Vivier 1978a,b]. Those investigations proved that, close to the pipe outlet, the hydro-sedimentary contamination related to the flooding of red muddy material (i.e., high sedimentation rate) precluded benthic meiofauna and macrofauna settlement along the canyon axis. In the surrounding areas, normal macrofauna with suspension and deposit feeders were able to thrive, despite the presence of in a thin layer of red mud. Fontanier et al. [2012] conducted an ecological study of foraminiferal faunas from two stations located at 725 m and 1528 m along the axis of the Cassidaigne Canyon (NW Mediterranean Sea) (ESSROV cruise, October 2011). At both studied sites, sediments were highly contaminated by iron, titanium, vanadium and chromium compared to normal hemipelagic sediments. At the shallower station located close to the pipe outlet, the living benthic foraminiferal community was characterized by very low diversity (only three species) and by the unusual dominance of Gyroidina umbonata (Silvestri, 1898) and Bulimina marginata d’Orbigny, 1826. The physical disturbance related to red mud deposition was likely the major hydro-sedimentary parameter precluding the settlement of diverse fauna. Conversely, the living foraminiferal fauna from the deeper site was typical of oligo-mesotrophic conditions prevailing in natural environments. There, bauxite residues had no environmental impact on foraminiferal faunas. Nearly one year later (September 2012), fourteen stations located between 288–2432 m water depth at varying proximity to the pipe outlet were sampled [Fontanier et al.2015]. Due to more extensive coring, Fontanier et al. [2015] evaluated the impact of red mud dispersal in the Cassidaigne Canyon, not along its axis but on its flanks, and its surrounding area (adjacent canyons and the deep basin). Deposits of red mud were observed in the Cassidaigne and Planier Canyons down to ∼2000 m (coverage area ∼900 km2). The diversity, composition and standing stock patterns for foraminiferal faunas in this area represented assemblages predominantly constrained by overall meso-oligotrophic conditions. The reduction of sedimentary organic detritus with varying water depth and the ecological constraint determined by bottom currents generated gradual changes in foraminiferal communities, regardless of red mud presence. Compared to the canyon axis studied by Fontanier et al. [2012], there was no obvious environmental impact of dispersed bauxite residues on benthic biodiversity at the sampling period (September 2012).
In September and October 2016, four years after the last foraminiferal investigation in the Cassidaigne canyon and its surrounding area (September 2012), and ten months after the cessation of red mud dumping (January 2016), more extensive core collections than those previously performed by Fontanier et al. [2012, 2015] were gathered in the frame of a statutory survey. Foraminiferal communities were sampled at 16 stations located between 265–2500 m with varying proximity to the pipe outlet (Figure 1; Table 1). Most of these sites are within the geographical zone where historical bauxite residues have been previously detected [Dauvin 2010; Fontanier et al.2012, 2015]. The main objective of our study is to determine the ecological patterns (diversity indices and faunal composition) of benthic environments some months after the cessation of solid waste dispersal in the Cassidaigne Canyon.
Bathymetry, study area and location of the 16 stations sampled during the 2016 oceanographic cruise (autumn 2016).
Water depth, coordinates and physiographic settings of all stations sampled during the 2016 oceanographic cruise (autumn 2016)
| Station | Sampling date | Latitude | Longitude | Depth (m) | Settings | Distance from the pipeline outlet (km) | Visual detection of red mud deposits |
|---|---|---|---|---|---|---|---|
| U03 | 31/08/2016 | 43° 07.05′ N | 05° 26.11′ E | 292 | Head of the Cassidaigne Canyon | 5.9 | Reddish brown surface layer (several cm) |
| SR2 | 02/09/2016 | 43° 07.31′ N | 05° 28.88′ E | 747 | Axis of the Cassidaigne Canyon | 2.4 | Reddish brown sediment |
| U05 | 03/09/2016 | 42° 59.40′ N | 05° 31.85′ E | 751 | Eastern flank of the Cassidaigne Canyon | 17.3 | No |
| SR1 | 02/09/2016 | 43° 00.13′ N | 05° 25.49′ E | 1553 | Axis of the Cassidaigne Canyon | 16.3 | Reddish brown surface layer (several cm) |
| U13 | 22/09/2016 | 43° 00.78′ N | 05° 45.54′ E | 952 | Western Branch of the Cap-Sicié Canyon | 25 | No |
| U06 | 01/09/2016 | 43° 02.34′ N | 05° 21.00′ E | 605 | Head of the eastern branch of the Planier Canyon | 16.6 | Reddish brown surface layer (several cm) |
| U07 | 01/09/2016 | 43° 00.09′ N | 05° 19.21′ E | 1056 | Eastern branch of the Planier Canyon | 21.2 | Reddish brown surface layer (several cm) |
| U08 | 02/10/2016 | 42° 57.43′ N | 05° 14.04′ E | 1530 | Axis of the Planier Canyon | 29.7 | Reddish brown surface layer (several cm) |
| U09 | 01/10/2016 | 42° 51.53′ N | 05° 14.58′ E | 1968 | Axis of the Planier Canyon | 37.5 | Reddish brown surface layer (several cm) |
| U10 | 30/09/2016 | 42° 49.22′ N | 05° 21.95′ E | 1800 | Connexion between both Marseille and Planier Canyons | 42.3 | Reddish brown patches at the sediment surface |
| U02 | 30/09/2016 | 42° 48.83′ N | 05° 29.58′ E | 2100 | Connexion between both Marseille and Planier Canyons | 36 | Reddish brown patches at the sediment surface |
| U11 | 03/09/2016 | 42° 46.22′ N | 05° 40.80′ E | 2222 | Connection between Marseille/Planier and Cassidaigne Canyons | 43.3 | Reddish brown surface layer (cm) |
| U12 | 07/10/2016 | 42° 49.01′ N | 05° 46.97′ E | 2290 | Connection between Marseille/Planier and Cassidaigne Canyons | 42.3 | Reddish brown patches at the sediment surface |
| U28 | 04/10/2016 | 42° 35.00′ N | 05° 05.00′ E | 1758 | Lower slope between Petit Rhône and Grand Rhô Canyons | 72 | No |
| U27 | 02/10/2016 | 42° 35.00′ N | 05° 30.00′ E | 2250 | Deep Basin | 61.6 | No |
| U26 | 07/10/2016 | 42° 35.00′ N | 05° 57.50′ E | 2432 | Deep Basin | 72 | No |
2. Study area
The Cassidaigne Canyon abuts the eastern Gulf of Lions and the Ligurian Sea (NW Mediterranean) (Figure 1 insert). The 200 m-deep canyon head borders the Cassis Bay at a distance of only 7 km from the coast and is characterized by a narrow canyon axis (1 km in width) (Figure 1).
The Northern Current (NC), which forms the northern branch of the cyclonic Liguro-Provençal Current (LPC), follows the continental margin from the Provence coast (France) to the coast of Catalonia (Spain) [Béthoux and Prieur 1983; Millot 1990]. The NC determines the general surface water circulation patterns. Below the surface waters (>200 m), spreads the modified Levantine Intermediate Water (LIW), which is characterized by a salinity maximum ( ∼38.5) and a relative temperature maximum (>13 °C). The Western Mediterranean Deep Water (WMDW) occurs below the LIW with a diffusive boundary at 500–800 m [Béthoux and Prieur 1983; Béthoux et al.2002]. It is generally characterized by a rather homogeneous temperature (∼13 °C) and salinity (38.40–38.45) [Béthoux and Prieur 1983; Béthoux et al.2002].
Our present study is based on sediment cores collected aboard the R/V FéliX during the monitoring oceanographic cruise, which took place in September and October 2016. Sixteen stations were sampled within and around the Cassidaigne Canyon (Table 1; Figure 1). Fourteen of these stations, starting with “U”, have already been studied by Fontanier et al. [2015] and the remaining two stations, SR1 and SR2, correspond approximately to sampling sites investigated in Fontanier et al. [2012]. Stations U03 (292 m) and U05 (751 m) are located at the head and on the eastern flank of the Cassidaigne Canyon. Stations SR2 (747 m) and SR1 (1553 m) are situated along the Cassidaigne Canyon axis. Stations U06–U09 are along the Planier Canyon between ∼600–2000 m water depth. Both stations U02 and U10 are located along the deep valley where both the Marseille and Planier tributary canyons converge (>1800 m). U11 and U12 (>2200 m) are under the influence of the Marseille/Planier/Cassidaigne Canyon system. U28, U27 and U26 represent a bathymetric transect of the interfluve between the Petit Rhône and Grand Rhône Canyons (˜1750 m) to the deep basin (˜2400 m). Station U13 is located in the western branch of the Cap-Sicié Canyon (France), less than 7 km from the coast and around 25 km south-east of the pipe outlet.
In accordance with previous studies by Fontanier et al. [2012, 2015], reddish brown surface sediment was observed at most stations providing (with other physicochemical proves) qualitative evidence regarding the geographical and historical dispersal of bauxite residues (Table 1; [CREOCEAN 2018]). Only stations U05 (725 m), U13 (958 m), U28 (1758 m), U27 (2250 m) and U26 (2432 m) were not contaminated by red mud deposits. Surface sediment (0–4 cm interval) Titanium (Ti) content, considered a geochemical proxy of red mud dispersal [Dauvin 2010], matches relatively well with visual observations of sediment-water interface (Figure 2). Extraordinarily high Ti values were recorded at station SR2 (∼32,000 μg⋅g−1 DW) and to a lesser degree, station SR1 (∼20,500 μg⋅g−1 DW) confirming that bauxite residues accumulated preferentially along the Cassidaigne Canyon axis. For comparison, the Ti content of the pipeline dispersed red mud before January 2016 was ∼70,000 μg⋅g−1 DW [SAFEGE 2011]. Nepheloid layers and sediment gravity flows are considered the main hydro-sedimentary processes responsible for transferring the bauxite-derived material from the pipeline outlet along the Cassidaigne Canyon axis [Dauvin 2010; Fabri et al.2013; Fontanier et al.2012, 2015]. Moderate to high Ti values were recorded at most of the other stations in adjacent canyons (between 3300 and 4400 μg⋅g−1 DW) even at great depths (∼5100 μg⋅g−1 DW at station U10, 1800 m). As suggested by Fontanier et al. [2015], the region’s episodically strong up- and down-welling currents coupled with efficient sediment transfer by both gravity and suspension flows could trigger the large spatial coverage of the natural and Ti-laden seafloor sediments. In contrast, samples from station U05 (725 m) and U13 (958 m) yielded relatively low Ti content (respectively ∼3400 and ∼3100 μg⋅g−1 DW) (Figure 2) [Fontanier et al.2012, 2015]. As already discussed in Fontanier et al. [2015], both stations U05 and U13 are located in canyon areas not accessible by the bauxite residue (Figure 2). Stations U11 (2222 m), U12 (2290 m) and U02 (2100 m) located at the deeper connections between the Marseille, Planier and Cassidaigne Canyons, also exhibit low Ti content (between 2700 and 3100 μg⋅g−1 DW). Sites U28, U27 and U26, located more than 60 km away from pipeline outlet at depths greater than 1700 m, logically present Ti content close to natural background levels (<2400 μg⋅g−1 DW), an order of magnitude lower than the highly contaminated station SR2.
Titanium (Ti) content in surface sediments (0–4 cm interval) collected at 16 stations (2016 oceanographic cruise, autumn 2016). The Ti content is indicated using a 3-class color code). This data was taken from the survey report by CREOCEAN (CREOCEAN, 2018).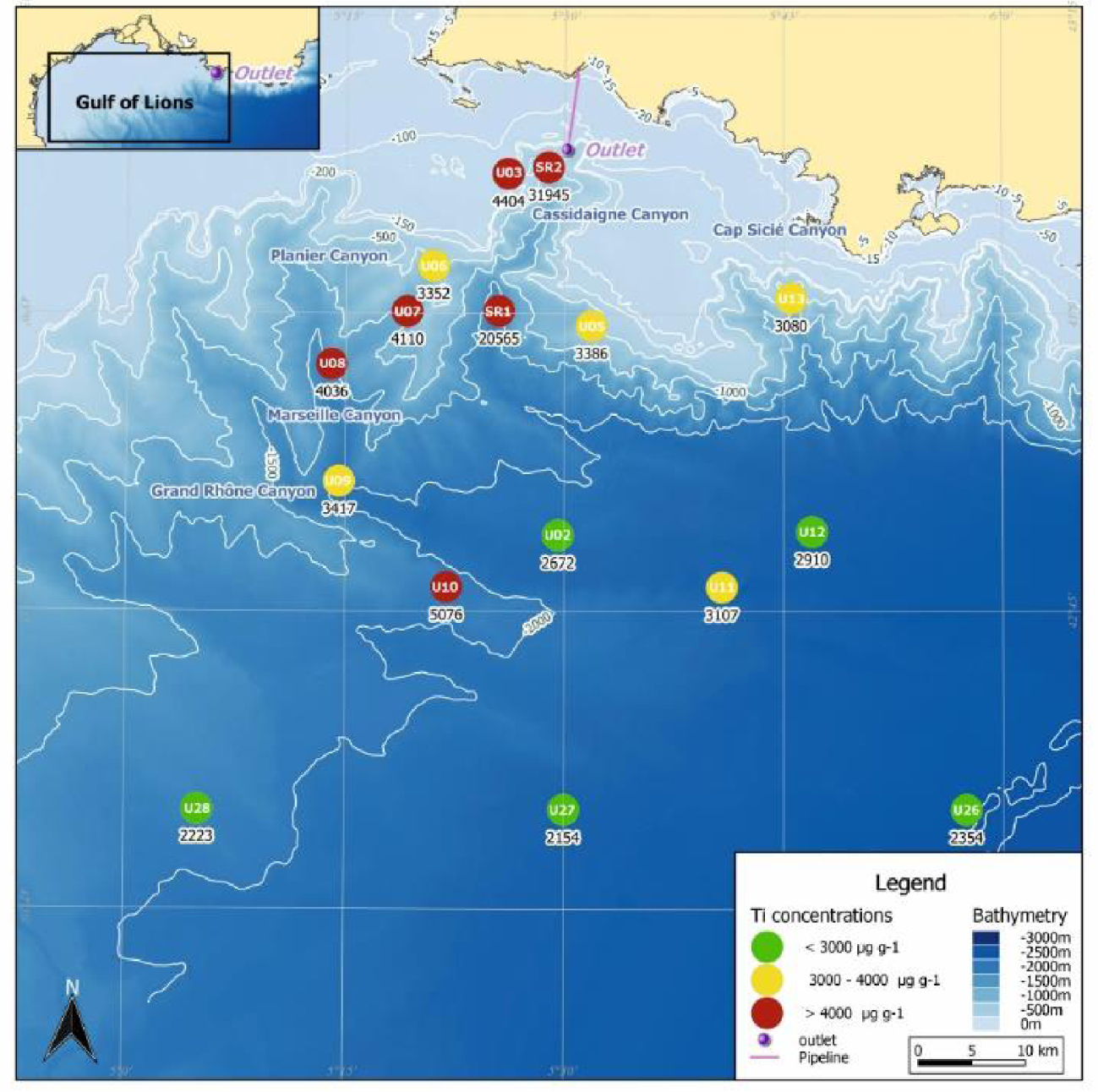
3. Material and methods
This study constitutes a snapshot of ecological conditions prevailing during September/October 2016 in the Cassidaigne Canyon and surrounding area. Many works on living foraminifera were performed in the Gulf of Lions and in the Ligurian Sea before our present study [Bizon and Bizon 1984; Contreras-Rosales et al.2012; De Rijk et al.2000; Fontanier et al.2008a,b, 2015; Goineau et al.2012, 2011; Schmiedl et al.2000]. They provide reliable information concerning what we might expect in terms of natural foraminiferal abundance and distribution in the region. Furthermore, a foraminiferal response to red mud pollution in the axis of the Cassidaigne Canyon has already been documented by Fontanier et al. [2012]. This work and other recent papers regarding foraminiferal recolonization in canyon settings [Duros et al.2011, 2013; Hess and Jorissen 2009; Hess et al.2005] provide a reliable basis on which to assess the potential impact of red mud dispersal on foraminiferal biodiversity and its potential resilience since January 2016, when red mud dispersal ceased).
Standing stocks (No. Ind. 100 cm-2) of living (stained) foraminiferal faunas at the 16 investigated stations. Stations are arranged by both physiographic setting and increasing depth.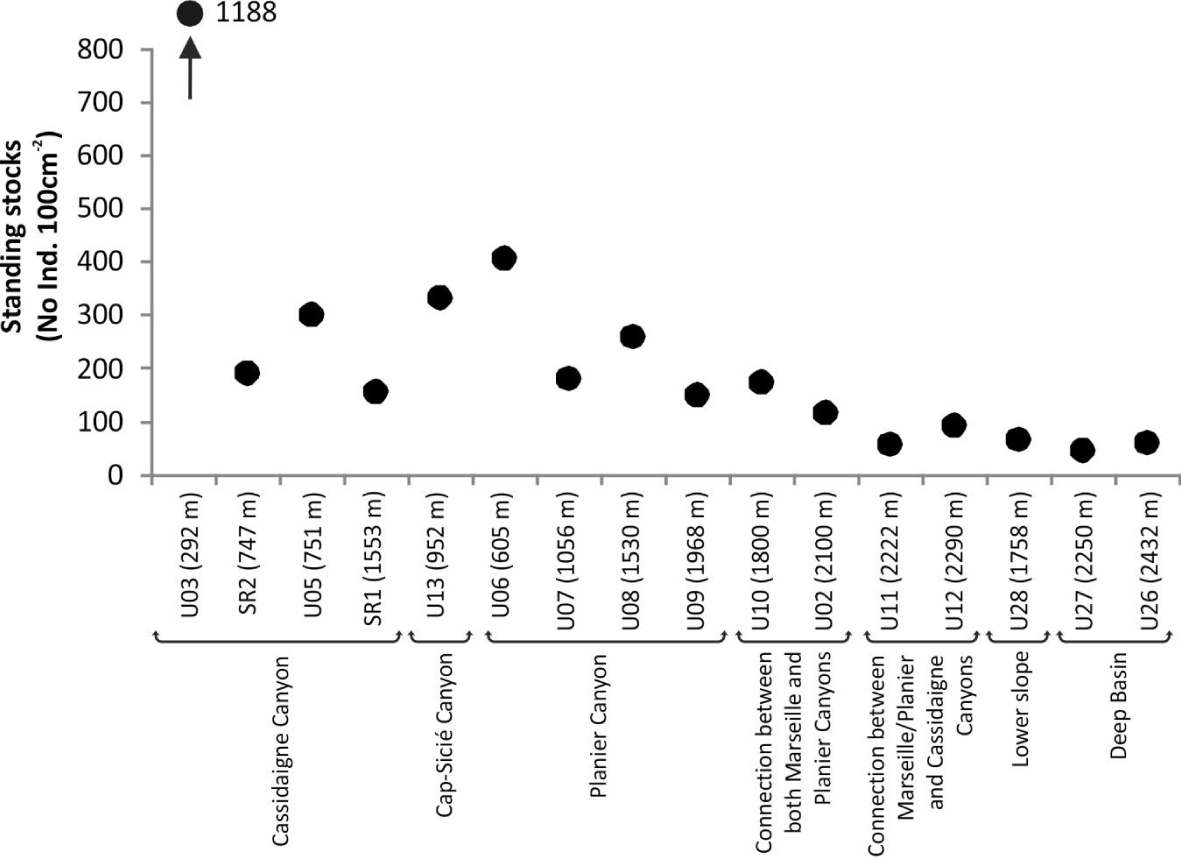
3.1. Samplings
Sediment samples were collected with a Barnett-type multiple corer equipped with Plexiglas tubes (9.3 mm internal diameter, surface area of 68 cm2) [Barnett et al.1984]. The multi-corer allowed sampling of the uppermost centimeters of the sediment column, the overlying bottom waters, and a comparatively undisturbed sediment-water interface. It was deployed once at each station. One core from each site was used for this foraminiferal study. The cores were sliced horizontally every 0.5 cm from the sediment-water interface (SWI) down to 2 cm. Because of meteorological constraints (strong swell), the multicorer could not be deployed at stations U13 (952 m), U12 (2290 m) and U26 (2432 m). There, sediment samples were collected with an USNEL box corer (surface area of 2500 cm2). A Plexiglas tube (internal diameter 9.3 cm, surface area of 68 cm2) was used to subsample a sediment core. The uppermost 2 cm were similarly sliced to the Barnett cores for foraminiferal analyses. All potential methodological biases related to foraminiferal sampling with a box corer have been discussed previously by Fontanier et al. [2015]. To understand overall ecosystem variability, triplicates are recommended at each sampling site [Schönfeld et al.2012]. Despite this, most ecological papers studying deep-sea living (stained) foraminiferal communities use only one core per site. To facilitate effective comparisons between previous work of this kind, we have also only used one core per site. Nevertheless, readers should consider our observations and interpretations with care as they may be biased by potential spatial (cm to m scale) variability that we cannot fully account for with our data sets.
3.2. Benthic foraminiferal analysis
Whilst on board, sediment samples dedicated to foraminiferal study were transferred to 250 cm3 bottles filled with 95% ethanol containing 2 g⋅L−1 Rose Bengal stain, commonly used to identify live foraminifera [Murray and Bowser 2000; Walton 1952]. All samples were gently shaken for several minutes to obtain a homogeneous mixture. One month after the cruise they were sieved through a 125 μm screen and the sieve residues were stored in 95% ethanol. Well-stained foraminifera (all chambers excluding the final stained bright pink) were sorted in wet samples and stored in Plummer slides. Strict staining criteria were applied and doubtful individuals without perfectly stained tests were not included. Non-transparent agglutinated and miliolid taxa were broken on many occasions for inspection of the interior of the test. Most live foraminifera were identified to species level. All data generated or analysed during this study are included in this published article (See Supplementary material). At each station, we calculated diversity indices including simple diversity S (representing the number of species), Shannon index H′ (log base e), Rarefied Species Richness E(S35) and Dominance index D [Hayek and Buzas 1997; Murray 2006]. These indices were based on counts of stained specimens from the four depth horizons analysed in each core. Census data are available on request to the first author of this publication.
4. Results
4.1. Foraminiferal standing stocks and diversity
Foraminiferal standing stocks ranged between ∼50 (U27, 2250 m) and ∼1190 (U03, 265 m) individuals 100 cm-2 (Figure 2). Values were lower (<100 individuals 100 cm-2) at depths greater than 2200 m compared to shallower stations. Simple diversity (S) varied between 7 (U26, 2500 m) and 61 (U03, 265 m) species (Figure 3). Diversity generally decreased with increasing water depth, with S values lower than 12 species below 2200 m. The only exceptions were recorded at stations SR2 (747 m) and SR1 (1553 m) located along the Cassidaigne Canyon axis where only 13 and 11 taxa were identified respectively. Shannon index H′ and Rarefied Species Richness E(S30) followed the same trend (Figure 3) as values were low at stations deeper than 2200 m compared to more shallow sites. Once again, stations SR2 and SR1 were exceptions with low H′ (<1.8) and E(S30) (<7) values corresponding to the very low simple diversity and strong dominance. Dominance index D and Shannon index values were inversely related.
4.2. Faunal composition
At the head of the Cassidaigne Canyon (station U03, 292 m), Melonis barleeanus (Williamson, 1858) (32%) and Valvulineria bradyana (Fornasini, 1900) (18%) dominated the living fauna (Figure 4). Along the axis of the Cassidaigne Canyon (station SR2, 747 m), Bulimina marginata d’Orbigny, 1826 (50%) and Gyroidina altiformis Stewart & Stewart, 1930 (27%) were dominant. At the same depth on the eastern flank of the Cassidaigne Canyon (station U05, 751 m), Bigenerina nodosaria d’Orbigny, 1826 (16%) and Rosalina bradyi (Cushman, 1915) (16%) were the most abundant living fauna. At station SR1 (1553 m) located along the Cassidaigne Canyon axis, foraminiferal fauna were dominated by Uvigerina mediterranea Hofker, 1932 (43%), Uvigerina peregrina Cushman, 1923 (19%) and Hoeglundinaelegans (d’Orbigny, 1826) (15%). In the Sicié Canyon, and along the Planier Canyon axis (<2000 m water depth), M. barleeanus was dominant with a relative contribution ranging between 10% and 30% at both stations U08 (1530 m) and U13 (952 m) respectively. Globobulimina affinis (d’Orbigny, 1839) was abundant (16%) in the Sicié Canyon (station U13, 952 m). Uvigerina mediterranea Hofker, 1932 and Uvigerina peregrina Cushman, 1923 were both substantial components (>10%) at stations U06, U07 U08 and U10 (ranging between 605–1800 m). Deeper than ∼2000 m, Nodellum membranaceum (Brady, 1879) was the dominant species with percentages between 11% (U09, 1968 m) and 83% (U26, 2432 m) (Figure 4) and agglutinated Lagenammina calcarea (Cushman, 1947) and Thurammina albicans (Brady, 1879) were secondary taxa.
Simple diversity S, Shannon diversity index H′, Rarefied Species Richness E(S30) and Dominance index D of living (stained) foraminiferal faunas at the 16 investigated stations. Stations are arranged by both physiographic setting and increasing depth.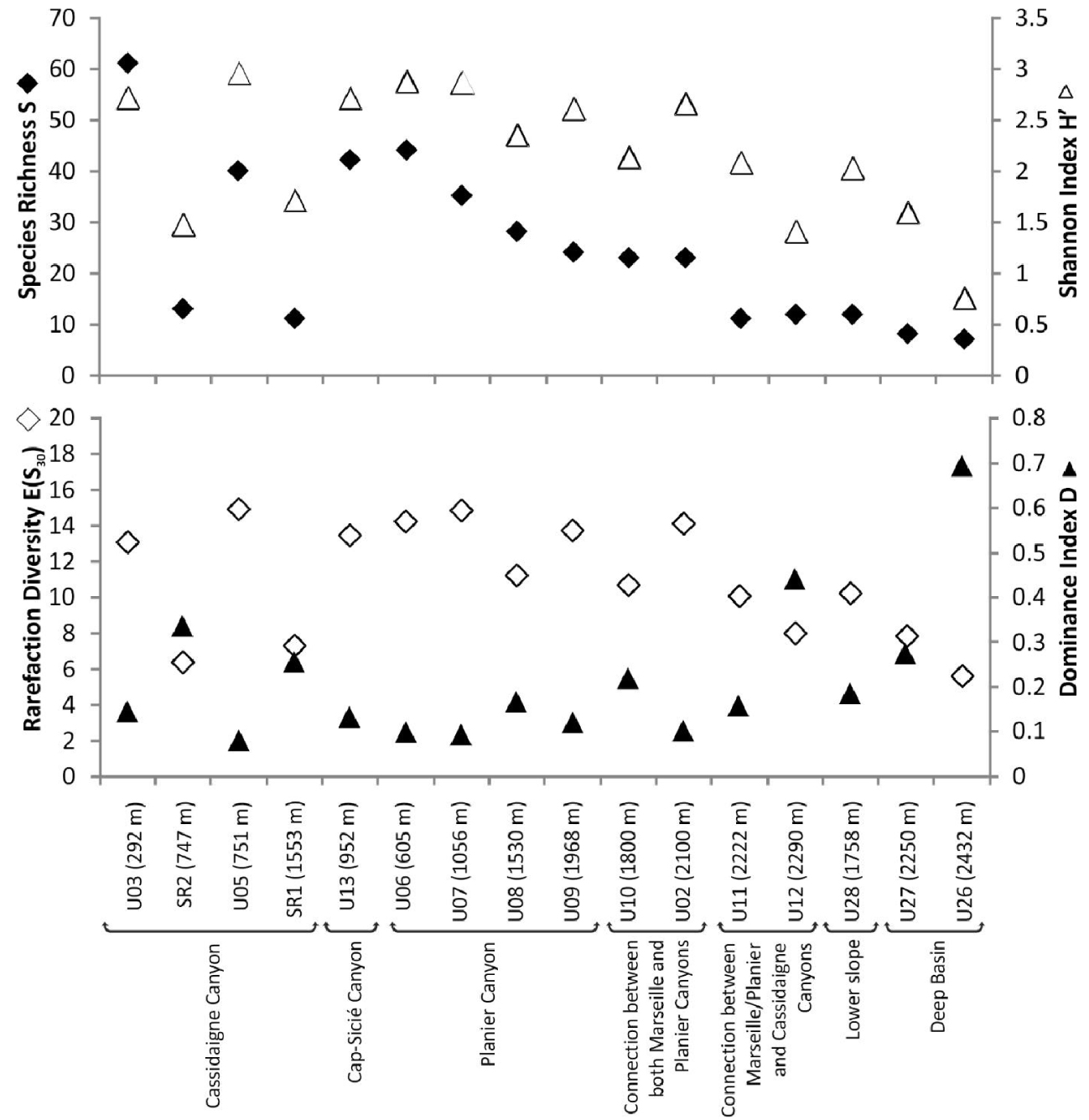
5. Discussion
5.1. Natural trophic control on foraminiferal faunas in the Cassidaigne Canyon surrounding (Figure 5figf5)
As already discussed by Fontanier et al. [2015], based on samples collected in September 2012, all stations except SR2 and SR1 located in the Cassidaigne Canyon axis, present a decrease in both foraminiferal density and diversity (S) with water depth (Figures 3 and 4). Low-diversity foraminiferal faunas are documented in the deeper basin and on the distal lower slope compared to more diverse communities from other shallower stations. This trend is likely related to the natural scarcity of food (i.e. sedimentary organic matter) at varying depths, which echoes (1) the natural decrease of exported primary productivity (i.e. fresh phytodetritus) with increasing water depth and (2) the naturally diminishing lateral advection of degraded organic compounds from neritic areas to deep-basin stations [Fontanier et al.2015].
Composition of benthic live (stained) foraminiferal faunas at the 16 investigated stations. Only major species (at least >5% at one site) are illustrated. Stations are arranged by both physiographic setting and increasing depth.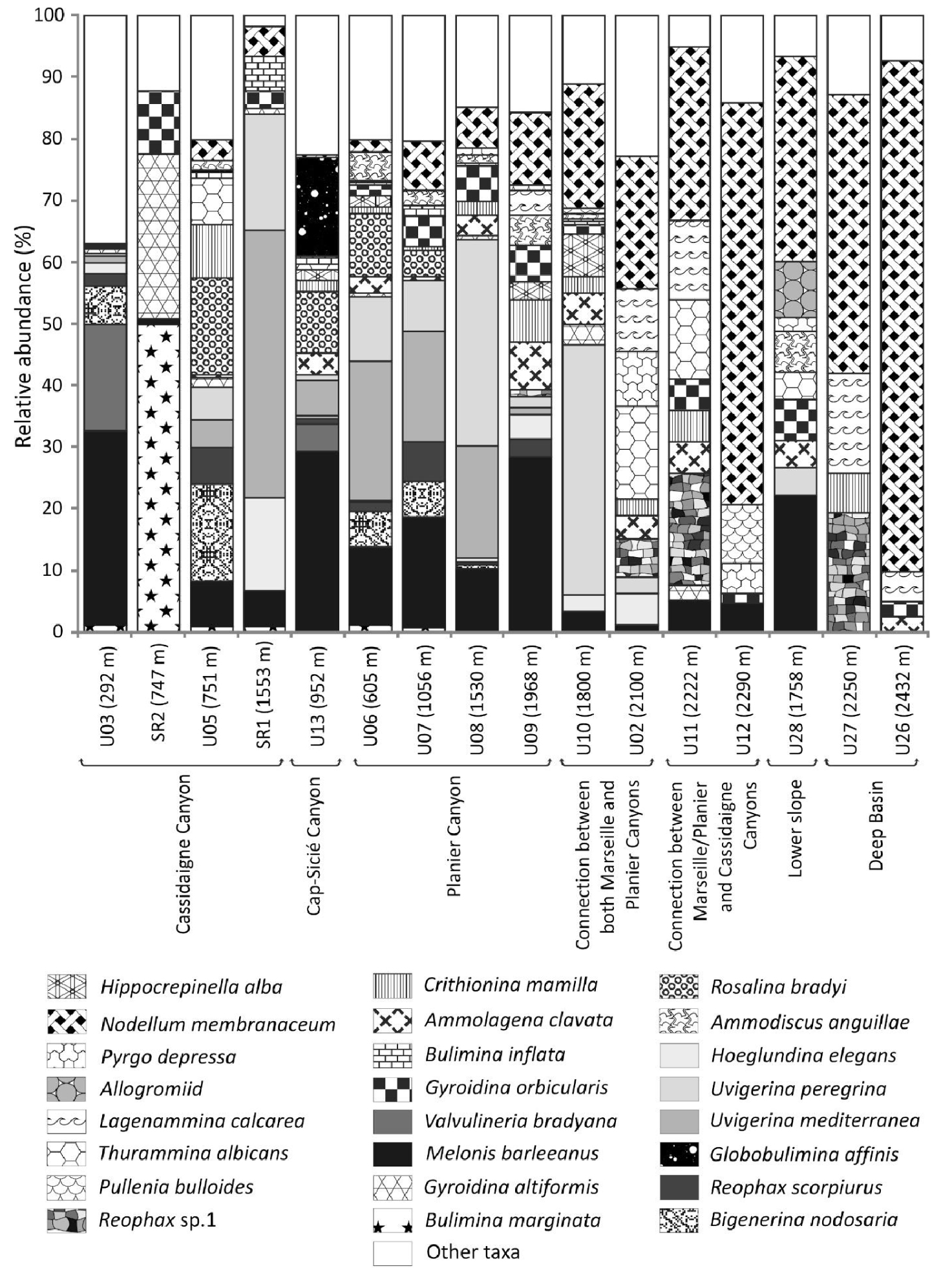
The faunal composition of the isolated foraminiferal communities confirms the major role of organic matter supply on the ecological status of benthic ecosystems. For instance, Melonis barleeanus is a major species at stations between ∼290–2000 m where the overall sedimentary input of organic matter is considered relatively high [Fontanier et al.2015]. Accordingly, this taxon is abundant in mesotrophic and well-oxygenated environments [Caralp 1989a,b; Duros et al.2011, 2013; Fontanier et al.2002, 2003, 2005, 2008a,b; Koho et al.2007; Kurbjeweit et al.2000; Licari et al.2003; Schmiedl et al.2000]. In both open slope and canyon settings, M. barleeanus thrives generally in deep microhabitats below the sediment-water interface where it feeds on degraded organic matter. This supports the assumption that most of the bathyal stations are characterized by the input of low-quality organic compounds, likely transported laterally by along-slope currents. Furthermore, the co-occurrence of U. mediterranea and U. peregrina is in agreement with bathyal faunas described in the western Mediterranean Sea. These uvigerinids are generally described as shallow infaunal species able to feed on relatively fresh organic detritus in mesotrophic ecosystems [Contreras-Rosales et al.2012; De Rijk et al.2000; Duros et al.2011, 2013; Eberwein and Mackensen 2006; Fontanier et al.2002, 2003, 2006, 2008b; Koho et al.2007, 2008; Schmiedl et al.2000]. Uvigerina peregrina is considered an opportunistic species feeding on freshly exported phytodetritus in canyon and slope environments whereas U. mediterranea is a reactive taxon able to grow and reproduce when food is available in the surface sediment [Duros et al.2011, 2013; Fontanier et al.2003]. To summarize, the fact that the M. barleeanus and uvigerinid faunal association originally found in the autumn 2012 biomonitoring [Fontanier et al.2015], still prevails in autumn 2016, shows that the mesotrophic conditions within the canyon systems adjacent to the Cassidaigne Canyon are independent of bauxite residues. Furthermore, the dominance of the outer-shelf/upper-bathyal species V. bradyana with M. barleeanus at the shallowest station U03 (292 m) indicates an ecosystem enriched in organic detritus [Fontanier et al.2002; Goineau et al.2011; Hess and Jorissen 2009; Langezaal et al.2006]. It is a common species in rich and diverse faunal assemblages encountered in canyon-head environments where there is no sediment redeposition [Hess and Jorissen 2009]. The co-occurrence of M. barleeanus and V. bradyana suggests that meso-eutrophic conditions prevail at the shallowest station. The remarkable presence of R. bradyi at stations U05 (751 m), U13 (952 m) and U06 (U06) is questionable. This euryhaline taxon is abundant in neritic areas with a preference for an epiphytic and/or epilithic life habit in inner shelf environments [Fontanier et al.2008a]. Whilst attached to vegetation, individuals of this species can be transported by bottom currents into canyons [Fontanier et al.2008a]. Therefore, the occurrence of R. bradyi at our sample sites further underlines a natural source-to-sink connection in terms of organic supply and sediment transfer between upper-slope environments and deeper adjacent shelves. In autumn 2012, the dominance of Globobulimina spp. and a relatively high organic content (1.4% DW) suggested a preferential focusing of organic compounds at station U13 [Fontanier et al.2015], a site located in the western branch of the Cap Sicié Canyon only 7 km from the coast. In autumn 2016, Globobulimina spp. remains a substantial component (20%) of the living faunal assemblage after M. barleeanus. This genus is considered a highly specialized taxon able to feed on low-quality organic detritus that collects within eutrophicated canyon depressions [Fontanier et al.2005, 2008a]. Below 2000 m, the foraminiferal community is dominated by N. membranaceum, P. depressa,L. calcarea, Thurammina albicans Brady, 1879 and Pullenia bulloides (d’Orbigny, 1846). Similarly, these observations coincide with the autumn 2012 faunal patterns and surface sediment organic carbon content [Fontanier et al.2015]. All above-mentioned taxa are typical of oligotrophic basins from the western Mediterranean Sea [Bizon and Bizon 1984; De Rijk et al.2000; Fontanier et al.2008b, 2012, 2015]. This suggests that our deeper sample sites (>2000 m) are affected by a limited input of organic compounds, likely related to either exported primary productivity or lateral advection of reworked phytal remains.
5.2. Questionable ecological resilience along the Cassidaigne Canyon axis
In autumn 2012, a 725 m-deep sample site was characterised by a very low-diversity fauna (S = 3 and H′ = 0.76) and dominated by the opportunistic and stress-tolerant species Gyroidina umbonata and Bulimina marginata [Fontanier et al.2012]. Considered pioneer taxa, the species found at this site indicate intense hydro-sedimentary pollution due to the red mud deposition along the axis of the Cassidaigne Canyon. Our results from autumn 2016 at site SR2 (747 m), located very close to the autumn 2012 site, show that diversity has increased (S = 13; H′ = 1.67). Bulimina marginata dominates, contributing 50% of the living fauna. This taxon is documented as an opportunistic species living in outer-shelf and upper-slope environments, at both early and advanced stages of recolonization in active canyons (e.g. [Fontanier et al.2003; Goineau et al.2011; Hess and Jorissen 2009; Hess et al.2005; Langezaal et al.2006]. Gyroidina altiformis constitutes 27% of the living community and is generally a very low contributor (<1%) to bathyal foraminiferal faunas in the Western Mediterranean al., 2013; Sea and the North-east Atlantic Ocean (e.g., [Duros et al.2011, 2013; Fontanier et al.2002, 2008a,b, 2015]). Its dominance with B. marginata at station SR2 may underline its exceptional ability as a pioneer species in a first step of sediment recolonization after extended extreme environmental stress. As described by Anschutz et al. [2002] and Hess et al. [2005] in Cap Breton canyon (NE Atlantic), the overall biotic recovery of foraminiferal fauna was uncompleted two years after recent turbidite deposition. Therefore, in our study area, only 10 months after a historical change in the nature of discharged industrial waste in the region (from the dumping of dense red mud to liquid effluent release), it is fairly natural to document at station SR2 an ongoing recolonization characterized by a low diversity fauna. In Figure 6, we illustrate the proportion of opportunistic, stress-tolerant foraminiferal taxa which were documented as potential recolonizers of freshly disturbed areas (Psammosphaera spp., Saccammina spp., Technitella spp., R. scorpiurus,Quinqueloculina seminula (Linneaus, 1758), G. umbonata, B. marginata) [Fontanier et al.2012, 2013; Hess and Jorissen 2009; Hess and Kuhnt 1996; Hess et al.2005; Kaminski 1985]. We added G. altiformis to this group as a potential stress-tolerant taxon. At station SR2, opportunistic and pioneer taxa constitute more than 80% of the fauna, indicating a stressed community is recovering from ecosystem upheaval. Yet at all stations except SR2, opportunistic recolonizers account for less than 5% of the living faunas (Figure 6) where benthic foraminifera thrive in relatively stable ecosystems and natural trophic conditions control diversity, density and composition.
Relative abundance (%) of opportunistic and stress-tolerant foraminiferal taxa that are considered potential recolonizers of freshly disturbed areas (Psammosphaera spp., Saccammina spp., Technitella spp., Quinqueloculina seminula, Gyroidina umbonata, Gyroidina altiformis, Bulimina marginata).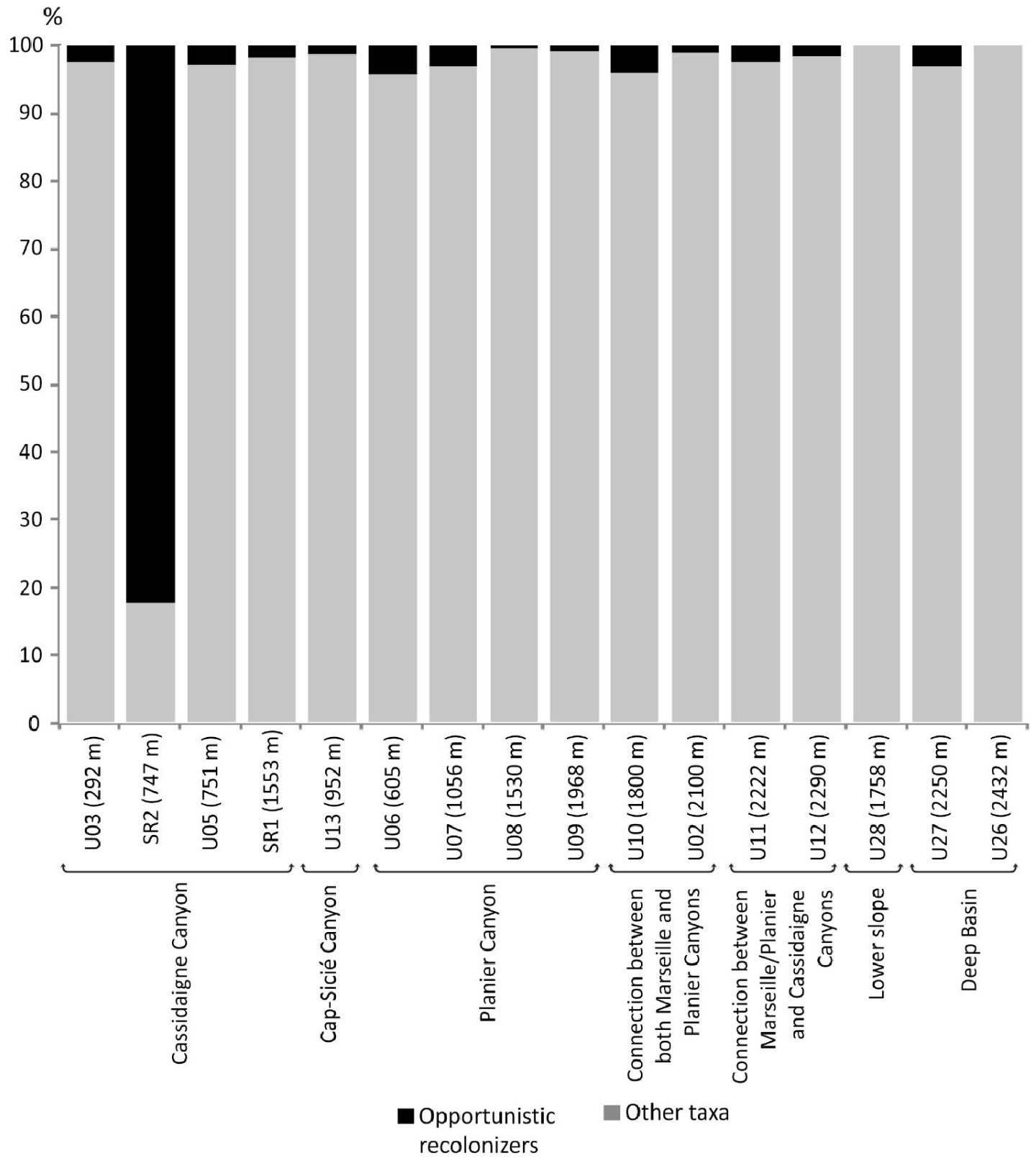
6. Conclusions
In autumn 2016, ten months after red mud dispersal ceased in the Cassidaigne Canyon, foraminiferal communities were sampled at 16 stations located between 265–2500 m water depth at varying proximity to the pipe outlet. Our ecological observations at the 725 m station (station SR2) located closest to the submarine pipe along the Cassidaigne Canyon axis show the highest concentration of the opportunistic and stress-tolerant species Bulimina marginata, commonly identified as a recolonizer of disturbed areas. At the other fifteen stations, foraminiferal standing stocks and simple diversity (S) decrease with increasing water depth and decreasing food input to the seafloor. There, the foraminiferal composition is characterized by a minor contribution of stress-tolerant species, echoing the overall meso-oligotrophic patterns of relatively stable ecosystems. Our study clearly shows that foraminiferal diversity close to pipe outlet in the Cassidaigne Canyon remains altered.
Acknowledgements
We thank the crew members of R/V “FéliX” (IXBLUE) and all scientific participants on the 2016 oceanographic cruise. C.F. (first author of this paper) and P.D. performed foraminiferal analyses in the framework of an industrial contract linking financially the FORAM Research Group (http://www.foram.eu.com) and CREOCEAN (http://www.creocean.fr) to ALTEO. In summer 2018, ALTEO allowed C.F. to use foraminiferal data for this publication. The Titanium dataset generated during the current study are not publicly available due contractual constraints linking CREOCEAN to ALTEO ALUMINA but are available from the corresponding author and the co-authors working in CREOCEAN on reasonable request. Finally, we want to thank both reviewers for their comments on the first manuscript.
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crgeos.5 or from the author.




 CC-BY 4.0
CC-BY 4.0