1 Introduction
It has been recently suggested that cells engaged in a given lineage could, under extreme experimental conditions, change their fate and redifferentiate across developmental barriers that were supposed before to be impossible to transgress. In the past few months, some of these results have been denied, though, and others where shown to reflect such rare phenomena that they hardly bear any real biological significance or potential therapeutic value 〚1〛. Some other related observations have not been criticised: undoubtedly, very low numbers of stringently sorted hematopoietic stem cells (HSCs) have regenerated functional hepatocytes in mice suffering acute liver failure 〚2〛. Yet, it remains to be determined whether this reflects an actual change in the differentiation program of blood-committed stem cells, or the co-purification with HSCs of more primitive stem cells endowed with broader developmental potential. We will herein review very recent results that suggest the role of a key ‘transdifferentiation’ event in the emergence of hematopoietic cells within the human embryo.
2 Intraembryonic origin of human definitive hematopoietic stem cells
At five weeks of human development, hematopoiesis has already significantly declined in the yolk sac and liver hematopoiesis arises. We have shown previously that at that stage, the floor of the dorsal aorta and vitelline artery is covered with hundreds of packed, rounded cells. These endothelium-adherent cells are by all means primitive hematopoietic stem cells, which express surface antigens, transcription factors and oncogenes similar to those expressed by their adult counterparts (Fig. 1) 〚3–5〛. These cells are first visible at day 27 of human gestation in the more rostral region of the aorta; derived cell clusters then increase in size and extend toward the pre-umbilical region of the aorta until day 40, after which they definitively disappear 〚6〛. The emergence of these cells within truncal arteries is indeed correlated with the appearance in that territory of functional hematopoietic progenitors, including high-proliferation potential colony-forming cells (HPP-CFCs), and more primitive long-term culture-initiating cells (LTC-ICs) 〚3〛.
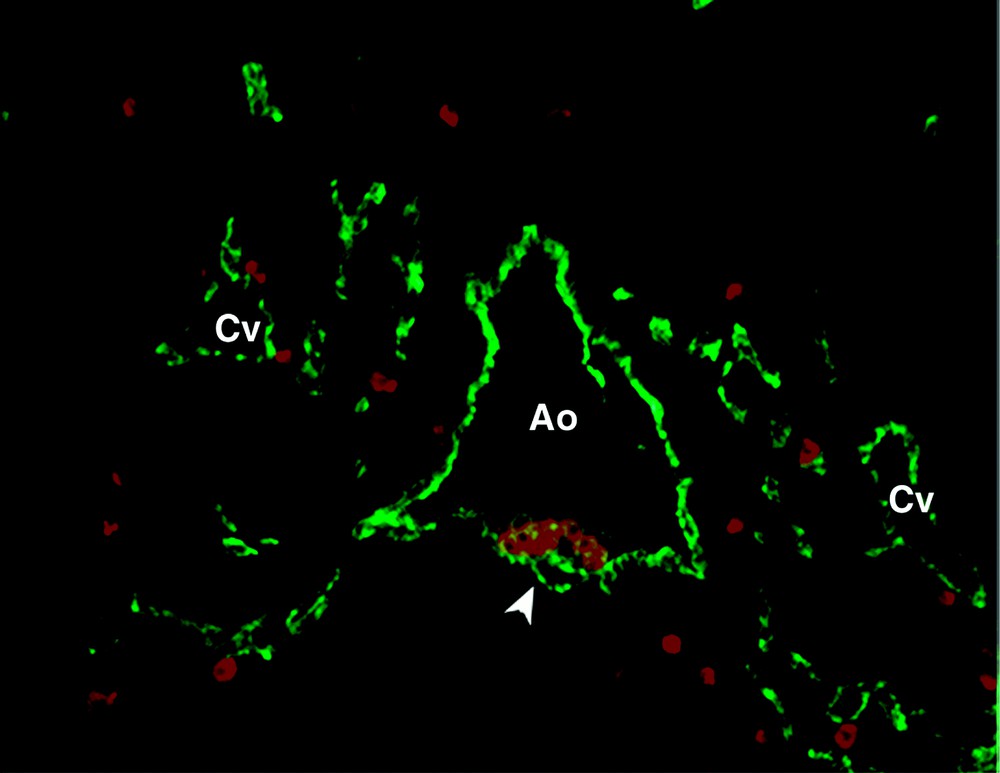
Transverse section of a 32-day embryonic human aorta (Ao) stained with anti-CD34 (green) and anti-CD45 (red) antibodies. Hematopoietic progenitors, being CD34+ CD45+, appear in orange, adhering to the ventral side of the aorta (arrow). Cv, cardinal vein.
Is this hematopoietic potential intrinsic in origin to intraembryonic territories or have these progenitors merely migrated from the yolk sac? To answer this question, we have assayed blood-forming potential in the human embryo and yolk sac as early as day 19 of development, i.e. before both are connected by the blood circulation, an event that takes place at day 21 of human gestation. The paraaortic splanchnopleura (PSp) and, at later stages, the derived AGM (aorta/gonad/mesonephros region) have been dissected and allowed to develop in the presence of MS-5 stromal cells, that support multilineage, myelo-lymphoid human hematopoiesis. T-cell potential was assessed by colonising NOD-SCID mouse foetal thymuses in vitro by cells derived from these territories.
The ability to establish long-term hematopoietic cell cultures was indeed present within the paraaortic splanchnopleura as early as day 19, the earliest stage tested, i.e. two days before blood circulation connects the embryo to the yolk sac and a week before stem cell clusters arise within truncal arteries 〚7〛. This shows that intraembryonic hematopoiesis arises autonomously, independently from yolk sac blood formation, and is in agreement with results obtained previously and simultaneously in the avian 〚8〛 and mouse models 〚9〛.
We have then further compared human intra- and extra-embryonic hematopoiesis in terms of lineage potential and found clear-cut differences between the yolk sac and PSp. In the above-described experimental setting, only intraembryonic territories yielded a multilineage progeny of myeloid, NK and lymphoid cells. In contrast, the yolk sac cultured in the same conditions generated only myeloid cells and NK cells. This basic difference was confirmed in all experiments performed from day 23 of development. The same observation was made as of T-cell potential ; only the PSp/AGM, and never the yolk sac, gave rise to T lymphocytes when used to colonize the foetal SCID/NOD mouse thymus 〚7〛.
Hence, to the classic succession of hematopoietic cell waves occurring in the human yolk sac, liver, thymus, and bone marrow, we have added a site of hematopoiesis born autonomously to the paraaortic splanchnopleura. This novel territory is most likely to play a critical role, if not the sole one, in the settlement of human definitive hematopoiesis 〚7〛.
We have also circumscribed more precisely the intraembryonic territory where hematopoietic cells emerge by dissecting, within the AGM, the embryonic aorta (A) away from the gonad (G) and kidney (M) rudiments and assaying those separately. Clearly, only the aorta is endowed with hematogenous potential 〚7〛.
3 Filiation of hematopoietic cells from embryonic vascular endothelium
At this point, we had determined the region of the embryo in which hematopoietic stem cells emerge, but their cellular origin remained unknown. An obvious observation, though, is that in the early embryo all blood cells form in the close vicinity of vascular endothelial cells, be it in the yolk sac, where blood islands adhere to newly formed vascular endothelial cells 〚10〛, or in the embryonic aorta, where hematopoietic cells emerge adjacent to pre-existing endothelial cells.
We thus aimed at testing the hypothesis that endothelial cells themselves are at the origin of embryonic hematopoietic cells. To this end, we sorted vascular cells from embryonic and foetal blood-forming tissues by flow cytometry. Vascular cells and hematopoietic cells share several markers such as CD34 and CD31 and presumably univocal endothelial markers such as VE-cadherin did not prove reliable for sorting endothelial cells. Eventually, the best recipe in our hands for endothelial cell sorting was the combination of CD34, or CD31, surface expression and absence of CD45, which marks the commitment to hematopoiesis. All blood-forming tissues in the embryo and foetus indeed contained CD34+ CD45+ hematopoietic progenitors and CD34+ CD45– endothelial cells, whereas non-hematopoietic tissues only contained the latter endothelial cells. Both cell subsets were sorted by FACS and it was confirmed by three-colour analysis that all sorted CD34+ CD45– cells are labelled with the Ulex europaeus lectin, an endothelial cell marker, whereas double-positive cells are not.
The absence of contaminating hematopoietic cells among sorted CD34+ CD45– endothelial cells was checked by PCR and, indeed, no CD45 messenger was ever detected within sorted single-positive cells. We also confirmed, functionally, that CD34+ CD45– endothelial cells were completely free of clonogenic hematopoietic progenitors, whereas CD34+ CD45+ cells expectedly contained such progenitors at a high frequency. When seeded in vitro in conditions of endothelial cell growth, sorted CD34+ CD45– cells did differentiate into adherent cells, which expressed markers of vascular endothelium such as CD31, Ulex europaeus ligand or von Willebrandt factor.
Consequently, sorted embryonic and foetal CD34+ CD45– cells do behave like true endothelial cells and are free of contaminating hematopoietic cells. However, when both single- and double-positive cells, i.e. endothelial cells and hematopoietic progenitors, sorted from the same blood-forming tissue, were seeded on MS-5 cells, both populations differentiated into hematopoietic cells, some of which formed typical cobblestone areas under the stroma (Fig. 2). Similar to whole, non-separated tissues 〚7〛, endothelial cells sorted from the yolk sac yielded myeloid cells only, whereas those sorted from the PSp/AGM gave rise to both myeloid and lymphoid cells.
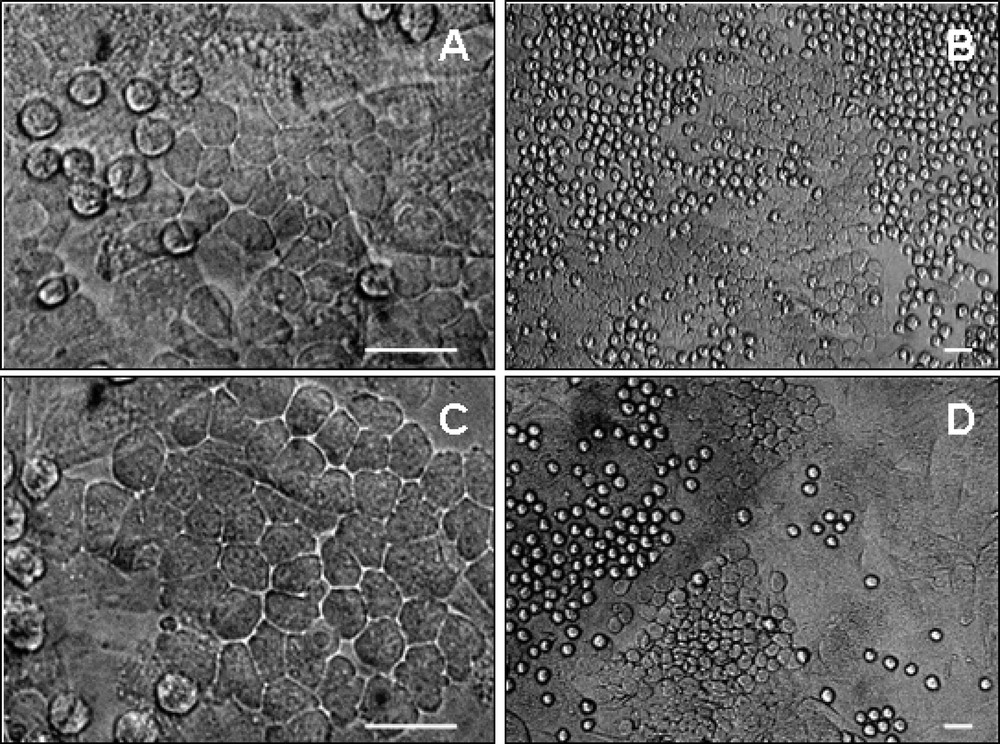
Colony formation on MS-5 stroma by 8-week foetal liver hematopoietic and endothelial cells. CD34+ CD45+ (A, B) and CD34+ CD45- (C, D) cells sorted from the foetal liver gave rise to colonies of packed rounded hematopoietic cells on MS-5 cells from day 3 up to day 35 of co-culture. Dark phase cobblestone-shaped colonies also developed underneath the stroma (arrows) from day 7 to day 35 of co-culture. Scale bar, 20 μm.
Such hematogenous endothelial cells were detected in primary sites of stem cell emergence, that is the yolk sac and PSp/AGM but also, less expectedly, in the embryonic liver and foetal bone marrow, where no hematopoietic cells are supposed to emerge intrinsically. In contrast, endothelial cells sorted identically from the lung, pancreas, spleen, thymus, foetal aorta and umbilical cord vein did not exhibit any blood-forming potential 〚11〛.
In order to estimate the frequency of blood-forming endothelial cells in embryonic and foetal hematopoietic tissues, the same experiments were performed again in limiting dilution conditions, seeding 2000 to 15 sorted cells per well in the presence of MS-5 stromal cells. A close correlation was observed to exist between the number of hematogenous endothelial cells and the actual blood-forming status of the tissue analysed, as a function of developmental age. In the yolk sac, this frequency was highest at 26 days of gestation and declined thereafter. Blood-forming endothelial cells were virtually undetectable in the 40-day yolk sac, which is not hematopoietic anymore. In the AGM, this frequency was highest, at about 1/120, around 30 days, but decreased dramatically at later stages to approach zero at day 40, which marks the end of aortic hematopoiesis 〚6〛. Of note, the frequency of blood-forming endothelial cells was significantly underestimated in these experiments, since the whole AGM was analysed, which contains many blood vessels in addition to the aorta. Finally, hematogenous endothelial cells were more rare, but definitely present in the embryonic liver and foetal bone marrow 〚11〛.
These results suggest that hematopoietic cells arise in embryonic truncal arteries by ‘transdifferentiation’ of pre-existing vascular endothelial cells. This notion was reinforced by semi-thin section histology analysis, showing that the endothelial lining is profoundly disorganised, or even no more present, underneath intra-aortic hematopoietic cell clusters 〚6〛.
4 Does hematogenous endothelium exist in the adult human bone marrow?
Most recently, M. Souyri and her colleagues in our laboratory have repeated the same experiments using, instead of embryonic and foetal tissues, normal adult bone marrow from which CD34+ CD45+ hematopoietic progenitors and CD34+ CD45– endothelial cells were sorted and co-cultured in the presence of MS-5 stromal cells. Unexpectedly, as was the case with embryonic and foetal blood-forming tissues, medullary endothelial cells also produced hematopoietic cells (unpublished results).
5 Conclusion
We have recently shown that a wave of hematopoietic stem cell emergence takes place within the human embryo proper, which most probably assumes a key role in the settlement of definitive hematopoiesis. Experiments reported herein suggest that vascular endothelium is a differentiation intermediate in the developmental sequence that drives extra- and intra-embryonic mesoderm into blood cells. This conclusion is in agreement with recent previous observations. Jaffredo et al. 〚12〛 have traced the progeny of vascular endothelium in the avian embryo with a fluorescent dye and shown that it includes blood cells. Similarly, mouse AGM endothelial cells sorted by VE-cadherin expression yielded blood cells in culture 〚13〛. It is still unclear whether all HSCs derive in ontogeny from pre-existing endothelial cells, or whether this represents a parallel pathway in developmental hematopoiesis. It is likely that only transgenic mouse models in which the endothelial cell lineage has been marked permanently will allow answering that question. Surprisingly, we also found hematogenous endothelial cells in primary hematopoietic organs, the embryonic liver and foetal bone marrow, which have long been known to depend on extrinsic stem cell seeding in order to sustain blood cell formation. Unexpected too was the identification of blood-forming endothelium in the adult bone marrow, an observation that can be related to the reported presence of BCR-ABL-expressing endothelial cells in chronic myeloid leukaemia patients 〚14〛. Retroviral marking experiments are in progress, which should allow finally establishing whether the vascular wall produces blood throughout ontogeny and postnatal stages.
Version abrégée
1 Introduction
Il est suggéré depuis quelques années que, dans des conditions expérimentales extrêmes, les cellules de certains lignages puissent, même dans l’organisme adulte, se redifférencier dans une autre voie. Certains de ces résultats ont été pourtant récemment niés. En outre, la plupart des phénomènes de cet ordre qui demeurent valides semblent survenir à une fréquence extrêmement faible. On ignore si ces observations reflètent une réelle redifférenciation ou la persistance dans l’organisme développé de cellules souches primitives, reliquats de l’ontogenèse. Nous présentons ici des résultats récents qui suggèrent que certaines cellules hématopoïétiques émergent, dans l’embryon et le fœtus humains, à partir de progéniteurs appartenant au lignage endothélial vasculaire.
2 Origine intra-embryonnaire des cellules souches de l’hématopoïèse humaine définitive
Nous avons précédemment décrit chez l’embryon humain un site inédit d’hématopoïèse situé dans la paroi ventrale de l’aorte et de l’artère vitelline. Une population de cellules souches hématopoïétiques y émerge et prolifère entre 27 et 40 jours de gestation. Ce sont les premières cellules souches multipotentes, lympho-myéloïdes, qui apparaissent au cours de l’ontogenèse humaine ; en effet celles qui se développent plus tôt dans le sac vitellin ont un potentiel de différenciation limité aux lignées myéloïdes.
Ce potentiel hématogène associé aux artères embryonnaires troncales est intrinsèque à l’ébauche de ce territoire. Nous l’avons en effet mis en évidence, dans un système de culture in vitro, dès le 19e jour de gestation, dans la splanchnopleure para-aortique, territoire présomptif de la région vasculaire où les cellules souches hématopoïétiques apparaissent huit jours plus tard.
Toutes ces données, ainsi que l’extrapolation de résultats convergents obtenus simultanément chez l’animal par d’autres groupes, indiquent que les cellules souches de l’hématopoïèse humaine définitive émergent dans l’embryon lui-même et non dans le sac vitellin, comme on le pensait précédemment.
3 Une filiation entre cellules endothéliales vasculaires et cellules hématopoïétiques, chez l’embryon et le fœtus humains
Nous avons émis l’hypothèse que, dans le sac vitellin comme dans l’aorte dorsale embryonnaire, les cellules hématopoïétiques émergent de la redifférenciation de cellules endothéliales préexistantes. Nous avons donc trié, par cytométrie en flux, les cellules endothéliales contenues, à différents stades, dans les tissus hématopoïétiques de l’embryon et du fœtus (sac vitellin, aorte dorsale, foie, moelle osseuse, thymus, rate) ainsi que dans des tissus non hématogènes, comme le poumon, le pancréas, l’aorte fœtale ou le cordon ombilical. Les cellules endothéliales ont été rigoureusement sélectionnées (CD34+ CD31+ CD45–) et l’absence de cellules hématopoïétiques contaminantes vérifiée dans tous les cas. Néanmoins, les cellules endothéliales triées à partir des tissus hématogènes primaires (sac vitellin, aorte et foie embryonnaires, moelle osseuse fœtale) ont donné naissance à une vigoureuse hématopoïèse in vitro. La fréquence dans ces tissus de cellules endothéliales hématogènes est très directement corrélée à leur activité hématopoïétique, en fonction des stades de la gestation. Les cellules endothéliales sélectionnées à partir de tissus non hématopoïétiques n’ont, en revanche, montré aucun pouvoir hématogène en culture.
En conclusion, il apparaît que les cellules souches hématopoïétiques dont nous avons montré l’émergence au sein de la paroi ventrale de l’aorte embryonnaire humaine y naissent de la « transdifférenciation » de cellules endothéliales préexistantes. Le même phénomène semble être aussi en partie mis en jeu dans le développement de l’hématopoïèse vitelline, hépatique et même médullaire. Des résultats préliminaires suggèrent même qu’une sous-population de cellules endothéliales vasculaires de la moelle osseuse garderait jusqu’à la vie adulte un potentiel hématopoïétique.



