1 Introduction
A common progenitor for angioblasts and hemopoietic cells, the hemangioblast, has been postulated to exist some 70 years ago. The idea that blood and vascular system share a transient ancestor was based on cytological observations in yolk sac blood islands. More recently, this conviction has been reinforced when several surface molecules and transcription factors were found to be expressed by both types of cells. However, experimental attempts to detect a mixed progeny have been frustrating: it has been difficult to find blood/endothelial colonies 〚1–4〛. This question offers more than theoretical interest, in view of the recent ideas to be presented during this meeting, namely that adult stem cells may display plasticity and that bone marrow Hemopoietic Stem Cells (HSC) may be able to repair other injured tissues, the case best monitored being that of hepatocytes 〚5〛. In particular, injected bone-marrow cells sorted for hemopoietic markers are able to repair injured endothelia 〚6, 7〛, raising the question whether hemangioblasts may stand at an important crossroad of cell differentiation and cell repair. We will review here our evidence about the lineage relationships between angioblasts and HSC during ontogeny. The experiments, carried out in the avian model, were focused on the secondary phase of HSC emergence, which occurs in the aortic region, immediately after a primary phase that occurs in yolk sac blood islands 〚8〛.
2 Role of the aortic region demonstrated in yolk sac chimeras
An experimental model using the quail/chick marker system 〚9〛 was devised, in which the E2 quail embryo was grafted on the E2 chicken extra-embryonic area 〚10〛; this type of chimera brought about indisputable proof that yolk sac hemopoietic progenitors were not self-renewable and were soon replaced by hemopoietic progenitors of intra-embryonic origin 〚11, 12〛. A similar approach, implemented in the amphibian embryo, arrived at a similar conclusion 〚13, 14〛. Finally, in vitro clonogenic assays as well as restoration of irradiated adults uncovered an intra-embryonic source of hemopoietic progenitors in the mammalian embryo 〚15–17〛.
Several early studies described ‘intra-aortic clusters’ in the embryos of phylogenetically distant species, provided that pertinent embryonic stages were investigated 〚18, 19〛. These clusters typically protrude from the ventral endothelium of the aorta (Fig. 1). They display affinity for hematopoietic cell markers, while affinity for endothelial cell markers disappear in the cluster region. These cytological observations were extended with demonstrations in the avian embryo 〚20〛 and the mouse embryo 〚15, 16, 21, 22〛, according to which cultured or engrafted aorta or aortic cells were able to produce a hemopoietic progeny. The conclusion was that the aortic endothelium is responsible for the production of the secondary hemopoietic progenitors.
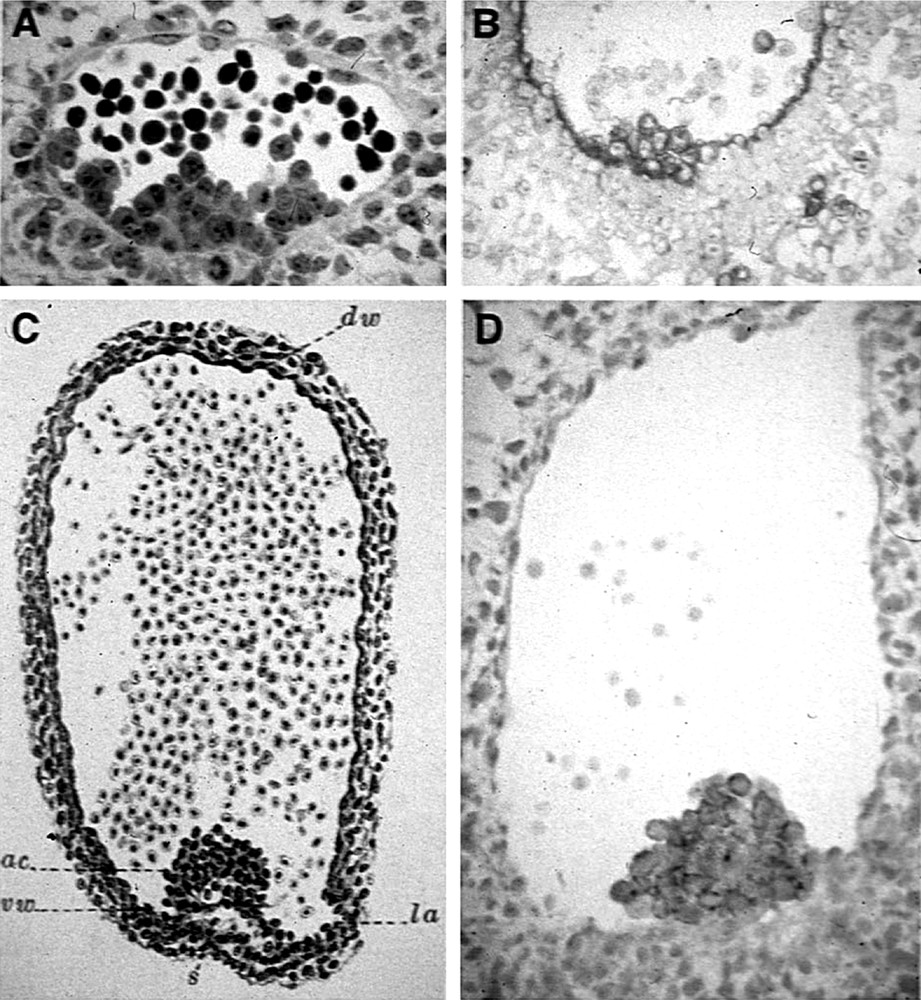
Intra-aortic clusters are typically found in vertebrate embryos: (A) in the mouse (E9 semi-thin section, toluidine blue; document kindly provided by Garcia-Porrero, see 〚36〛); (B) in the quail (E3, QH1 antibody/alkaline phosphatase); (C) in the pig (9 mm, hematoxylin and eosin, from Emmel 〚18〛); (D) in the E35 human embryo (CD45 antibody/alkaline phosphatase; document kindly provided by Tavian, see 〚17〛).
3 Why is the hemogenic potential of the aortic endothelium ventrally restricted?
This question was first asked by Jordan 〚23〛: “there seems to be no escape from the interpretation of such clusters as derivatives from the ventral wall of the aorta. The further question then arises: why is the hemogenic capacity of the endothelium of the aorta limited to the ventral wall in the region of the mesonephroi?” We addressed this question by means of orthotopic exchanges of rudiments between quail and chick embryos. We already knew that splanchnopleural mesoderm was endowed with a powerful hemogenic potential 〚24〛. Furthermore, several investigators had shown that somites could give rise to angioblasts in great numbers 〚25〛. Thus, we replaced one or two of the latest-formed somites of a chicken host with equivalent somites from a quail; alternatively, we grafted quail splanchnopleural mesoderm on top of the chick host splanchnopleura (Fig. 2) 〚26, 27〛. The cell progeny from the graft was followed by means of the QH1 antibody, which has affinity for quail hemopoietic and endothelial cells. The results were clear cut: somite-derived angioblasts invaded the body wall and limb bud at the level of the graft and integrated into the roof and sides of the aorta, but never migrated into the visceral organs nor into the floor of the aorta, and never gave rise to hemopoietic clusters. In contrast, cells from the splanchnopleural mesoderm invaded both limb buds/body wall and visceral organs; they also settled in the whole aortic endothelium, giving rise in the floor – and there only – to hemopoietic clusters. Thus, (1) only splanchnopleural mesoderm has a hemogenic potential; (2) this potential becomes expressed solely in the floor of the aorta. These experiments discriminate a dorsal angioblastic lineage from a ventral hemangioblastic lineage (Fig. 3) and show that, for the hemopoietic potential to become expressed, cells from the splanchnopleural mesoderm must receive a secondary signal, when they integrate into the floor of the aorta.
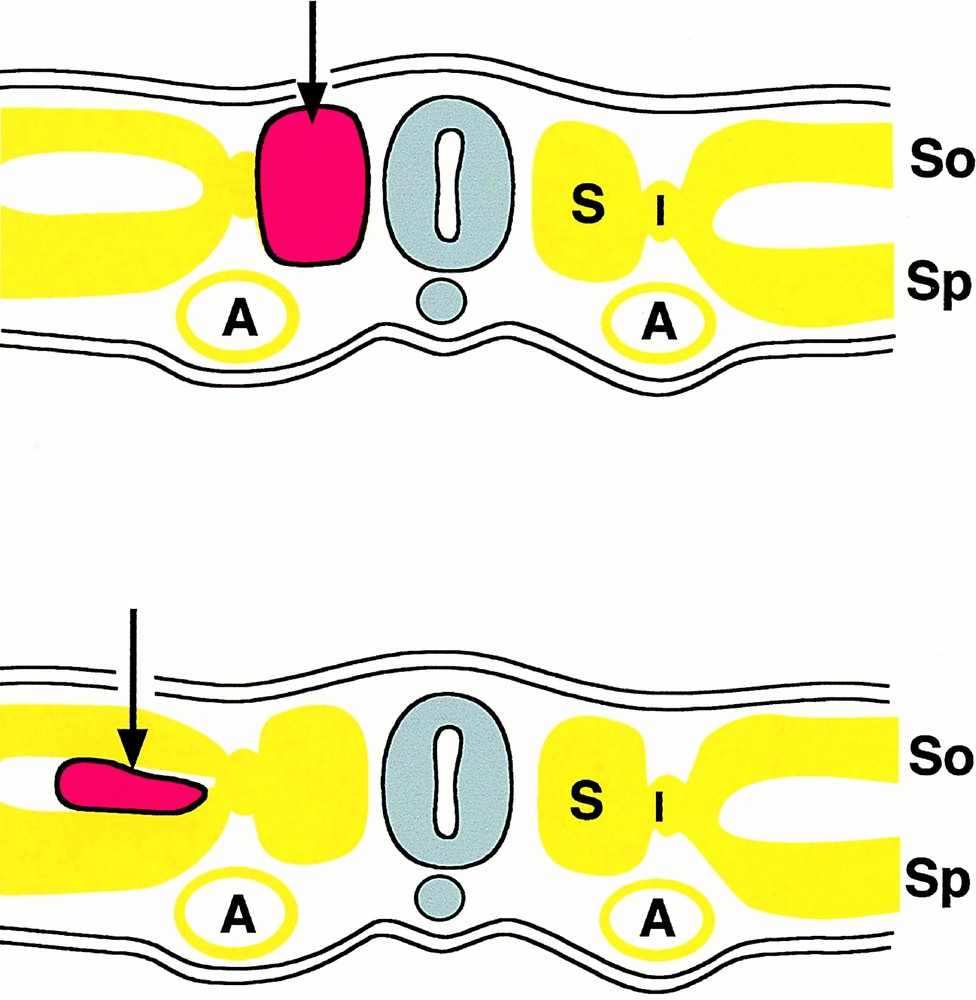
Scheme of grafts used to probe the angiogenic/hemangiogenic potential of somites (top) or lateral plate mesoderm (bottom). The grafts are depicted in red. A: Aorta; S: somite; So: somatopleural mesoderm; Sp: splanchnopleural mesoderm.
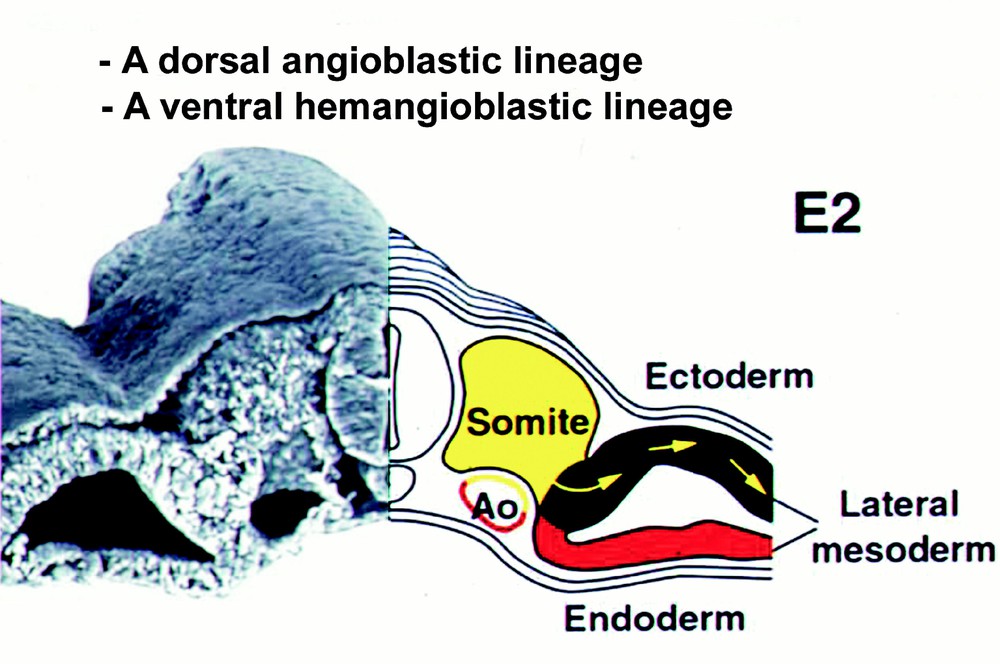
How we interpret the origin of the vascular and blood system. Note the mosaic origin of the aorta.
In order to obtain experimental support for this interpretation, the mesoderm was pre-treated either by in vitro overnight contact with one of the epithelial germ layers of the embryo, or by overnight culture on growth factor-containing semi-solid medium 〚27〛. Indeed, either coculture with endoderm or with VEGF, bFGF or TGFβ coaxed the progeny of somitic mesoderm into integrating into the floor of the aorta and giving rise to hemopoietic clusters. Inversely, the progeny of splanchnopleural mesoderm lost the capacity of moving into the aortic floor when treated by contact with ectoderm or pre-cultured on EGF or TGFα-containing medium.
The nature of the growth factor(s) driving, during normal development, the commitment to the angioblastic or hemangioblastic potential cannot be inferred from these experiments. It is known, however, that VEGF is expressed by the early endoderm 〚28, 29〛 and by the mesonephros 〚30〛 in close vicinity to the cluster-bearing endothelium at the right time of development. Thus, VEGF is a good candidate.
The developmental expression pattern of VEGF-R2, a receptor expressed on hemangioblasts 〚3, 31〛, also points to a crucial role of VEGF. Jaffredo and colleagues (unpublished data) used a method that amplifies weak signals to study the affinity of the early mesoderm for the VEGF-R2 specific antibody 〚3〛. At the definitive primitive streak (Fig. 4), the receptor is uniformly distributed over cells of the whole lateral plate mesoderm in the caudal half of the blastodisk, indicating the commitment of the median germ layer chiefly to hemopoiesis. Indeed a large number of hemopoietic or endothelial colonies can be obtained from VEGF-R2+ sorted cells 〚3〛. At the 10 somite-stage, (Fig. 5) the blastocoelic cavity has formed; these cells have redistributed to the lower (splanchnopleural) layer of the mesoderm in contact with the endoderm, and have almost disappeared from the upper (somatopleural) layer in contact with the ectoderm. In the anteriormost region, VEGF-R2+ cells are seen streaming out of the somites (Fig. 5, level A).
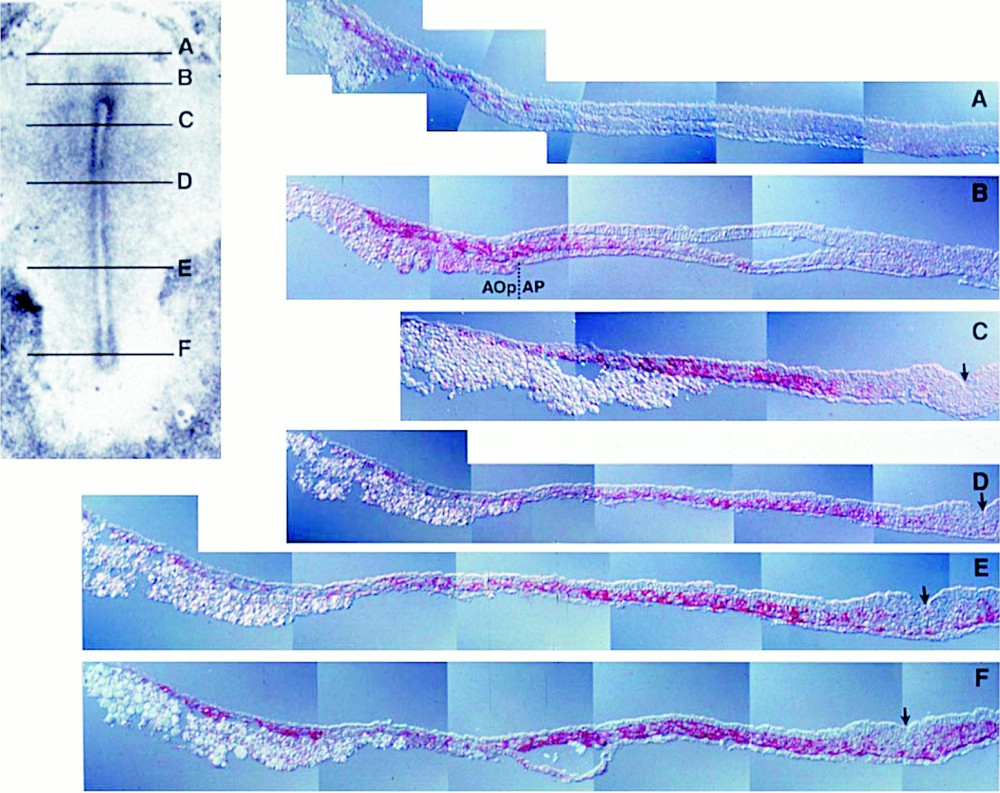
VEGF-R2+ cells (red) displayed at the definitive primitive streak stage in the chick embryo (VEGF-R2 antibody/tyramide signal amplification/alkaline phosphatase in pseudo-colour and Nomarski optics). The levels of the transverse sections (right) are indicated on the whole mount view (left). At this stage, all or most cells in the posterior lateral plate (C–F) mesoderm are VEGF-R2+. Negative upper and lower layers are respectively the ectoderm and the endoderm. The arrow indicates the primitive streak through which cells ingress forming the mesoderm. Aop/Ap: area opaca/area pellucida. The anti-VEGF-R2 antibody was a kind gift from Anne Eichmann.
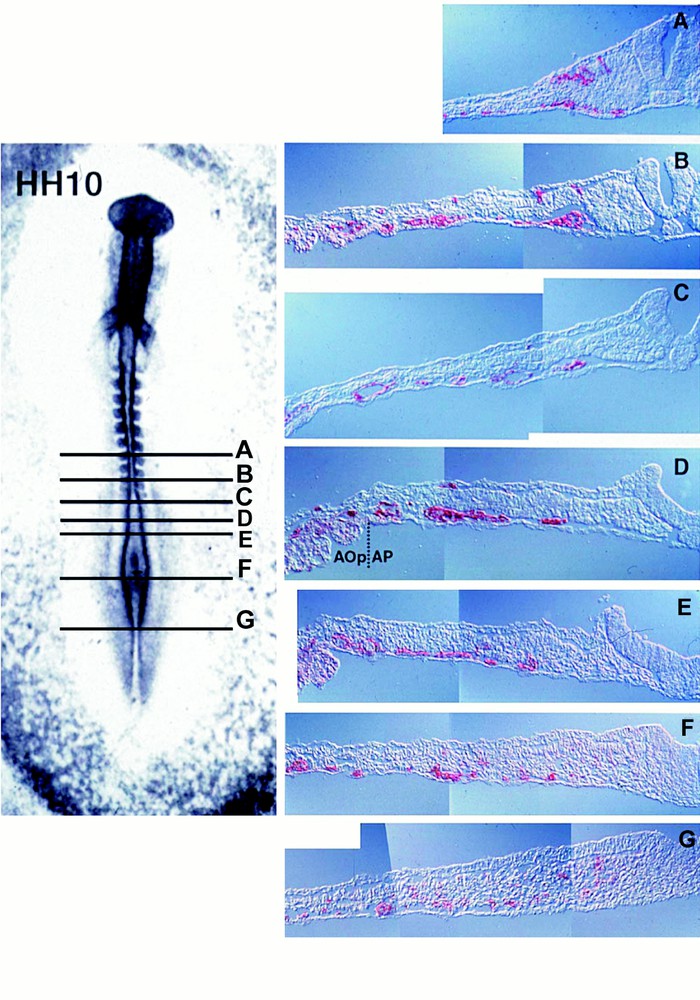
VEGF-R2+ cells at the 10 somite stage (Hamburger and Hamilton-HH-10). Note that positive cells are condensing towards the endoderm, especially in sections D and E where vessels are shaping up. In the anterior most section (A), i.e., in the more evolved region of the embryo where the neural tube has closed, VEG-R2 cells are streaming out of the somite.
Together, these features point to an influence of endoderm-secreted VEGF: according to our interpretation, the hemangioblastic potential, ubiquitously distributed in the early mesoderm, later concentrates in the lower layer. While endoderm exerts a positive influence on survival and multiplication of these cells and probably also attracts them, the ectoderm probably exerts a negative influence.
4 Lineage relationship between endothelium and hemopoiesis
The peculiar insertion of the aortic clusters in the aortic floor endothelium and their time-restricted development make this site interesting for lineage tracing. This was accomplished using two vital probes. Acetylated Low Density Lipoproteins (acLDL) are endocytosed by endothelial cells and macrophages through specific receptors. AcLDLs, injected in the heart of 13 somite embryos, labelled within two hours the whole endothelial tree prior to cluster appearance. If the embryos were sacrificed 24 hours later (stage HH 19), the clusters, forming well after endothelium labelling and acLDL clearance from the blood, were double positive for acLDL and CD45, a leukocyte-specific antigen 〚32〛. The second probe (a non-replicative retroviral vector, derived from the spleen necrosis virus and encoding the lacZ reporter gene) had the advantage that it labelled cells more durably, and the inconvenience, regarding our goal, that only rare cells were labelled. The vector was injected at the same stage and through the same route as acLDL, it required eight hours to become integrated and expressed in endothelial cells and thereafter was cleared from the blood. Labelled cells remained detectable several days after injection. Twenty-four hours after injection, isolated cells, or small groups of cells (2 to 4), were labelled in the vascular tree, some of them among unlabelled cells in clusters. Four days after the injection (E6), the extensive hemopoietic foci, developed in the dorsal mesentery at that stage 〚33〛, contained many double-labelled βgal+/CD45+ cells 〚34〛. Thus, like the clusters, the hemopoietic foci derive from aortic endothelial cells (Fig. 6).
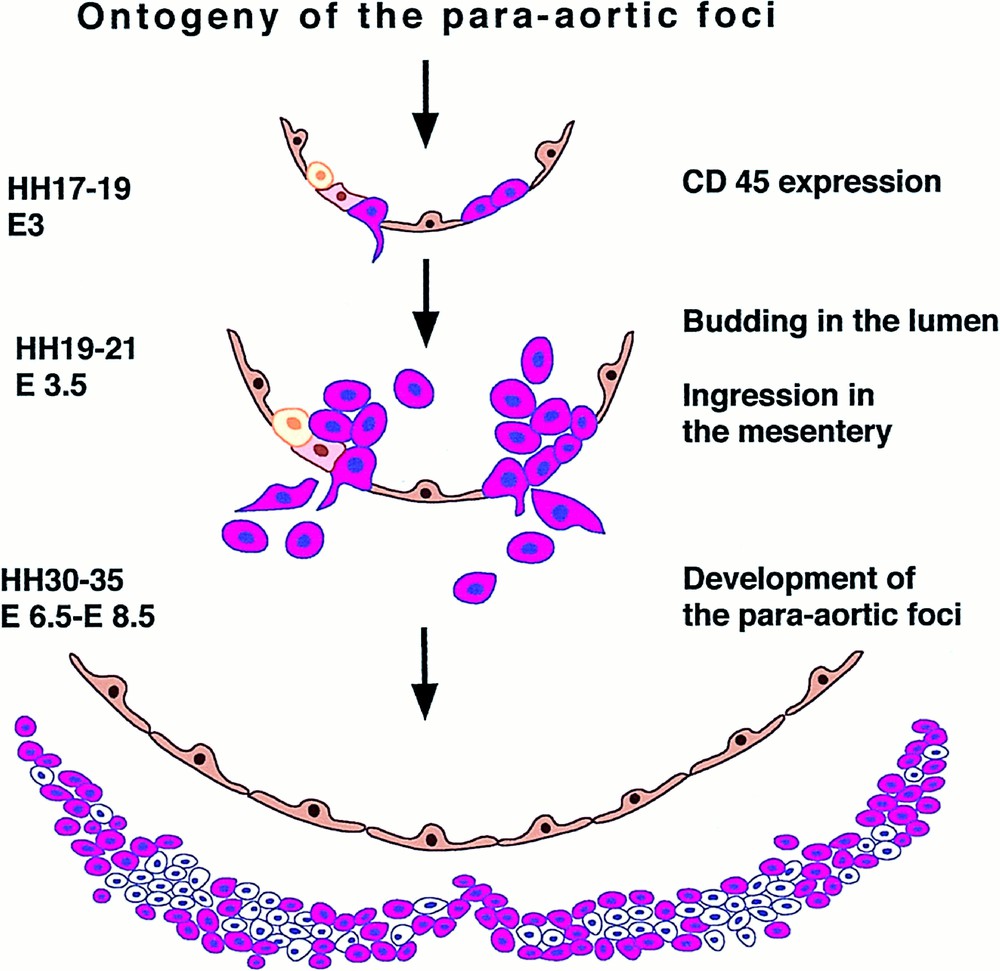
Scheme depicting the origin of the intra-aortic clusters (E3) and para-aortic foci (E6) from the floor of the aortic endothelium, as demonstrated by E2 in vivo labelling. CD45+ cells (white blood cells and progenitors) are shown in red, while VEGF-R2 positive cells are shown in brown (document kindly provided by Jaffredo, see 〚34〛).
5 Expression of hemangioblastic specific transcription factors
Several genes encoding transcription factors have been identified by virtue of their implication (following translocations or mutations) in leukaemias. The encoded proteins participate in DNA-binding complexes that transactivate hemopoietic and/or endothelial specific genes. The commitment to a specific differentiation pathway depends on the combination of factors involved in the complexes. Based on the expression patterns and on the effects brought about by knockout, these genes are thought to mediate angiogenesis, hemopoiesis, or both and the activity of some of them may turn on at the hemangioblastic stage. Some of these gene activities are already required for yolk sac hemopoiesis (SCL/tal1, GATA2, LMO2), while that of others become active at the foetal liver stage (Runx1, c-myb). Their combinative involvement has been worked out in vitro on cell lines or on embryonic stem cells differentiating to hemopoiesis 〚35〛. Because of their tightly controlled development in time and space, the clusters are obvious candidates from which to work out the fine-tuning of these gene activities as hemopoiesis proceeds. Bollérot and Jaffredo (unpublished data) carried out an in situ hybridisation study of these activities between stages HH17 (precluster stage) and 19 (full cluster stage). Only Runx1 and c-myb expressions are completely restricted to the ventral aortic endothelium (Fig. 7). GATA2 and SCL display a strong signal on clusters, but are also irregularly expressed on other endothelia. LMO2 is strongly expressed by all endothelia, comprising the cluster-bearing stretch of ventral aortic endothelium. Runx1 becomes expressed prior to the formation of the clusters. More surprisingly, so does c-myb, even though the activity of this gene is supposed to be involved in the amplification of the hemopoietic progenitor population rather than in its commitment. Finally, it is interesting to note that Gata-1, implicated in erythroid terminal differentiation, is never expressed in the clusters, while PU-1 is. These latter activities indicate that the progenitors become committed in the clusters, but do not find there appropriate conditions for erythropoiesis. Instead, they may remain uncommitted, before moving into other microenvironments, or undergo granulopoiesis, as indicated by PU-1 transcription.
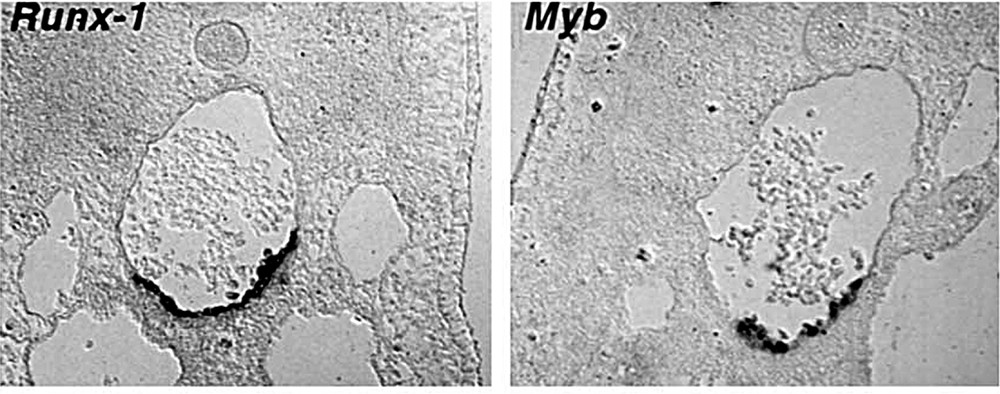
Expression of hemopoietic-specific transcription factors Runx-1 and c-myb is restricted to the aortic floor, in the developing clusters (Bollérot and Jaffredo, unpublished results).
6 Conclusion
Orthotopic transplantations of rudiments between the chicken and the quail embryo made it possible to establish that the aortic endothelium is mosaic in origin, explaining the restriction of the hemopoietic commitment to the floor. Tracing studies led to the conclusion that this endothelium emits hemopoietic progenitors and should thus be considered as a ‘hemogenic endothelium’. Other experiments point to crucial influences of endoderm and ectoderm that can be reproduced with growth factors, suggesting that a cascade of signals may be required to promote hemopoietic commitment in this site. Genes encoding transcription factors specific for hemopoiesis become activated a few hours before the appearance of clusters, sharply marking the conversion of the endothelial phenotype into the hemogenic phenotype. In other experimental settings where the plasticity of endothelial cells to hemopoietic cells (or vice-versa) can be substantiated, these gene activities might be used as markers to discriminate steps in transdifferentiation.



