1 Introduction
A major goal of conservation biology is the preservation of genetic diversity in order to maintain the evolutionary potential of the species [1]. Hence many conservation genetics studies have focused on assessment of genetic diversity within and between populations of rare species, and on mating systems and the dynamics of small population size as major influences on the level of genetic diversity within species. Whilst these genetic analyses makes an important contribution to conservation and management strategies, phylogenetics, the use of genetic data to determine evolutionary relationships between populations and species, can also make significant contribution to conservation. Phylogeny, and its application in phylogeography, can provide valuable insights into how current genetic patterns have developed through evolutionary history. This knowledge provides the broad evolutionary context for the conservation and management of the genetic resources of the flora in a region. In addition phylogeny can also contribute to practical outcomes in the conservation of specific taxa. Phylogenetic studies below species level are valuable for identifying different genetic lineages with unique evolutionary histories as conservation management units or evolutionary significant units (ESU's) [2,3]. A knowledge of phylogenetic relationships between species allows determination of the phylogenetic value of species to assess priority for conservation activity [4], and also enables appropriate comparisons to be made between rare species and their widespread relatives in assessment of their genetic diversity [5]. Knowledge of phylogeny and the differentiation of genetic and/or taxonomic units is valuable for assessing taxonomic status. This is critical for species complexes where genetic and morphological variation have not been resolved. The flora of south-west Western Australia has been subject to evolutionary forces over long time frames and phylogenetic analysis provides a knowledge of evolutionary relationships that is particularly valuable in the conservation and management of this diverse flora. Unlike many areas of the world the south-west of Western Australia has had a stable geological history with no major glaciation events [6]. The subdued landscape has no significant mountains, and is dominated by a semi-arid zone between the high rainfall mesic area in the extreme south west corner and the desert arid zone in the north and east [7]. However, the area has experienced climatic oscillations during the Pleistocene due to cyclic contraction and expansion of the arid and mesic zones, particularly in the transitional semi-arid zone [6]. The area has a highly diverse and largely endemic flora characterised by both relictual taxa that have persisted through landscape stability and lack of glaciation, and more recently evolved components that have resulted from adaptation to a more arid environment [7,8]. A complex mosaic of soil types and habitats has led to a high degree of natural isolation and fragmentation within the flora, resulting in significant local endemism and a high turnover of taxa across the landscape, affecting both relictual and recently evolved species. This review examines aspects of phylogenetics that are relevant to the conservation of a diverse and ancient flora in a fragmented landscape.
2 Phylogenetics and taxonomic status
While the goal of conservation is the preservation of variation rather than taxonomic units [9], in practice some means of categorizing diversity into units on which specific conservation actions can be focused is required. Currently, conservation management is usually focused on taxonomically defined species although populations can be listed for conservation in some organisms and countries [10,11]. Taxonomy is most often based on morphological similarity and while this approach will generally define cohesive biological units there may be many exceptions. Taxonomies that do not reflect phylogenetic entities will hinder conservation efforts [10].
The flora in south-west Western Australia contains many species complexes with a high degree of morphological variation. In many of these complexes some taxonomic resolution has been achieved but others remained unresolved and phylogeny can assist in the resolution of taxonomies that reflect evolutionary history in these complexes. Eucalyptus angustissima is one such species complex where taxonomic units have been defined but there are still some uncertainties about the taxon boundaries. The complex contains three species, one of which has two subspecies. The four taxa are morphologically similar varying in leaf and fruit size. They all occur in flat low lying habitat but occupy different niches within that habitat. Three of the four taxa are rare and restricted, E. foliosa is known from two populations but E. misella and E. angustissima subsp. querenda are only known from one population each. The other taxon, E. angustissima subsp. angustissima, has a wider distribution but is still localised. Studies of two other eucalypt species complexes in Western Australia have shown little genetic differentiation between the taxa [12,13] and their taxonomic status is being revised (D. Nicolle, pers. com.). This has led to questions about the genetic distinctness of the taxa in the E. angustissima complex. If there is also little differentiation within this complex the three taxa that are currently considered rare would have a low priority for conservation and their taxonomic status may also be reconsidered. A phylogenetic study of the E. angustissima complex using nuclear RFLP loci showed that the taxa were all genetically distinct (Fig. 1). In addition the two subspecies of E. angusissima showed the greatest genetic differentiation of all the taxa. The phylogeny of the taxa is consistent with their current taxonomic status except that the level of differentiation between the two subspecies of E. angustissima suggests that they should be recognised at species rather than subspecies rank. This means that the conservation listing of the rare species in this complex should be retained, although further field survey is required to confirm their geographic distribution and abundance, and hence their conservation status.
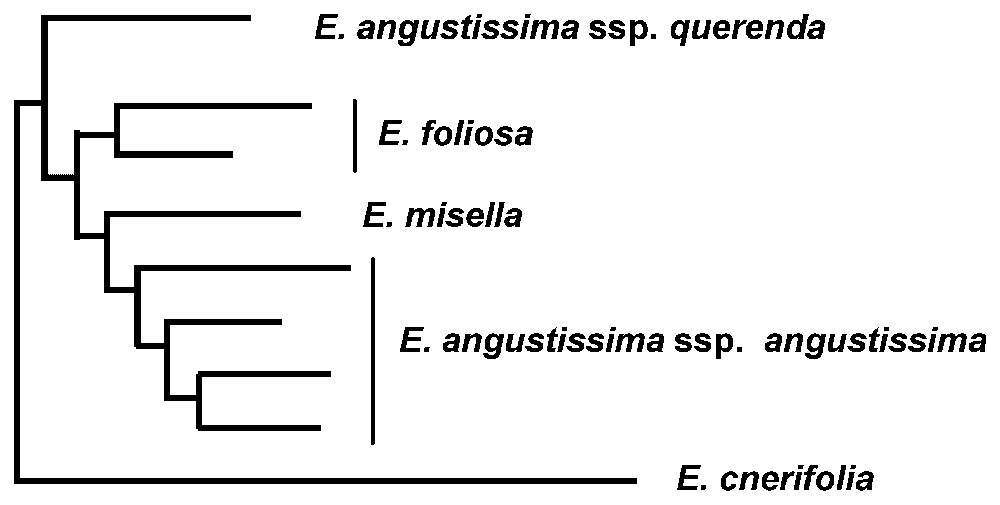
Phylogenetic tree of taxa in the Eucalyptus angustissima complex using nuclear RFLPs.
In contrast to the localised taxa in the E. angustissima complex many other species complexes are widespread but they may also harbour unrecognised rare and restricted species. The rare Acacia sp. “Dandaragan” is a recently recognised species which is currently gazetted as a phrase name and listed as threatened flora with a IUCN and state ranking of critically endangered. However, there is some uncertainty about its taxonomic status in relation to the common A. microbotrya from which it has been separated. Acacia sp. “Dandaragan” is restricted to a ridge outside Dandaragan on western edge of the range of the widespread A. microbotrya complex, and may be a morphological variant of, or the end of a morphological cline from, A. microbotrya. A morphological and genetic study of A. sp. “Dandaragan” and several populations of the two described variants of A. microbotyra, showed A. sp. “Dandaragan” to be morphologically and genetically distinct from the A. microbotyra variants [14] and confirmed the independent status of the taxon. The identity and conservation status of rare species may be masked by unresolved morphological variation present in species complexes such as A. microbotrya and phylogenetic studies provide assistance in resolving these taxonomic ambiguities so that the conservation status of units is appropriately recognised.
3 Identification of evolutionary significant units within species
In addition to providing a basis for identification of biologically cohesive units phylogenetic analysis can also identify populations or lineages within species that are genetically distinct. If these distinct lineages are rare or threatened they may require protection and conservation action as evolutionary significant units (ESUs). The evolutionary history of the south-west of Western Australia would be expected to lead to significant effects on the structuring of genetic diversity within the flora. In a naturally fragmented landscape many species persist in geographically restricted distributions and genetic studies on Western Australian species that are rare and restricted, particularly those with disjunct distributions, have often revealed genetic lineages within these species, e.g. Acacia anomala, Stylidium coroniforme, S. nungarinenese, Banksia cuneata and Lambertia orbifolia [3]. These genetic lineages have been identified despite the absence of morphological differentiation between the population groups in each species [3] although differences in reproductive strategy have been observed in A. anomala, with the northern populations exhibiting sexual reproduction while the southern populations reproduce by clonal propagation [15]. In one of these species, L. orbifolia, the differentiation between the lineages was also examined using cpDNA markers. Lambertia orbifolia is a large bird pollinated woody shrub that occurs in two population clusters at Narrikup and Scott River Plains which are approximately 200 km apart. An isozyme study [16] found very high genetic divergence between the two population clusters (Nei's genetic distance=0.252) and phylogenetic analysis of the species showed the differentiation of the Narrikup population from those at Scott River Plains (Fig. 2a). Analysis of the populations using cpDNA markers confirmed the evolutionary distinctness of these lineages [17]. In this study eight mutations were detected within the species but six of them were specific to the Narrikup populations and distinguished them from the Scott River Plains populations (Fig. 2b). Thus the maternal phylogeny confirmed the evolutionary distinctiveness of the lineages identified using nuclear markers. The two population clusters can be considered as separate conservation units since they meet the criteria suggested for ESUs [18] as they have significant allele frequency differences in the nuclear genome and reciprocal monophyly of populations for a maternal uniparentally inherited marker. Based on these studies the Narrikup populations are now informally considered a separate taxon under the phrase name L. orbifolia “subsp. orbifolia”. This has had practical implications for the conservation of these populations. The species Lambertia orbifolia is listed as threatened with an IUCN and State ranking of endangered, but, due to the large number of critically endangered species in Western Australia, it is not considered a high priority for conservation and recovery action. However, the Narrikup populations are small, occur on degraded roadsides and are threatened by disease. Since their informal recognition as a separate subspecies these populations have been ranked critically endangered and are now being targeted for recovery action by translocation to a safe, disease-free site.

Phylogenetic trees of populations of Lambertia orbifolia using (a) nuclear markers and (b) cpDNA markers. Solid bars on lines represent mutations, dotted bars on lines represent the loss of mutations.
The confirmation of evolutionary distinct lineages in L. orbifolia suggests that the lineages identified in other species will also be evolutionarily distinct and indicates that genetic processes associated with historical eco-geographical barriers to gene flow may have lead to the formation of distinct evolutionary lineages within many species. These lineages often warrant separate conservation management [10] and their identification ensures that the protection of distinct lineages is addressed by enabling conservation activity to be focused at this level where necessary.
While practical conservation outcomes are usually focused on rare and restricted species, ESUs may also be present in the genetic resources of widespread species. Most studies of the nuclear genome of widespread species in Western Australia have confirmed little genetic differentiation between populations [12,13,19] but phylogenetic studies using cpDNA have shown substantial differentiation in the chloroplast genome in these species [20,21]. The indicates that widespread species have also been affected by historical isolation and fragmentation which is still detectable in the slowly evolving chloroplast genome even though more recent gene flow has obscured this effect in the nuclear genome. This pattern of historical isolation and fragmentation is consistent with what might be expected based on the biogeographical history of the region. Recognition of evolutionary lineages within widespread species is also important for ecosystem management especially in the conservation and rehabilitation of patches of remnant native vegetation in the semi-arid zone now dominated by agricultural crops.
4 Priority setting in conservation
Conservation management operates in a framework of priority setting due to competition for land use and limited funding for conservation activities [11]. In most cases the driving force behind priority setting is the degree of threat to specific taxa [7]. However this assumes that all species are equal and several authors have suggested that this is not the case [9,22–24]. Biodiversity holds different values for different people and assessment of value can be based on various criteria including cultural, economic, ecological or phylogenetic. Assessing biodiversity value using phylogenetic diversity incorporates an evolutionary perspective, whether priorities are placed on phylogenetic distinctiveness [24], character richness [9] or taxa from rapidly evolving clades [25].
Phylogenetic distinctness will be particularly important in an ancient flora where the phylogenetic value of relictual species and recently evolved species are quite different even though both types of species may be rare and restricted. Two rare species of Acacia occurring at the north-western edge of the range of their more common widespread relatives were thought to represent recent speciation due to their morphological similarity, restricted distributions, and occurrence in an area of high speciation. A study of the phylogenetic relationships using chloroplast DNA between the pairs of taxa showed that they had quite different evolutionary patterns [4]. The rare species Acacia sciophanes shares a common ancestor with its more common relative A. anfractuosa (Fig. 3a) but the two species have diverged relatively recently [4]. In contrast phylogenetic relationships between the second pair of species with a similar distribution pattern in the same area showed that the rare A. lobulata was genetically very distinct from the more widespread A. verricula [4]. Acacia lobulata was also distinct from several other taxa from the A. flavipila alliance including an eastern Australian species, A. ixiophylla, which coalesced to a common ancestor with A. verricula and another species in the alliance (Fig. 3b). Acacia lobulata does not have close affinities with any other species and may represent a relictual species with no extant close relatives. Acacia sciophanes has a higher priority for conservation than A. lobulata based on degree of threat. However the phylogenetic distinctness of A. lobulata as a unique and ancient lineage should also be recognised in any decisions regarding its conservation status.
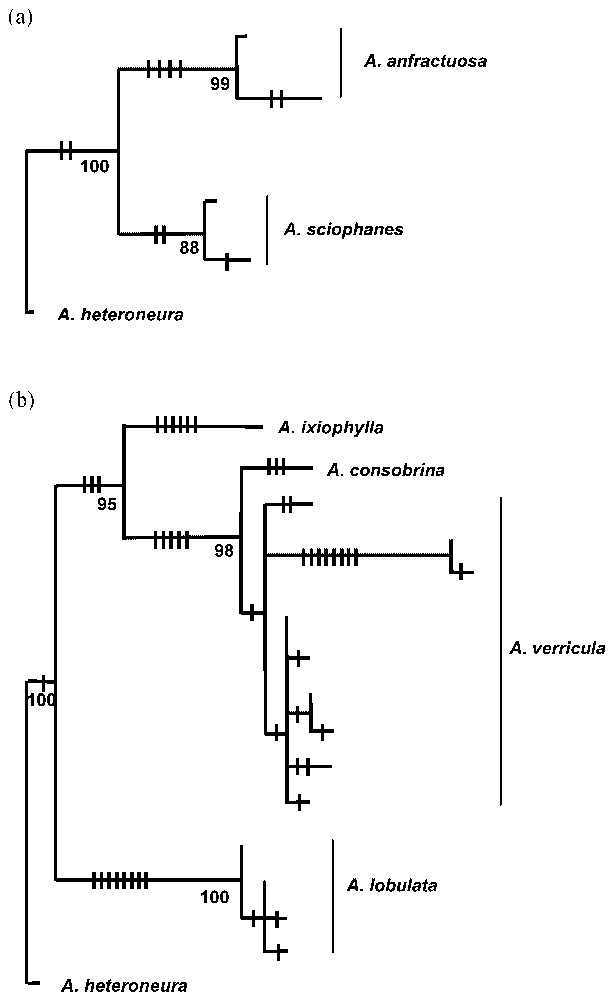
Phylogenetic parsimony tree of chloroplast haplotype relationships in (a) Acacia sciophanes and A. anfractuosa and (b) A. lobulata and A. verricula. Bars on lines represent mutations. Acacia heteroneura was used as the outgroup and is from the same alliance as A. sciophanes and A. anfractuosa.
Another rare species that also occurs at the edge of the distribution of its presumed widespread relative is A. oldfieldii which is restricted to two populations in the Moresby Range north of Geraldton. Acacia oldfieldii is morphologically similar to the widespread complex A. acuminata, but a nuclear study showed it to be significantly divergent from other taxa in that complex [26]. A phylogenetic study using cpDNA confirmed the distinctiveness of A. oldfieldii as it had a high number of mutations differentiating it from the other taxa in the A. acuminata complex [21]. The degree of divergence of A. oldfieldii from A. acuminata was unexpected given the low level of morphological differences between them and appears to be another example of a relictual species with a restricted distribution. These studies show that similarities based on morphology and geographic distributions are not necessarily reliable in identifying relictual species or more recent speciation events. Identification of phylogenetically distinct relictual taxa will enable the value of these species to be taken into account when setting conservation priorities.
5 Phylogenetics and species comparisons in conservation
Species phylogenies also have value in assessing levels of genetic diversity in rare species. Methods of assessment of genetic diversity require some reference point and most studies have made comparisons between rare species and average values for other species. Such comparisons have often shown rare species to have lower levels of genetic diversity compared to widespread plants with similar life history traits [27], however, the range in levels of diversity varies greatly. Rather than comparisons to a mean for rare or widespread species, Karron [28] pointed out that it was more logical to make comparisons between congeneric species. Comparison between rare species and a widespread common relative helps to elucidate the particular aspects that limit the success of rare species [5] and this applies to comparisons of ecological parameters as well as genetic diversity. However Felsenstein [29] has also suggested that comparisons of congeners should use fully resolved phylogenies to ensure that the studies utilize the full power of phylogenetically independent comparisons. Genetic studies on several Acacia species have shown that rare acacias in Western Australia are not depauparate in genetic diversity compared with the levels of diversity generally observed in Acacia species, including many from eastern Australia [30]. However, these rare species do show lower levels of diversity when compared directly to their widespread relatives in phylogenetic independent comparisons [30]. Properly resolved phylogenies make it easier to identify species pairs between which appropriate phylogenetically independent comparisons can be made for both genetic and ecological data. Such comparisons enable the particular aspects of the species biology that may be a factor in its rarity to be more readily determined.
6 Conclusions
Phylogenetic analysis is particularly valuable in an ancient, stable landscape such as the south-west of Western Australia. Phylogeny provides many insights into the evolutionary history of species that can assist in making generalisations about genetic patterns since not all species can be studied extensively. This evolutionary perspective on biodiversity places the conservation and management of plant genetic resources within the broad context of a dynamic and interactive ecosystem. In addition phylogeny can make practical contributions to the conservation and management of the flora particularly in clarifying taxonomic ambiguities and identifying evolutionary significant units. Identification of relictual and recently derived species allows the phylogenetic distinctness and value of relictual species to be recognised leading to conservation action if necessary. Phylogenetics also assists other conservation biology studies by providing information on appropriate species comparisons for genetic and ecological parameters.



