1 Introduction
The bitterling is considered as a fish species of high conservation status, listed in the ‘Habitats’ Directive (Annex II) and Bern Convention (Annex III). It is listed in the French Fishes Red list as a vulnerable species according to UICN criteria [1,2] and it is protected by French legislation because of its fragmented distribution [3]. The sensitivity of the species to the degradation of its habitat is relatively little documented, and is accentuated by its remarkable life traits: the relationship with freshwater mussels (Unionidae) [4,5]. Complex interactions exist between bitterling and several freshwater bivalve species since female bitterlings deposit eggs into the gills of a mussel, which are fertilized by sperm released by males in the inhalant siphon of the host species [6–10]. Furthermore, larvae of the mollusc (glochidia) also develop in the gills of bitterling and other Cyprinid species [11] and thus constitute an unusual case of a “two-way parasitism” according to Combes [12].
All studies dealing with bitterling have focused on the relationships with freshwater mussels, the oviposition site choice and their consequences for population dynamics [8–10]. Except for the study of Reynolds [7] on the effects of phosphorus pollutants on bitterling and freshwater mussels, there is no study on the habitat use by this fish species and habitat variables influencing its spatial distribution.
Within the framework of a program on predator-prey interactions [13,14] an investigation on the fish community in the Bourgneuf Marsh enabled us to exploit a long-term database (1987–1991 and 1996–2001) on the bitterling population. The objectives of the present study are to identify firstly the trends of the bitterling population, and secondly the interactions between the presence of bitterling and landscape habitat variables in this man-made ditch network. Finally we discuss the conservation strategies for bitterling and the role of human activities in the future of this fish species.
2 Materials and methods
2.1 Study site
The Bourgneuf Marsh is located in western France (47°N, 2°W), near the Atlantic coast. It covers 16 000 ha of former salt marshes that were embanked during the 11th century for salt production. Nowadays, it forms a mosaic of extensive pastures with scattered pools (in the brackish-water part) and drained by a complicated ditch network [15,16] covering 6.7% of the freshwater marsh area, and connected naturally to a river (the Falleron) and artificially by a pump to the Loire River system. The study of the bitterling population was conducted in a representative 3660 ha sampling area in the north of the freshwater part of the Bourgneuf Marsh.
2.2 Habitat variables chosen
We extracted environmental data concerning a 5-year period (1987–91) and a 6-year period (1996–2001). Each year 20 to 30 ditches were sampled in June, including some ditches that were sampled every year from 1997 to 2001 (n=11). Thus a broad range of habitat types was investigated. At each sampling station, quantitative and qualitative habitat variables were noted. The ditch width, the water depth and the substrate depth, always composed of mud, were measured to the nearest centimetre. Vegetation cover was visually estimated at each sampling station and defined using five semi-quantitative categories of coverage: 1 (absence or <5%), 2 (5–25%), 3 (26–50%), 4 (51–75%) and 5 (>75%). Three vegetation types were distinguished to characterize different types of habitat. Emergent plants were recorded on the bank of the ditch and were essentially composed of Carex spp. and Juncus spp. Floating-leaved plants (mainly Lemna spp. and Hydrocharis morsus-ranae) sometimes completely covered the water surface of the sampling station. Finally several submerged macrophytes developed in the water column (see [17] for more details on the aquatic plant assemblage). All plant species were grouped according to the three kinds of vegetation. Ditch clearance (presence/absence) was classified into four categories: 0 (no clearance), 1 (1 year ago), 2 (2–3 years) and 3 (>4 years). Finally each sampling station was classified according to its degree of connection to the river: 1 (the main river), 2 (secondary network, ditches directly connected to the river) and 3 (tertiary network, ditches connected to the secondary network).
2.3 Fish sampling
In each ditch, a sampling station was delimited by two 5 mm mesh straight nets set 30 m apart. In these stations, we applied the removal method [16,18,19] using an electric fishing apparatus (EFKO F.E.G. 8000). Fishing conditions were defined to estimate fish densities using the Carle & Strub method [20]. After capture, each fish was identified and immediately returned to the water. When the capture probability was below 0.20, the density was obtained by adding the different samplings [20].
2.4 Data processing
Firstly, mean density and frequency of occurrence were used to compare trends in the bitterling population in the set of stations that were sampled in every year from 1997–2001 (n=11). Prior to investigating the relationships between fish data and habitat variables, the densities of bitterling were transformed into presence/absence for all stations sampled in 1996–2001 (n=112) and continuous habitat variables (ditch width, water depth and substratum depth) were converted into categories. We related bitterling occurrence to each habitat variable by generating an ecological profile as illustrated in Grenouillet [21]. The ecological profile was based on χ2 tests performed on categories of each variable showing preferences or avoidances of bitterling for each separate variable. Then we carried out a logistic regression selecting all habitat variables to build a predictive model of the occurrence of bitterling in the Bourgneuf Marsh.
Finally a Multiple Correspondence Analysis (MCA) was performed on the set of effective variables from the ecological profile procedure to classify the ditches sampled and to support previous statistical investigations. As the number of stations having the same categories for the remaining variables was low for some combinations, the number of categories was limited for all habitat variables (see the results section). Analyses and graphics were performed using ADE4 [22] and SYSTAT 9.0 for the ANOVA and logistic regression procedures.
3 Results
3.1 Trends in the bitterling population
There were no bitterling in the marsh during the 1987–91 period; the species appeared later since it was detected between 1996 and 2001. Both mean density and frequency of occurrence of the bitterling population increased from 1997 to 2001 (Fig. 1A). The fish population was small in 1997–98 with a low density (2.4±2.7 and 0.3±0.5 individuals/100 m2 respectively), although the frequency of occurrence was relatively large in 1997 (45%). The bitterling population rapidly increased and colonized the study area during the three last years (1999–2001). The frequency of occurrence seemed to reach a threshold (nearly 75%) in 2000 and 2001. At the same time the density of fish continued to increase significantly (one-way ANOVA, F=2.95, p=0.03 for the 5 years) with a higher density in 2001 (61.7±47.0 individuals/100 m2) compared to 1997 and 1998 (p=0.04 and p=0.03 respectively from Tukey HSD tests). The stabilization of the number of ditches colonized by bitterling was supported by the fact that once the fish species was noted in a ditch, it was noted during the following years (Fig. 1B) in the same ditch. Furthermore some ditches were never colonized by bitterling in 1997–2001 despite the expansion of the population size.
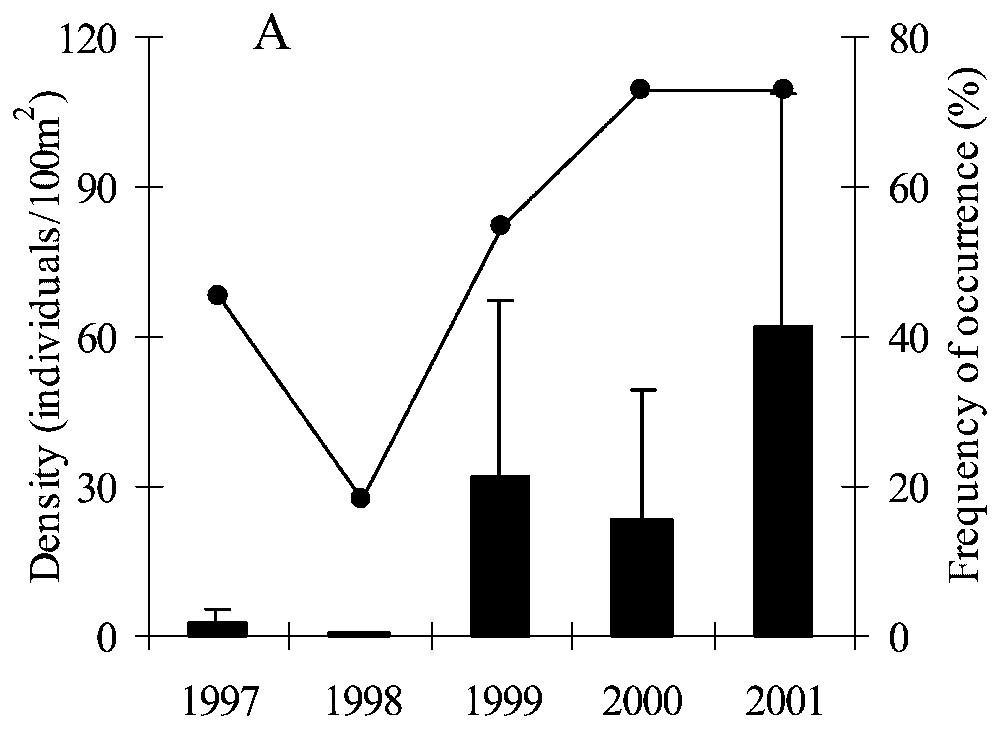
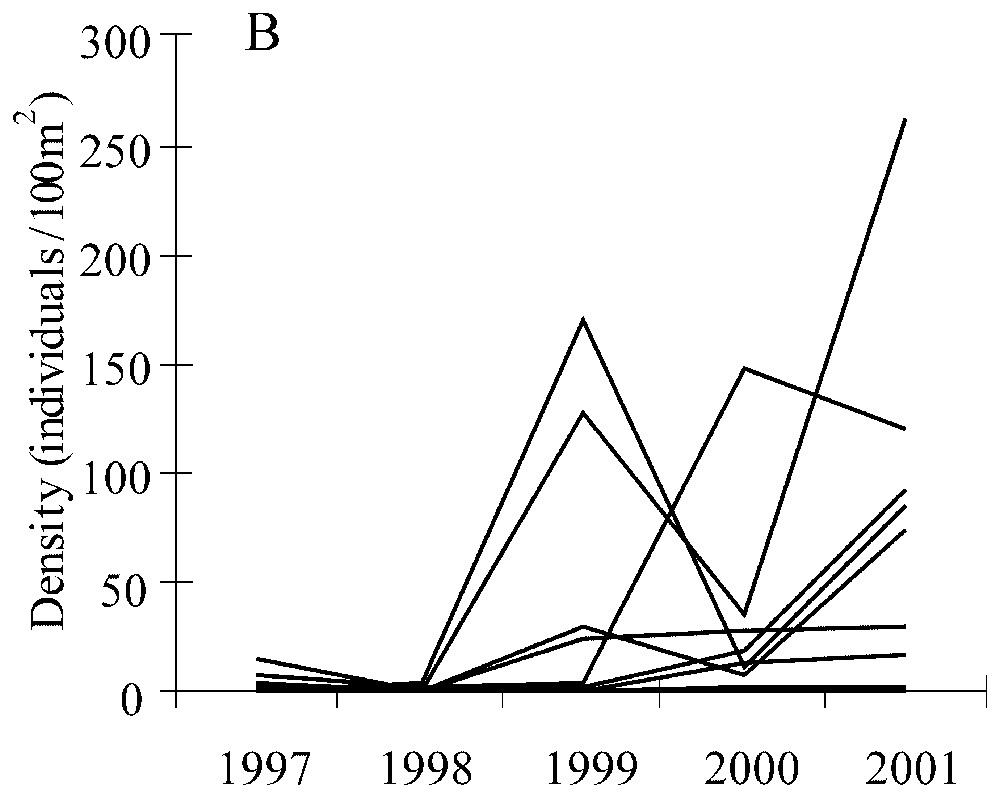
Trends in the bitterling population from 1997 to 2001 for the set of stations sampled in each year (n=11 for each year). (A) Mean density (individuals/100 m2, histograms) and frequency of occurrence (line); (B) density of bitterling for each station.
3.2 Habitat selection by the bitterling population
The ecological profile shows the relationships between the occurrence of bitterling and each separate habitat variable (Fig. 2). The patterns obtained revealed that the substrate (mud) depth and the covers of floating-leaved and emergent plants produced no significant response. However, the year effect was confirmed since bitterling was preferentially recorded in the three last years. Positive associations were noted with large (>5 m), deep (water depth >0.7 m) ditches, ditches with a limited cover of submerged plants and ditches which had been cleared within the last 2 or 3 years and belonging to the main and above all the secondary ditch networks. In contrast, bitterling significantly avoided narrow, shallow ditches, ditches with a very high cover of submerged plant obstructing the water column (>75%), and ditches not cleared and belonging to the tertiary network.
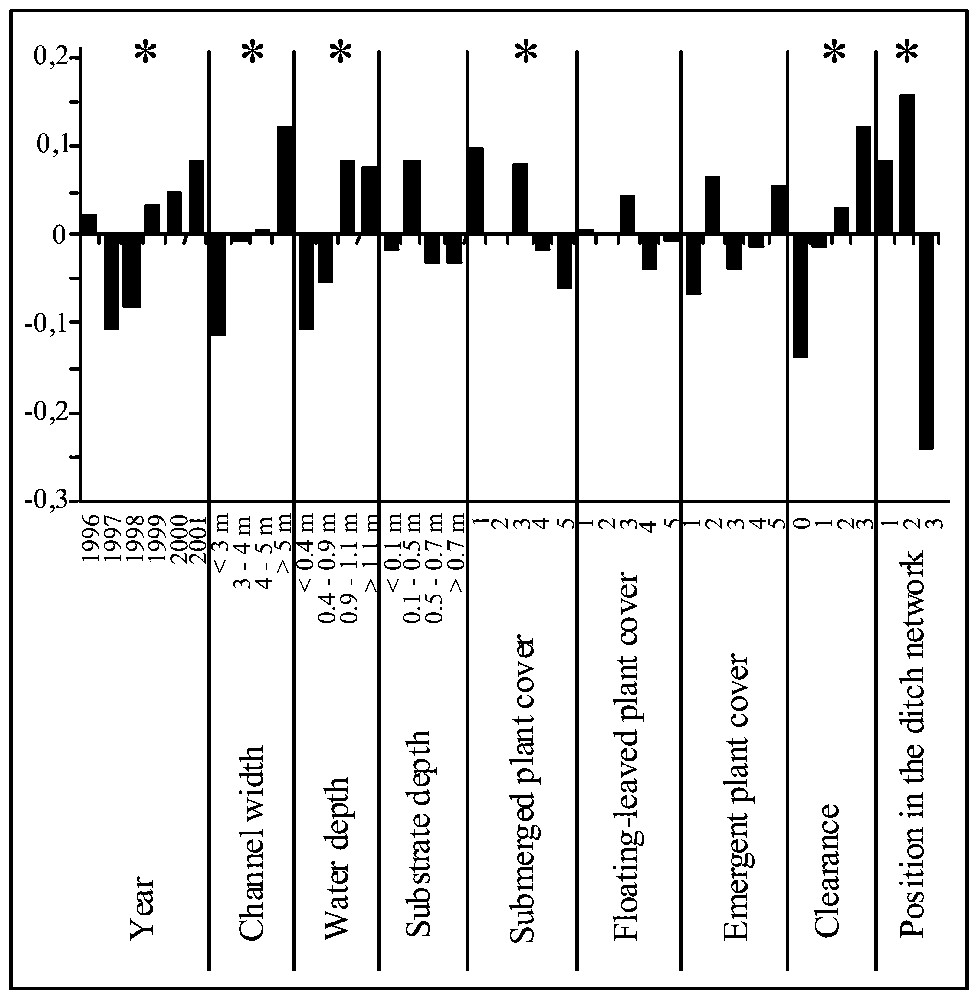
Ecological profile and χ2 associations for bitterling. Each histogram represents the difference between the frequency of bitterling in the group of stations with each category and the frequency of that species in all samples. Significant associations (p<0.05) between bitterling and habitat variables are indicated with an asterisk.
The logistic regression did not show any significant responses of bitterling to habitat variables, except for the ditch width (t=−2.121, p=0.03). The likelihood-ratio, testing the hypothesis that all coefficients of habitat variables are zero, and the deviance explained (Mc Fadden's Rho-squared=0.073) supported that no predictive model can be built. In fact, the colonization process during the five last years probably inhibited this predictive process, since ditches not colonized by bitterling in 1996 and 1997 were colonized during the following years without significant environmental change.
Nevertheless the MCA reinforced the results of the ecological profile by classifying the ditches according to the set of habitat variables. Because of the methodological constraint previously mentioned, the analysis was performed on a matrix composed of 112 stations and 6 variables (ditch width, water depth, submerged plant cover, clearance and position in the ditch network) totalling 14 categories (see Fig. 3). The first two axes described the data structure well, accounting respectively for 33 and 18% of the total inertia. The Gauss curves show factorial scores of the categories of each habitat variable on these axes (Fig. 3). The amplitudes of the curves are proportional to the category weights and the correlation ratio expressed as a percentage identify those variables that are taken into account by each axis. The analysis classified the ditches into three categories: (i) narrow and shallow ditches connected to the secondary network and characterized by a high cover of submerged plants and which are not cleared, (ii) the main river or secondary ditches (large and deep) which are managed (clearance) with a small amount of vegetation and (iii) intermediate ditches belonging to the secondary or tertiary network and not necessarily cleared. This classification showed a heterogeneity in the structural variables and human management of ditches belonging to the secondary or tertiary network.
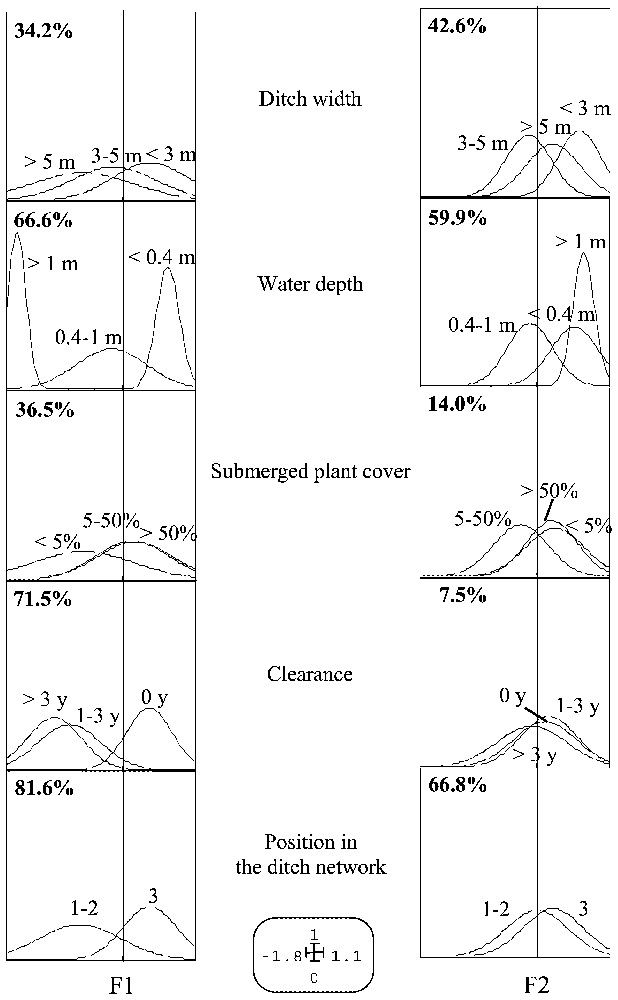
MCA of the six habitat variables showing significant response of bitterling (see Fig. 2). The Gauss curves represent factorial scores of the modalities of each habitat variable on the two first axes. The amplitudes of curves are proportional to the category weights and the correlation ratio expressed as a percentage identify those variables that are taken into account by each axis.
4 Discussion
Fish sampling on this representative area of the Bourgneuf Marsh over more than ten years demonstrated the recent settlement and expansion of a bitterling population. This result is all the more interesting as bitterling seems to be a vulnerable species not only in France but also in many European countries [1] although some populations would be unknown and the species would be perhaps less threatened than it is stated. Furthermore, the absence of bitterling during the first period of the sampling between 1987 and 1991, its sudden appearance in 1996, and the parallel increase of its occurrence and density between 1996 and 2001 leading to the saturation of the sampling area in 2000 and 2001 show the high colonisation capacity of a suitable area by this species. Bitterling represented the most frequent species in 26% of sampling stations in 2001 (unpublished data). This unknown capacity seems to be very useful for the management and the conservation of the species.
The absence of bitterling during the first period is surprising and seems not to be due to the absence of Anodonta, well represented in all marshes of the region where organic sediment is abundant. Moreover, the mussel parasitism is non specific and other fish species, especially Cyprinids, can be hosts for Unionidae larvae [9,23]. So mussels can be present without bitterling whereas this latter needs the presence of sufficient large mussels (40 to 70 mm [24]). Published studied on other sites have suggested that inorganic pollution [4], eutrophication and habitat destruction [7] are responsible for the decline of the mussels and thus of the bitterling [11,25]. However these factors have not greatly affected the Bourgneuf Marsh, particularly the tertiary network. Furthermore, our study shows another surprising inverse relationship with the habitat preferences of Anodonta, i.e. the organic muddy sediment in tertiary ditches and related factors such as vegetation.
The appearance of the species in the Bourgneuf Marsh was first observed after a campaign of ditch clearance in 1995 and 1996 on all the primary and secondary network to prevent flooding in the marsh by enhancing the water circulation and to improve the water quality [26]. So the previous absence of bitterling in the Bourgneuf Marsh could be due to adverse ecological conditions for this species, such as oxygen deficiency in summer and/or a too low connectivity in the network of ditches. The clearance campaign should have suppressed such limiting factors and allowed the colonisation by bitterling although the mussel population probably suffered from local disappearance due to these engineering operations and the reduction of organic sediment. Bitterling was not recorded in abundance for 1 or 2 years after clearance because of unsuitable habitats encountered by mussels and the absence of adult mussels for spawning [23]. Bitterling could only colonise in subsequent years probably owing to the delay in the settlement of a well-grown mussel population after the return of suitable conditions (accumulation of organic matter on the substrate).
The ecological profile and indirectly the classification of ditches based on habitat variables (MCA), in spite of the unsuccessful predictive model of occurrence of bitterling, showed positive preferences of the fish for large, deep ditches with a limited cover of submerged plants. These ditches have generally been cleared in the last 2 or 3 years and were connected to the main river. Clearance probably favoured connections between many ditches and consequently the establishment of the bitterling population in the secondary network from the main river. However, the tertiary network which is the most dense (85%) but also not managed (no clearance) is characterized by major silting up with a dense cover of submerged plants that leads to frequent oxygen depletion [17] greatly affecting the occurrence of several fish species.
This preliminary study showed the importance of clearance in the trends of a bitterling population in a man-made ditch network by favouring suitable habitats for the fish species. Shallow ditches with a very high cover of submerged plants obstructing the water column become more suitable for bitterling when they are cleared and can be colonized by the fish during subsequent years. So a prolonged period without engineering operations would lead to adverse conditions for bitterling and also possible isolation of local populations in the ditch network. Consequently clearance plans need to be defined for conserving the population of this species in the Bourgneuf Marsh by taking into account the cycle of silting up of ditches and favouring the colonization of the tertiary network.
Nevertheless further experiments would be needed firstly to take into account the mussel population dynamics in the habitat-bitterling interactions, and secondly to study the fish population in a framework of a multi-scale approach in order to test the effect of the connections between ditches in this large and complicated network at a subsequent sequence in the colonization process.
Acknowledgements
We are particularly grateful to J. Le Gentil, P. Boury and numerous students for assistance in fieldwork. We also thank R. Britton for correcting the English version.



