1 Introduction
The cormorant (Phalacrocorax carbo) is an avian top-predator of fish with two recognised sub-species found in Europe, P. c. carbo and P. c. sinensis [1,2]. Traditionally, P. c. carbo is marine and breeds on the coasts in the British Isles, France, Norway and Iceland, whilst P. c. sinensis inhabits various freshwater habitats across continental Europe [3]. It has received considerable attention in the literature, mainly because of concern over its increasing continental population since the 1980s [4–6] that has locally led to conflicts with fishery activities due to predation on some commercially valuable fish species [7–10]. The cormorant is an opportunist feeder relying mostly on a few often-dominant prey species for the majority of its diet [11–13]. It uses flexible foraging techniques adapted to habitats and prey types [14–16]. The recent habit of social fishing, corresponding to the formation of dense flocks of predators in order to concentrate and exploit common prey, enables cormorants to exploit turbid waters where high stocks of shoaling fish species, mainly Cyprinids, occur [17]. On the other hand, solitary (individual) fishing is more adapted to isolated prey fish in clear waters [18] and/or confined areas [19].
Several studies have shown that cormorants are able to forage up to 20–25 km from their wintering roosts [20] or breeding colonies [21–25]. The major findings on the use of feeding areas by cormorants are that there are: (1) seasonal changes in the use of feeding grounds in relation to possible changes in food availability in these areas during the breeding season, and (2) increased energy costs in relation to foraging trip distances. However no attention has been given to the relation between habitat use and fishing strategies (individual vs. social).
In the present paper, we investigated the feeding ground use by inland breeding cormorants in a complex spatial configuration of habitat patches compared to what has been described in some other related studies [22,23] and also where the two types of feeding strategies occur. Here cormorants are faced with various feeding habitat opportunities: the use of several wetlands in the vicinity of the colony site where only individual fishing is used (visual observations) and also the use of the lake waters where the colony is established and where social fishing predominates [11]. In this context, we addressed two specific questions: (1) To what extent cormorants exploit the lake and adjacent feeding areas?, and (2) Do their foraging decisions vary throughout the breeding season, and in-between years? The results are discussed in relation to the two feeding strategies used by cormorants and factors governing the choice of feeding grounds throughout the breeding season.
2 Methods
2.1 Study site
Lake Grand-Lieu is a shallow eutrophic natural freshwater ecosystem (1.5-m average depth) located in western France (47°05′N, 1°39′W). It covers 4000 ha in summer and 6300 ha in winter as a result of flooding of surrounding peaty wet grasslands. About 20 km of the lake margin, with an area of 2000 ha, are covered by a floating peat fen, which becomes progressively exposed in summer. About half of the permanently flooded central area of the lake (2000 ha) is covered from April to October by floating-leaved macrophytes, and the central 1000 ha of open-water lack vegetation. Cormorants establish their extensive colony in trees located into the southwestern area of the peat fen, whereas they use the fringe of the peat fen as a roosting site during the day. Large numbers of cormorants feed in the permanently flooded area (see results section) in contrast to temporarily flooded adjacent grasslands, where cormorants are rarely recorded [26].
This part of western France is characterized by a diversity of permanent rivers and water bodies bordering the Atlantic coast that are enclosed in arable lands (Fig. 1). It includes notably 16 000 ha of polders and former salt marshes (the Bourgneuf marshes) forming a mosaic of pastures with scattered pools and a dense network of narrow ditches (mainly
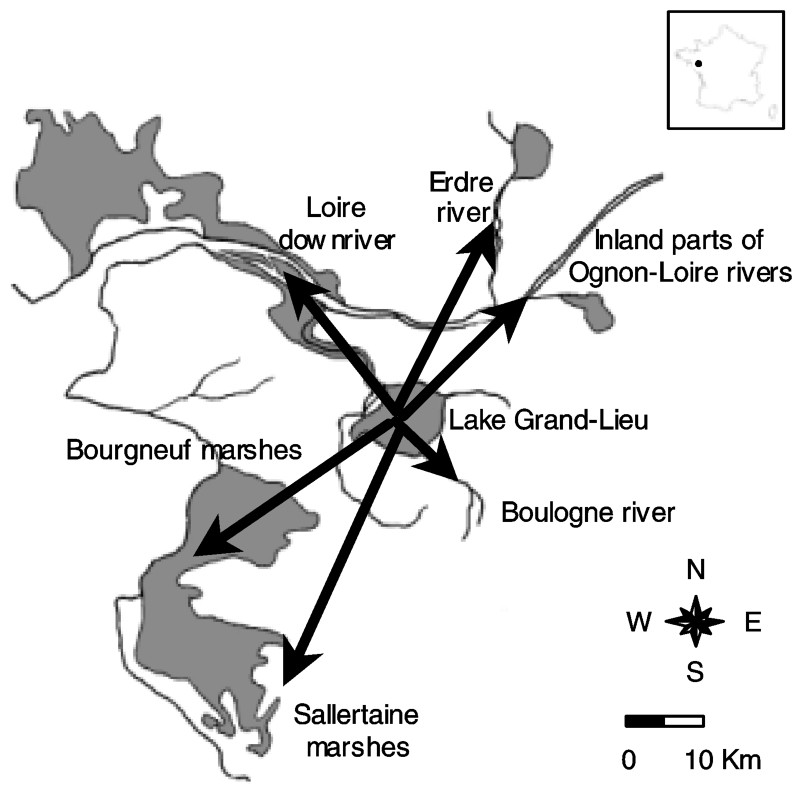
Location of feeding areas in the vicinity of the cormorant colony (Lake Grand-Lieu).
2.2 Foraging activity of the cormorant colony
Fieldwork was conducted during the breeding season in 2001. Because of the complex situation with respect to two kinds of foraging trips, the cormorant activity was studied using two distinct methods. With the aim of documenting the pattern of foraging activity towards nearby rivers and water bodies and their relative significance, we monitored departure–arrival (OUT/IN) directions of cormorant trips for 4 h in the morning (8:00–12:00) twice a month on average during the breeding season (April–August). Cormorants flying out and back to the lake were counted for periods of 15 min from four spots chosen according to visibility and general flyway of all birds. Using this method, all birds entering or leaving the lake were counted. The same methodology was previously used in Lake Grand-Lieu for the cormorant in 1996 [28], and also for the grey heron [29]. We assume that cormorants leaving the colony site keep the same bearing until they reach a feeding site, and respectively, birds travel fairly straight back to the nest from a precise feeding area. Previous radio-tracking studies on cormorants [30] and shags [31,32] support this assumption, since most of the time, during foraging trips, birds fly directly to a specific area. Patterns of nest attendance/presence with time of day vary between cormorant colonies [15,23,33] and sometimes no particular pattern of foraging activity exists [22]. In Lake Grand-Lieu, Marion [28] did not show any significant pattern of foraging activity by cormorants, although many birds left the lake in the morning (
To document cormorant foraging activity on the lake, we carried out regular counts of cormorants in the whole system, except the colony area, in late morning about once a week from May to mid-September. Counts were performed by three observers to minimize the risk of error arising from bird movements within the lake. Cormorants were counted individually, or generally in units of 10 when flocks were larger than 100, then classified as roosting birds on trees at the fringe of the peat fen, and either isolated or flocks of fishing birds in the central area of the lake. Visual observations showed that most roosting birds feed in the lake and are thus included as foraging activity.
To investigate the extent of the two foraging strategies throughout the breeding season, and because we used two kinds of data (numbers of trips for 4 h and instantaneous counts of birds on the lake waters), we converted these data into separate index numbers [35,36]. Index numbers are the ratio between the number of cormorants counted for a given date and that for a baseline date. We chose 17 May (the first date included in both monitoring methods) as the baseline date.
We also compared changes in foraging activity according to the fledging period. As the colony area was extensive and not completely accessible without disturbing breeding birds, fledging was determined on a set of 103 nests (19% of the breeding population size). This monitoring was conducted simultaneously with the cormorant counts on the lake (once a week from May to mid-September).
3 Results
3.1 Foraging activity towards the wetland complex
Foraging flight numbers towards all directions outside the lake were relatively stable in April–May (485–604), peaked in mid-June (IN+OUT flights=1028) before a strong decrease in July–August (277–89 flights). The numbers of OUT flights were generally larger than numbers of IN flights, because some birds did not return within the four observation hours (52.6–65.2% of total flights, mean ±SD=58.8±3.0%, n=8 dates) and even accounted for almost the entire movement numbers in late August (97.8%, n=89 flights). From a total of 1745 IN and 2404 OUT flights, three main foraging directions were observed during the breeding period: the Bourgneuf marshes (58.0% IN/57.9% OUT), the inland parts of Ognon-Loire rivers (28.3/20.2%) and the Loire estuary (7.8/13.9%) including the drainage channel flowing from the lake to the Loire estuary 25 km away (Fig. 2). Three minor directions were also found: the Sallertaine marshes (3.1/4.0%), the Boulogne River (2.4/3.3%) and the Erdre River (0.4/0.7%). Almost no significant changes in the flight directions were observed between dates (Spearman rank correlations, all with P<0.05). The only two significant differences were found on 24 April and 24 August (rs=0.26, P>0.05, n=6 feeding areas) when a limited proportion of flights towards the Bourgneuf marshes was observed in late April (17.2/47.0%, n=326/481 for IN/OUT flights) and more cormorants reached the Boulogne River in late August (16.6/22.2%, n=18/81 for IN/OUT flights; Fig. 2). Furthermore, no difference was found between 1996 (n=8591 flights) and 2001 (n=4149 flights) in the significance of the feeding areas outside the lake throughout the season (rs=0.77, P<0.05, n=6 feeding areas).
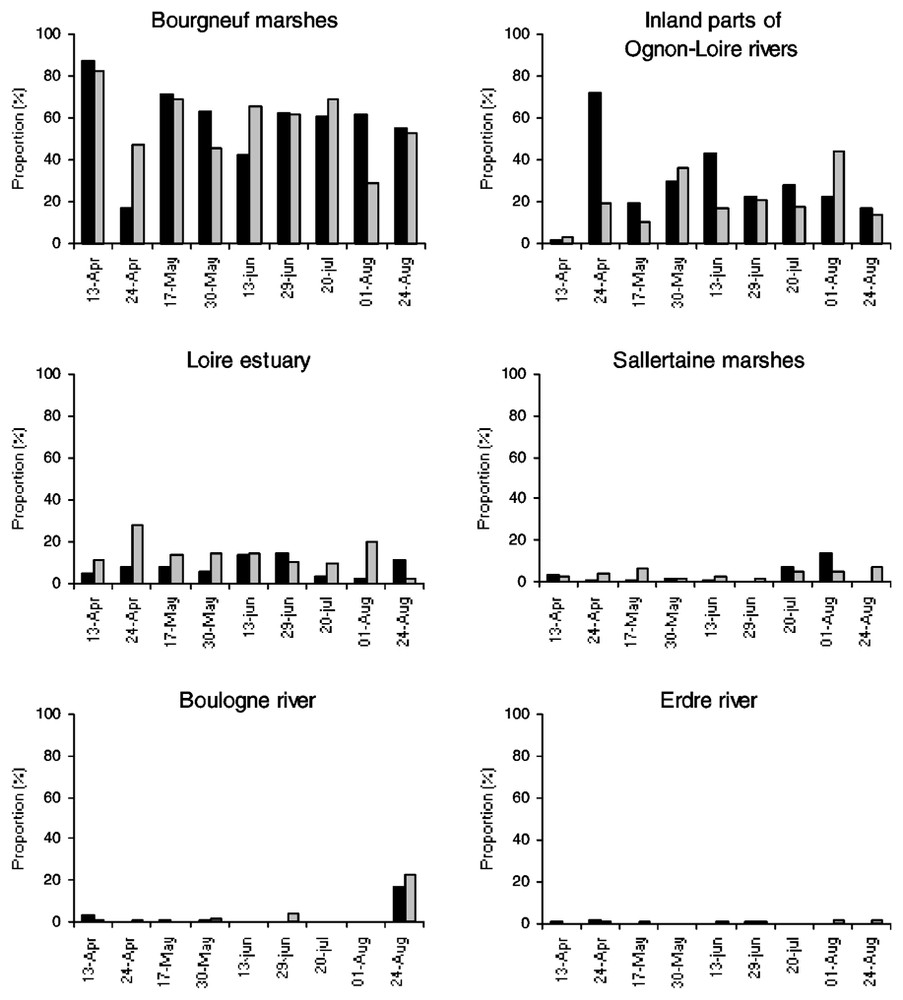
Relative abundance of feeding trips towards each habitat patch according to dates. Cormorants entering or leaving the lake are represented by dark and grey histograms respectively.
3.2 Cormorant numbers on the lake
As with foraging activity outside the lake, cormorant numbers within the lake increased during the breeding season with a maximum of 1390 birds noted in late June (Fig. 3). Most of the cormorants indulged in fishing activity (61–91%), except for two dates (7 June and 12 July), when large flocks of roosting birds were observed (55 and 45% respectively), probably as a result of cormorants returning from social fishing. Social fishing (300–833 birds) was observed at each date and was the main foraging activity in the lake (84.9±4.0% on average). Flocks of birds were usually located in unvegetated areas either within the floating vegetation beds or in the central part of the lake.
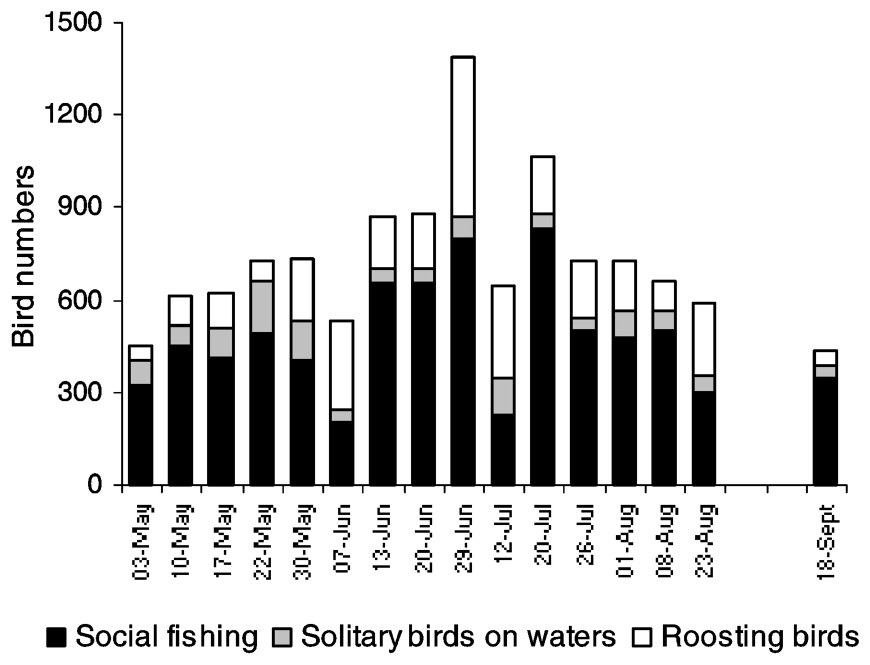
Cormorant numbers recorded on Lake Grand-Lieu (except the colony area) from May to September in 2001. Social/solitary fishing and roosting cormorants were distinguished.
3.3 Foraging activity and fledging period
The increase in foraging activity on and outside the lake reflected variations in fledging (Fig. 4), but whereas the index numbers of cormorant outside the lake peaked just before the maximum of young fledging, the index numbers on the lake peaked when fledging was at its maximum and when most chicks had fledged (80% of total fledging, n=178). Even though the methods used to investigate foraging activity were different, changes in index numbers suggested an increasing proportion of cormorant activity within the lake after the chick-rearing period compared to the use of surrounding wetlands. Movements to surrounding areas rapidly decreased to a low level at the end of the fledging period, whereas total cormorant numbers exploiting the lake decreased more slightly. No fundamental changes in the within-lake index numbers were noted throughout the breeding period, except at the fledging peak.
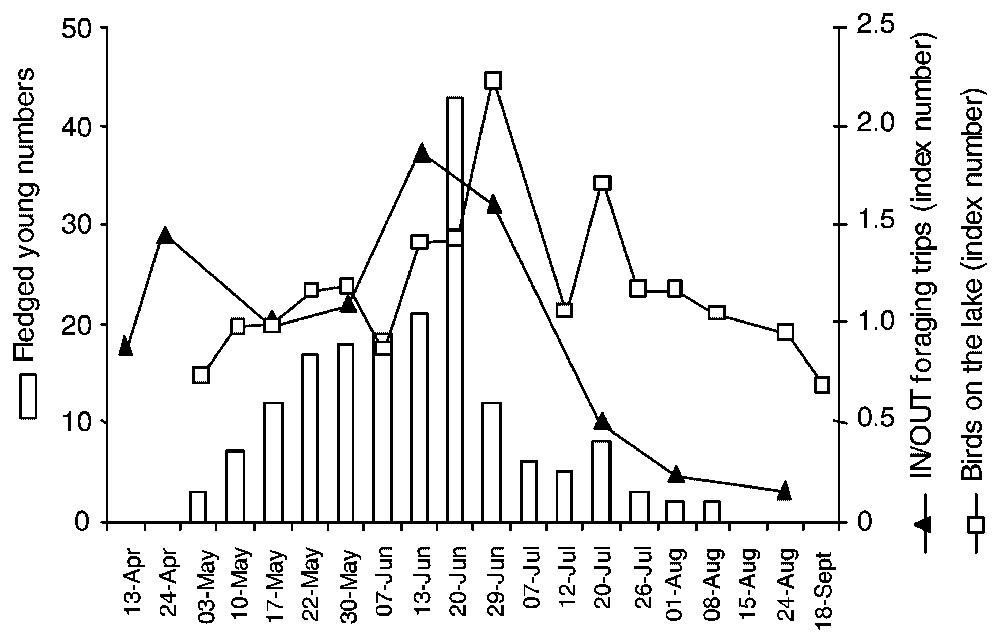
Foraging activities on and outside the lake and fledging in cormorants in 2001. Foraging activities (index numbers) were quantified as the ratio between the number of cormorants counted for a given date and that for a baseline date. Fledging was monitored on a set of 103 nests and the baseline date for calculating index numbers was 17 May.
4 Discussion
4.1 Assumptions used in monitoring methods
The investigation of the exploitation of feeding areas by cormorants outside the lake is based on the assumption that individual birds fly directly to a specific area keeping the same bearing. This is supported by some previous studies on marine predators using a more direct approach [30–32]. For the shag, there are small deviations, presumably associated with some meteorological conditions (F. Daunt, Personal communication). In the present study, cormorants face highly distinct and separated feeding patches enclosed in arable lands. For this reason, we consider that direct flights by cormorants to a specific area (feeding or colony sites) strongly prevail, even if it cannot be excluded that a few cormorants may have changed their travel bearing from the colony to feeding areas.
In other respects, concerning the fishing behaviour, we also assume that cormorants exploit feeding areas outside the lake using solitary fishing. The most attractive feeding area (the Bourgneuf marshes) is composed of a dense network of narrow ditches inhibiting social fishing. The use of this foraging technique by cormorants was verified by visual observations. Moreover, the efficiency of social fishing depends on the necessity for individual cormorants to synchronise their movements. There is evidence of synchronised massive flights of cormorants in the lake, where social fishing is the rule. In contrast, birds entering or leaving the lake were generally alone (mean=1.3 to 2.1 individuals (range=1–22) and 1.2 to 1.9 individuals (range=1–34) respectively for all dates).
4.2 Factors governing feeding ground selection by cormorants
In the present study the range of foraging flight distances (up to 25 km around Lake Grand-Lieu) is similar to values generally observed in other colonies, where cormorants use distinct inland feeding habitat patches (usually
The choice of feeding areas is influenced first of all by energy factors [22]. Predators must make a trade-off between the benefits of the use of food resources and costs of travel distances. For instance, Veldkamp [24] noted that even though breeding cormorants of the Wanneperveen colony in The Netherlands were able to reach the large Lake IJsselmeer (about 30 km), they exploited two small lakes in the vicinity of the colony (within 12 km) as foraging areas. Some authors have even suggested that the increased time to cover long distances between the colony site and the feeding areas may exceed the energy costs for rearing chicks [22,42,43]. For the grey heron, Marion [41] and van Vessem [40] explained the spatial distribution of breeding birds by a trade-off between costs and benefits of exploiting foraging habitats.
In the present study, cormorants are faced with two contrasting foraging strategies with respect to energy considerations: social fishing (84.9±4.0% in cormorant fishing numbers) associated with short trips (0.3 to 4.0 km from the colony) to increase fishing efficiency in the turbid waters of Lake Grand-Lieu although prey (i.e. mainly pelagic shoaling fish species [44]) have a low energy content (4.2–4.6 kJ g−1 [22,39]), or solitary fishing which is used in the feeding areas outside the lake resulting in increased foraging trip energy costs that might be balanced by high energy content prey (e.g., 7.92 kJ g−1 for eel [39]). Here, since social fishing prevails, most cormorants might compensate for lower energy supplies by a higher mean number of feeding trips, five according to Carpentier [45], compared to 2–3 trips per day recorded by Platteeuw [22] for a similar mean brood size of 2–2.5 fledging youngs [45,46].
Our results are in accordance with two previous experiments carried out in the Grand-Lieu colony [45, unpublished data]. In these studies, several patterns of duration of foraging activity were clearly observed. Nevertheless, a major proportion of foraging trip durations seemed to result from fishing activity on the lake. Moreover, data from automatic nest balances (unpublished data) show that, in all cases, individual breeding birds make short and long foraging travels (foraging activities on and outside the lake) to various degrees, suggesting possible flexible behavioural responses of cormorants to local conditions.
It is surprising that the Bourgneuf marshes were the major feeding ground outside the colony site although it is the furthest away area with probably high related energy costs. Nevertheless, these marshes are a very attractive foraging ground. They are the largest wetland in the proximity of Lake Grand-Lieu and are characterized by a dense network of narrow and shallow ditches where prey is easy to catch by cormorants. Furthermore, high-energy-content fish species, such as eels, represent a large proportion in the fish stocks (23.5% in total abundance in 1996–2001 [47, unpublished data]) compared to what is found in Lake Grand-Lieu (9.3% in 2000–2001, [19]). So cormorants might take advantage of the use of this feeding area in spite of high energy costs with respect to travel distances. The maintenance of a dual foraging strategy by cormorants throughout the chick-rearing period could results from a compromise between the necessity for adults to feed their chick frequently (short foraging trips) and the need to deliver prey of high energy value. This latter requirement could only be achieved by solitary fishing in some feeding area types. In some aspects, this is similar to what was also found in seabirds combining short and long foraging travels throughout the chick-rearing period [31,48]. The use of different feeding areas and prey types could be one way for parents to compensate for any decrease in food availability. In Lake Grand-Lieu, cormorants do not suffer from food depletion but rapidly face a reduction in the accessibility of prey when the extensive beds of macrophytes develop during the breeding period. Foraging activity only occurs in open waters where fish stocks are the lowest [19] and the exploitation of other feeding areas might be an advantage.
In the future, some additional investigations would be needed to complete these results studying at the individual level and with a more direct approach: (1) the hypothesis of fidelity to feeding areas outside the lake, and (2) the extent of short and long foraging trips by parents throughout the breeding period.
Acknowledgements
We are grateful to A. Iribar, S. Marzin, T. Geslin, P. Boury, V. Lague, and L. Penin for monitoring feeding trips in 2001, and S. Reeber for providing data on cormorant trips outside the lake in 1996. We also are grateful to T. Bregnballe and an anonymous referee for comments on earlier drafts of the paper. R. Britton made linguistic improvements.


