Version française abrégée
Dans les sites hydrothermaux le long de la dorsale du Pacifique oriental et sur la ride des Galapagos, le genre Bythograea Williams, 1980, est polytypique, avec quatre espèces déjà décrites, et offre une large distribution. B. thermydron Williams, 1980, espèce type [1–10], est l'espèce dominante sur de nombreux sites entre 21°N et 18°38′S ainsi que sur la ride des Galapagos, mais elle est absente dans le Sud de la dorsale du Pacifique oriental. B. microps de Saint Laurent, 1984 [2–4,6,8,10] a été observée à 21°N, 13°N et 9°50′N sur l'EPR. B. laubieri Guinot et Segonzac, 1997 [8–10] a été récoltée entre 11°S et 21°S, mais serait complètement absente plus au nord. B. intermedia de Saint Laurent, 1988 [3,5,8,10], originaire de la ride des Galapagos, est une espèce très mal connue et jamais retrouvée depuis sa description. Celle-ci avait été basée seulement sur six stades jeunes crabes (l'holotype est un spécimen de 4,5×6,8 mm, mais largeur de 6,3 mm indiquée dans la légende des figures par de Saint Laurent [3]) et une mégalope (paratype), trouvés mélangés avec des spécimens de B. thermydron dans la collection originale étudiée par Williams en 1980 [1]. Les caractères de l'adulte de B. intermedia sont donc totalement inconnus.
Nous décrivons ici deux nouvelles espèces de Bythograea : B. vrijenhoeki n. sp. (Figs. 1–6), récoltée sur la dorsale du Pacifique oriental au sud de la microplaque de l'ı̂le de Pâques (31°–32°S), et B. galapagensis n. sp. (Figs. 8–12), trouvée sur la ride des Galapagos, dans le site Rose Garden, d'où proviennent des paratypes de B. thermydron [1,11]. Nous les avons comparées aux adultes des autres Bythograea connues, à l'exception de B. intermedia, sur laquelle n'ont pu être pris en compte que les seuls caractères de la mégalope et des juvéniles. C'est dans le seul souci de faire connaı̂tre sans tarder la morphologie de l'espèce de Bythograea qui, sur la ride des Galapagos, cohabite avec B. thermydron, que B. galapagensis n. sp. a été établie ici, avec une réserve justifiée.
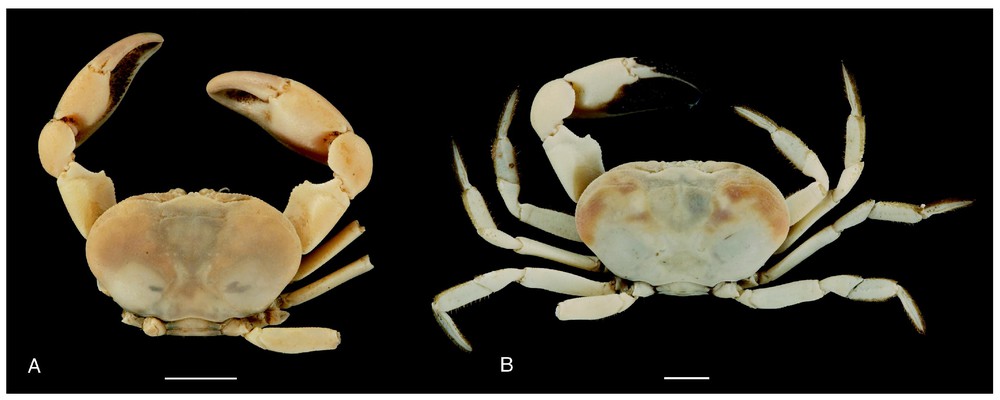
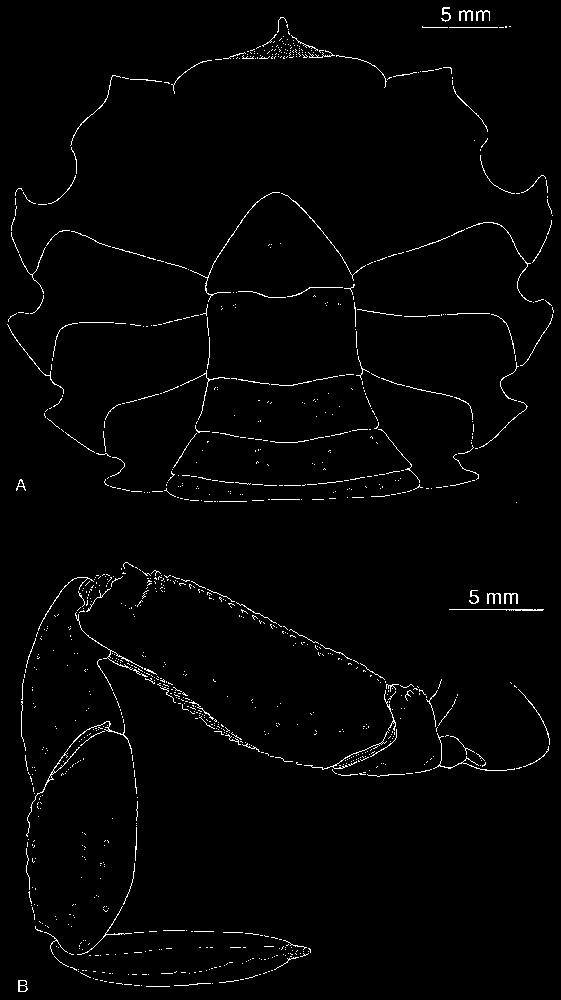
Bythograea vrijenhoeki n. sp. A, holotype, male 29.6×52.3 mm, Alvin Divin 3340, 31°51′S–112°02′W, 2334 m (MNHN-B28751); B, paratype, female 25.7×47 mm, Alvin Dive 3338, 31°09′S–111°55′W, 2334 m (MNHN-B 28754). Dorsal views. Scale bars: 1 cm.
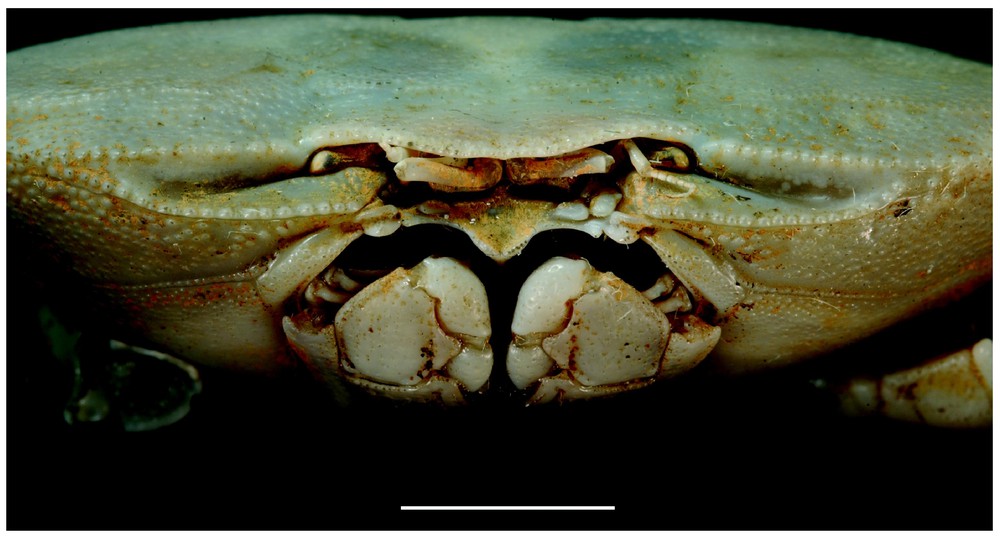
Bythograea vrijenhoeki n. sp., paratype, female 24.4×43.7 mm, Alvin Dive 3338, 31°09′S–111°55′W, 2334 m (MNHN-B 28754): frontal view. Scale bar: 1 cm.
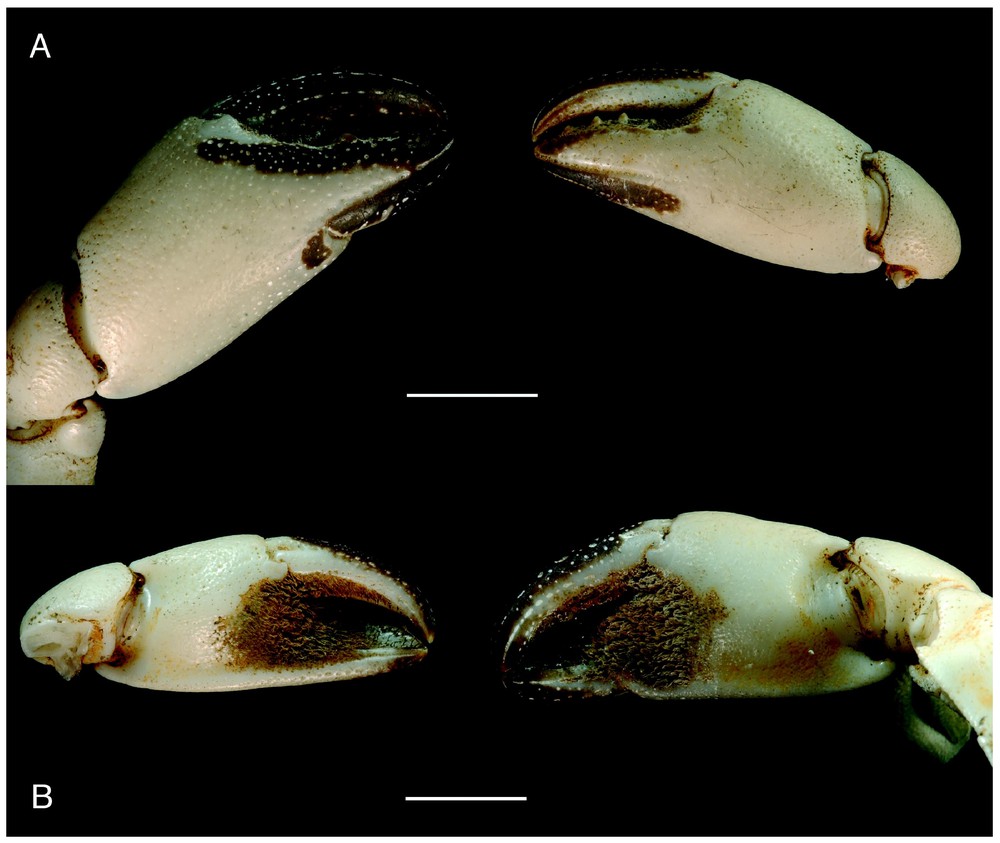
Bythograea vrijenhoeki n. sp, paratype, female 24.4×43.7 mm, Alvin Dive 3338, 31°09′S–111°55′W, 2334 m (MNHN-B 28754): chelae, external (A) and internal (B) views. Scale bars: 1 cm.
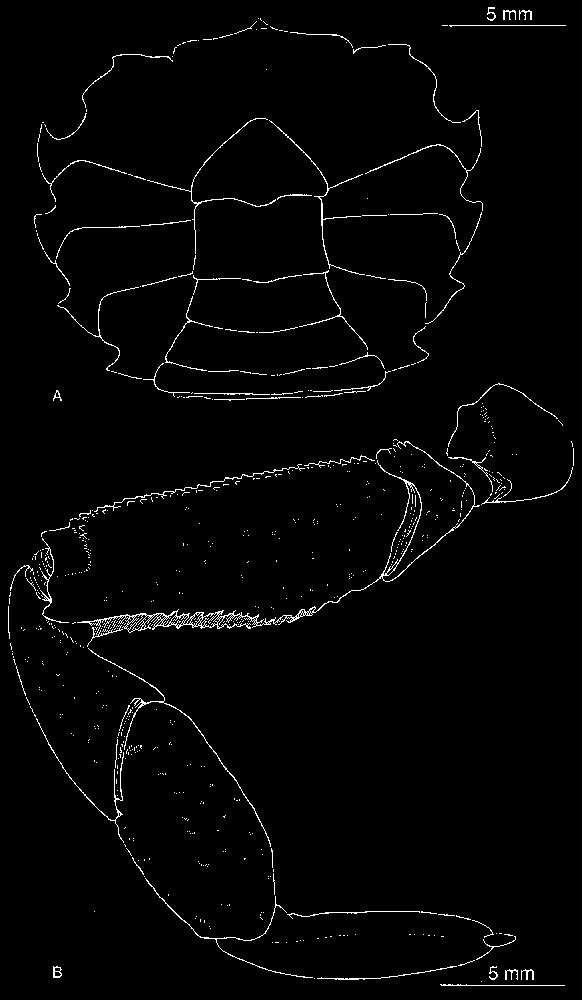
Bythograea vrijenhoeki n. sp. A, holotype, male 29.6×52.3 mm, Alvin Dive 3340, 31°51′S–112°02′W, 2334 m (MNHN-B 28751): thoracic sternum and abdomen. B, paratype, male 24.2×42.4 mm, Alvin Dive 3341, 31°51′S–112°02′W, 2333 m (MNHN-B 28757): left P5. Scale bars: 5 mm.
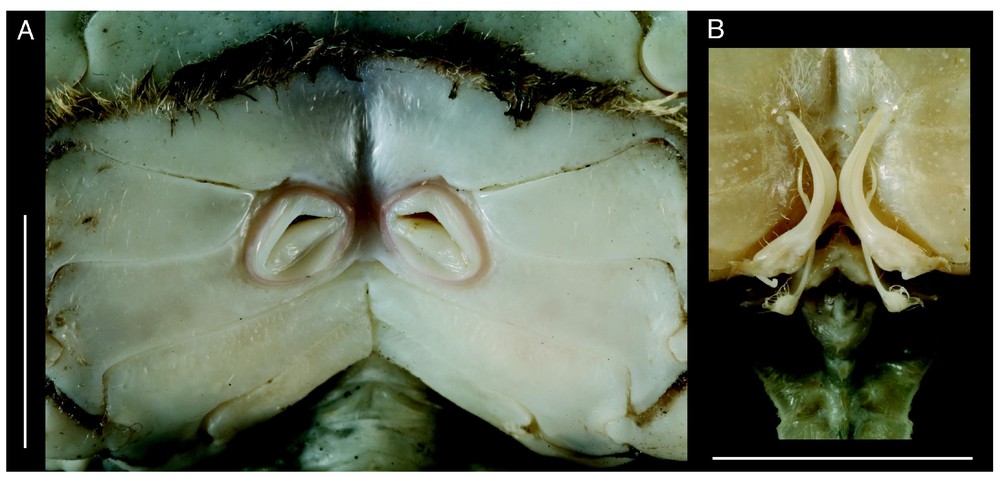
Bythograea vrijenhoeki n. sp. A, paratype, female 24.4×43.7 mm, Alvin Dive 3338, 31°09′S–111°55′W, 2334 m (MNHN-B 28754): vulvae. B, holotype, male 29.6×52.3 mm, Alvin Dive 3340, 31°51′S–112°02′W, 2334 m (MNHN-B 28751): G1 and G2 in situ. Scale bars: 1 cm.
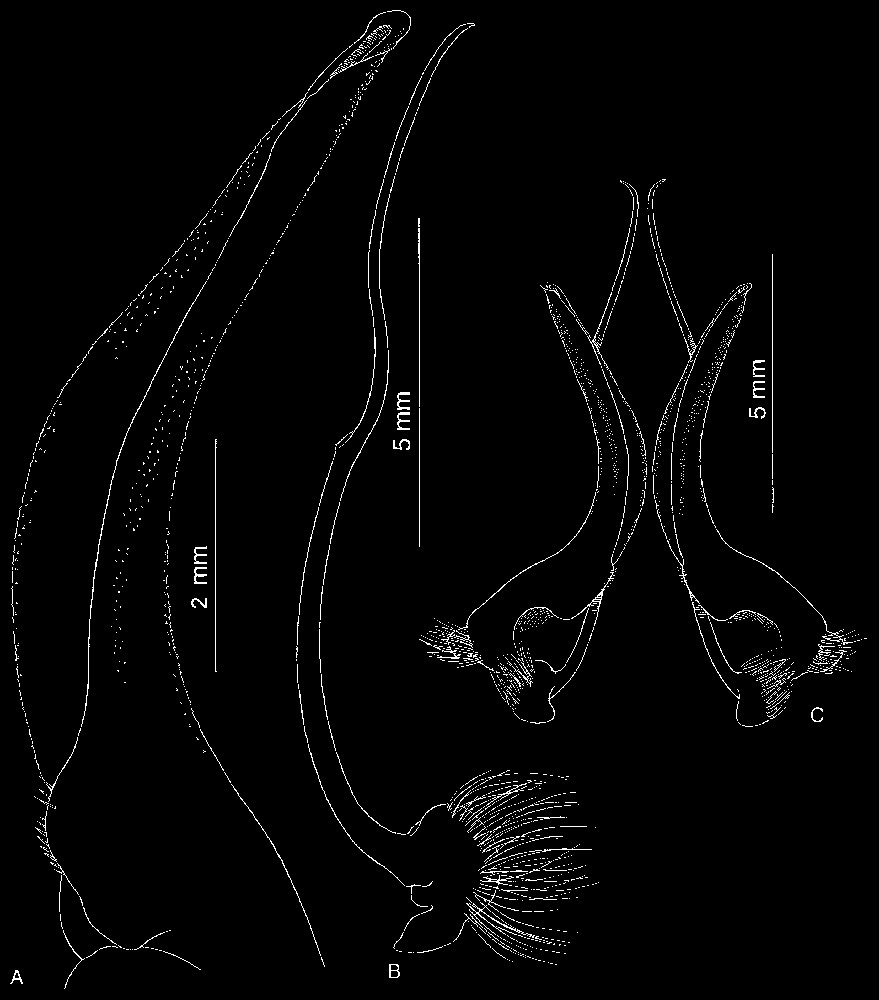
Bythograea vrijenhoeki n. sp., paratype, male 24.2×42.4 mm, Alvin Dive 3341, 31°51′S–112°02′W, 2333 m (MNHN-B 28757). A, G1; B, G2; C, G1 and G2 in situ. Scale bars: 2 mm (A), 5 mm (B, C).

Bythograea galapagensis n. sp., holotype, male 29.6×52.3 mm, Alvin Dive 2224, 0°48.2′N–86°13.9′W, Rose Garden, 2461 m: dorsal view (MNHN-B 28725). Scale bar: 1 cm.
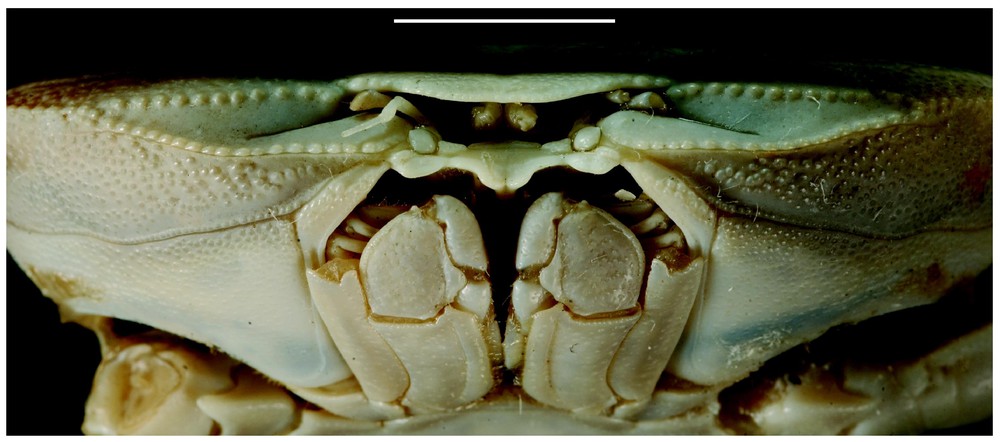
Bythograea galapagensis n. sp., holotype, male 29.6×52.3 mm, Alvin Dive 2224, 0°48.2′N–86°13.9′W, Rose Garden, 2461 m: frontal view (MNHN-B 28725). Scale bar: 1 cm.
Bythograea galapagensis n. sp., holotype, male 29.6×52.3 mm, Alvin Dive 2224, 0°48.2′N–86°13.9′W, Rose Garden, 2461 m (MNHN-B 28725). A, thoracic sternum and abdomen; B, left P5. Scale bars: 5 mm.
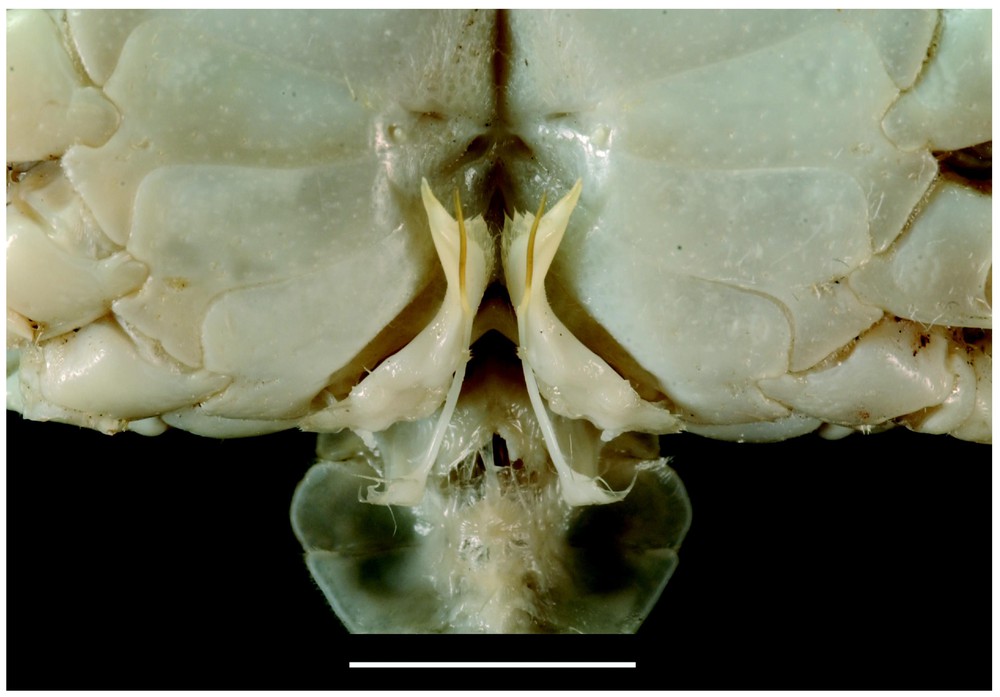
Bythograea galapagensis n. sp., holotype, male 29.6×52.3 mm, Alvin Dive 2224, 0°48.2′N–86°13.9′W, Rose Garden, 2461 m: G1 and G2 in situ (MNHN-B 28725). Scale bar: 1 cm.
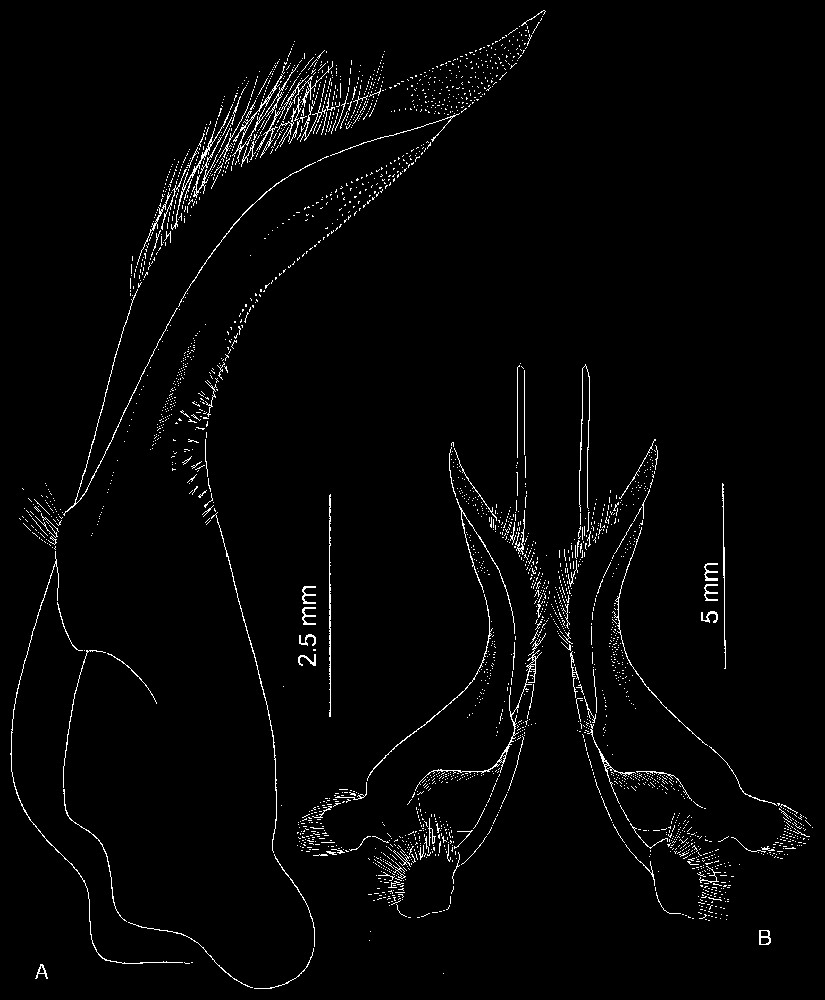
Bythograea galapagensis n. sp., holotype, male 29.6×52.3 mm, Alvin Dive 2224, 0°48.2′N–86°13.9′W, Rose Garden, 2461 m: A, G1; B, G1 and G2 in situ (MNHN-B 28725). Scale bars: 2.5 mm (A), 5 mm (B).
Bythograea laubieri a été parfois récoltée sur les mêmes sites que B. vrijenhoeki n. sp. (et qu'Allograea tomentosa [9]) dans le Sud de la dorsale du Pacifique oriental (31°09′S–111°55′W, Alvin Dives 3338 et 3339, 2334 et 2338 m, MNHN-B28764 et B28765), ce qui étend sa distribution géographique méridionale. B. vrijenhoeki n. sp. et B. laubieri (Fig. 7) partagent de nombreux caractères de la carapace, de la région oculaire, des maxillipèdes, du sternum thoracique, ainsi que des chélipèdes, avec notamment des plages sétifères et colorées sur les pinces. Les différences morphologiques concernent le premier pléopode sexuel mâle (régulièrement incurvé, plutôt étroit et seulement un peu effilé chez B. vrijenhoeki n. sp. ; subdroit, fort, avec une partie subdistale épaisse et un long apex très effilé chez B. laubieri), la plaque suborbitaire (lisse chez B. vrijenhoeki n. sp., granuleuse chez B. laubieri), l'ornementation de la carapace et des chélipèdes, la forme des pinces (main plus forte chez B. laubieri, Fig. 7), les proportions des pattes ambulatoires (plus courtes et plus épaisses chez B. laubieri, mais avec une ornementation similaire de soies souples et régulièrement disposées), le telson mâle (triangulaire chez B. vrijenhoeki n. sp., ovalaire chez B. laubieri).
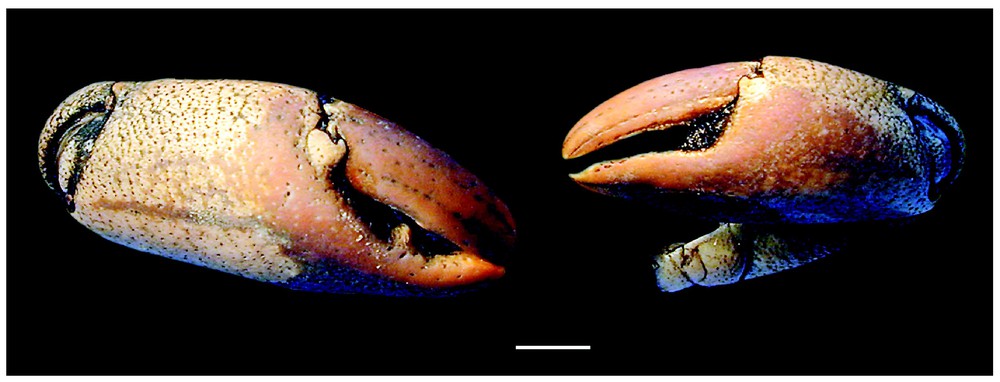
Bythograea laubieri Guinot and Segonzac, damaged female, about 60-mm wide, Alvin Dive 3283, 21°S, Hector vent (MNHN-B 28622): chelipeds of the largest individual known in the species. Scale bar: 1 cm.
Bythograea galapagensis n. sp. est proche de B. thermydron. Les différences portent principalement sur les pléopodes mâles (G1 torsadé, glabre et à extrémité arrondie chez B. thermydron, peu incurvé, sétifère et pointu chez B. galapagensis ; G2 plus allongés et s'entrecroisant chez B. thermydron), la plaque suborbitaire (plus développée chez B. thermydron), les pédoncules oculaires (légèrement plus épais et avec la cornée plus élargie chez B. thermydron), le telson mâle (plus allongé et en triangle pointu chez B. thermydron), et les proportions des pattes ambulatoires (beaucoup plus allongées et grêles chez B. thermydron, néanmoins avec une ornementation de touffes espacées de soies raides similaire à celle de B. galapagensis).
L'adjonction des deux espèces nouvelles ne modifie pas la diagnose du genre Bythograea, qui reste caractérisé par la présence d'une plaque suborbitaire et par un degré de réduction oculaire variant selon les espèces. À côté de B. microps, qui est à part, deux groupes d'espèces sœurs sont clairement reconnaissables. D'une part, B. thermydron et B. galapagensis n. sp., qui cohabitent sur la ride des Galapagos, et, d'autre part, B. laubieri et B. vrijenhoeki n. sp., qui cohabitent dans le Sud de la dorsale du Pacifique oriental. Le premier pléopode mâle ainsi que la sétation et les proportions des pattes ambulatoires témoignent de l'étroite relation entre les deux groupes d'espèces : B. thermydron et B. galapagensis n. sp. (groupe thermydron : G1 torsadé et en forme de S, pattes ambulatoires allongées et garnies de touffes espacées de soies raides) ; B. laubieri et B. vrijenhoeki n. sp. (groupe laubieri : G1 pas ou peu incurvé, pattes ambulatoires courtes et couvertes de soies courtes et régulières).
La comparaison des séquences de l'ADN mitochondrial (cytochrome oxydase I) [12,13] confirme la nouveauté de Bythograea vrijenhoeki n. sp. et de B. galapagensis n. sp., ainsi que leur degré de parenté [14] avec les autres espèces de Bythograea, genre maintenant composé de six espèces (Fig. 13). La divergence entre B. galapagensis n. sp. et B. thermydron est de 9,6 %, celle entre B. vrijenhoeki n. sp. et B. laubieri de 7,3 %. Ces valeurs sont similaires à celles rencontrées dans d'autres groupes d'Invertébrés marins [20,21], y compris chez des Brachyoures : 7,2–17,2 % dans le genre Cancer [22], 9,9–28,4 % pour des espèces sympatriques non hybrides du genre Potamonautes [23].
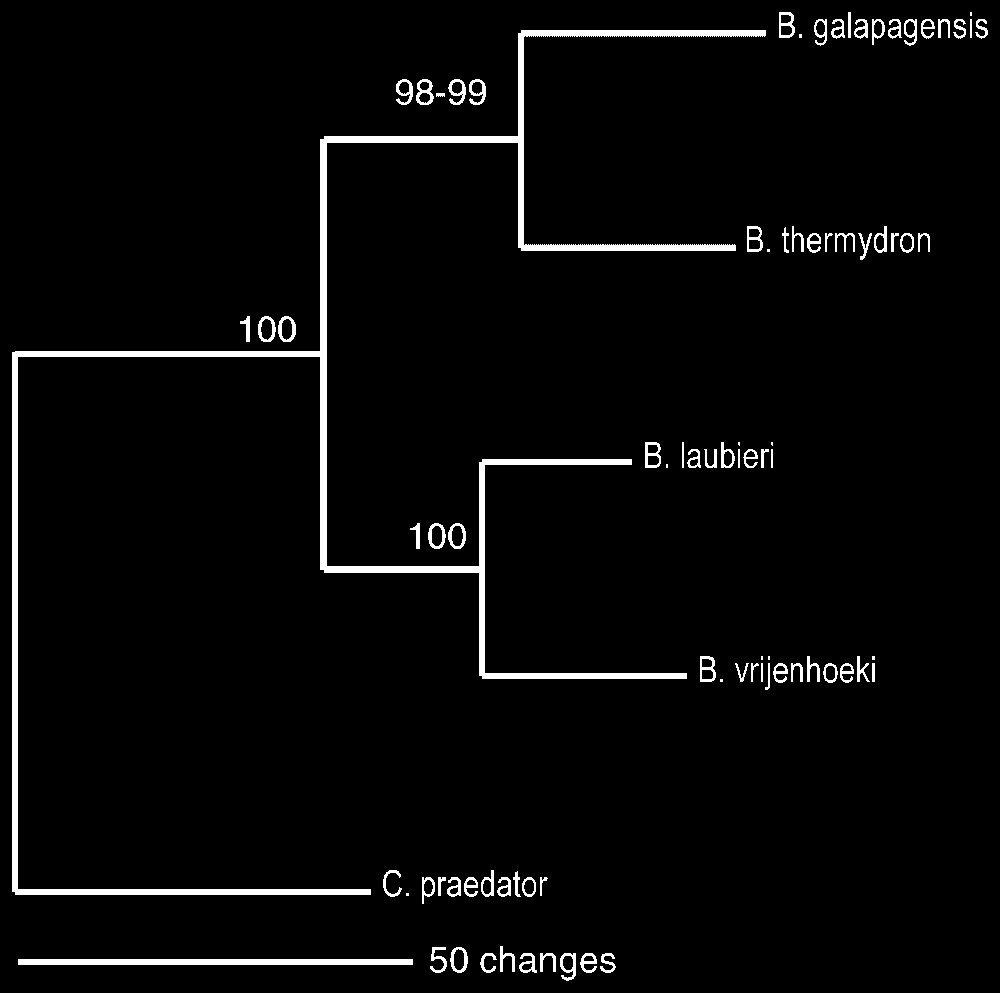
Phylogenetic relationships between Bythograea thermydron Williams, B. laubieri Guinot and Segonzac, B. vrijenhoeki n. sp., and B. galapagensis n. sp., with Cyanagraea praedator de Saint Laurent as outgroup. The phylogenetic tree was obtained through maximum parsimony method. Minimum evolution and neighbour-joining methods produced the same topology.
La divergence moyenne entre le groupe B. thermydron/B. galapagensis n. sp. et le groupe B. laubieri/B. vrijenhoeki n. sp. est de 14,5 %, ce qui est consistant avec les comparaisons morphologiques et confirme bien la présence de deux groupes d'espèces sœurs. Pour chaque groupe nous avons trouvé chez les espèces les plus cosmopolites une divergence intraspécifique plus marquée que chez les espèces à distribution plus restreinte, à savoir un maximum de 2 % pour B. thermydron, 1,7 % pour B. laubieri, 0,5 % pour B. vrijenhoeki et 0,3 % pour B. galapagensis.
Malheureusement, l'analyse biomoléculaire non concluante de B. intermedia (matériel type) en vue d'une comparaison avec les adultes découverts dans le même site (Rose Garden) sur la ride des Galapagos n'a pas permis de statuer sur leur éventuelle identité, ce qui nous a amenés à décrire ces derniers sous le nom de B. galapagensis n. sp. Seuls la récolte de séries complètes lors de futures expéditions sur la ride des Galagapos ou l'élevage de B. intermedia en laboratoire, de la mégalope jusqu'au stade adulte, permettront de décider de la validité de B. galapagensis n. sp.
1 Introduction
Three genera of the family Bythograeidae Williams, 1980, are exclusively present at hydrothermal vents on the East Pacific Rise and Galapagos Rift. Cyanagraea de Saint Laurent, 1984, is probably monotypic, with C. praedator de Saint Laurent, 1984, distributed between 13°N and 18°S on the East Pacific Rise (EPR) [2,4–7]. Allograea Guinot, Hurtado and Vrijenhoek, 2002, is also a monotypic genus, with A. tomentosa Guinot, Hurtado and Vrijenhoek, 2002, found only at vents south of the Easter Microplate (31°–32°S) on the southern East Pacific Rise [9]. Bythograea Williams, 1980, is a polytypic genus, with four widely distributed species. B. thermydron Williams, 1980 [1–10], type species, is the dominant crab species on most EPR hydrothermal vent sites between 21°N and 18°38′S and on the Galapagos Rift, although it has not been found at vents south of the Easter Microplate (31°–32°S), on the southern East Pacific Rise. B. microps de Saint Laurent, 1984 [2–4,6,8,10] has been observed at 21°N, 13°N and 9°50′N on the EPR. B. laubieri Guinot and Segonzac, 1997 [8–10] has been collected only in hydrothermal vents on the southern EPR between 11°S and 21°S, but it is completely absent from northern East Pacific Rise localities. B. intermedia de Saint Laurent, 1988 [3,5,8,10], found at vents on the Galapagos Rift, is a poorly known species since its description was based on six early crab stages and a megalopa, all mixed with B. thermydron specimens from the original collection of the Galapagos Rift studied by Williams [1]. Since this species has never been reported again, the characteristics of the adults of B. intermedia are totally unknown. Therefore, it is impossible to make direct comparisons of adults of B. intermedia with other Bythograea species.
Herein we describe two new species of Bythograea, B. vrijenhoeki n. sp. from vents on the southern East Pacific Rise, south of the Easter Microplate, and B. galapagensis n. sp. from vents on the Galapagos Rift. Based on a mitochondrial gene, we present preliminary results on DNA sequence divergence and phylogenetic analyses among Bythograea crabs that confirm the new status of these taxa. DNA data support the phylogenetic relationships proposed for these new species with other Bythograea species based on morphological comparisons. The genus Bythograea thus consists of six described species. Because the uncertainty of the B. intermedia adults and the lack of good DNA samples from the megalopa and juveniles used to describe this species, we cannot rule out that the species we describe here as B. galapagensis n. sp. may be indeed B. intermedia. This matter certainly warrants further studies.
We also report an extension of the known range of Bythograea laubieri, which was found together with B. vrijenhoeki n. sp. (and also Allograea tomentosa [9]) in the material examined from 31°–32°S vents on the southern East Pacific Rise, south of the Easter Microplate.
2 Material and method
Specimens used in this study were collected with the aid of the manned submersible Alvin. Samples used for the description of Bythograea vrijenhoeki n. sp. (11 females and 10 males) were collected during Alvin dives 3337 (01/13/1999), 3338 (01/14/1999), 3339 (01/15/1999), 3340 (01/16/1999), 3341 (01/17/1999), and 3342 (01/18/1999), at hydrothermal vents on the southern East Pacific Rise (31°–32°S–112°02′W) at a depth of 2338 m. Examination of videos from Alvin dive 3337 revealed that B. vrijenhoeki individuals were observed in large numbers on a mussel field and in lava pillars near vent chimneys, where few Allograea tomentosa crabs, anemones, numerous small white worms, and big white gastropods were also observed. The small white worms may well be ‘spaghetti worms’ (Saxipendium coronatum) that were collected in large numbers during dives 3337 and 3338. The white gastropods were recognized as members of the family Buccinidae, tentatively identified as Eosipho sp. These gastropods were collected in large numbers during dives 3337 and 3339. During dives 3337 to 3342 were also observed several individuals of Alvinella pompejana Desbruyères and Laubier, 1980, and A. caudata Desbruyères and Laubier, 1986 in the chimneys, and in the periphery dense clumps of Oasisia, Tevnia, and Riftia vestimentiferan tubeworms and Bathymodiolus mussels.
Samples used for the description of B. galapagensis n. sp. (2 females and 1 male) were collected during Alvin dive 2224 (05/29/90) at hydrothermal vents on the Galapagos Rift (0°48.2′N–86°13.9′W), at a depth of 2461 m.
Specimens were frozen at −60 °C soon after retrieval for genetic analyses and subsequently transferred to alcohol for morphological description. We extracted total DNA of B. thermydron (five individuals), B. laubieri (five individuals), B. vrijenhoeki n. sp. (two individuals), and B. galapagensis n. sp. (two individuals) from a claw muscle sample, following the manufacturer's protocol for the DNEASY kit (Qiagen, Inc., Valencia, CA). An approximately 710-bp fragment of the mitochondrial cytochrome c Oxidase Subunit I gene (mtCOI) was amplified using published primers and PCR conditions [12]. Both strands of each PCR product were sequenced on an ABI 377 automated sequencer (Perkin-Elmer/ABI, Foster City, CA). Sequences were proofread, aligned and assembled using Sequencher v. 4.1 (Gene Codes Corp., Ann Arbor, MI). Assembled sequences were truncated to 659-bp, a fragment that contained only clear and readable nucleotides. We compared DNA sequence divergence among these species using Kimura-2-parameter [13] nucleotide sequence divergence, estimated with the phylogenetic program PAUP∗ v. 4.0b8 [14]. We also used PAUP to infer evolutionary relationships among these four Bythograea taxa. We conducted maximum parsimony (MP), minimum evolution (ME), and neighbour-joining (NJ) analyses. The MP and ME analyses were executed with heuristic searches using 50 stepwise random additions and TBR branch swapping. Bootstrap support values were calculated from a 50% majority-rule consensus tree, based on 1000 bootstrap replicates. The MP analyses assumed equally weighted characters and equally weighted substitution types. Both ME and NJ analyses assumed Kimura-2-parameter distances. Trees were rooted using one individual sequence of Cyanagraea praedator as outgroup.
Abbreviations: mxp1, first maxilliped; mxp3, third maxilliped; G1, male first pleopod; G2, male second pleopod; P3, P4, P5, third, fourth, fifth pereopods. Measurements are given in millimetres (mm) for carapace, length × width. Material used in this study is deposited in the ‘Muséum national d'histoire naturelle’, Paris (MNHN); two paratypes of Bythograea vrijenhoeki n. sp. were deposited in the USNM, Washington.
3 Systematic description
Family Bythograeidae Williams, 1980
3.1 Bythograea vrijenhoeki n. sp.
Type material. Southern East Pacific Rise, Alvin Dive 3340, 31°51′S–112°02′W, 2334 m, 01/16/1999: holotype, male 16.8×29.5 mm (MNHN-B28751). Alvin Dive 3341, 01/17/1999: allotype, female 24.1×42.3 mm (MNHN-B28752).
Other material examined. Southern East Pacific Rise, Alvin Dive 3337, 31°09′S–111°55′W, 2335 m, 01/13/1999: 1 male 15.6×27 mm, 2 females 15.6×27.3, 16.3×28.1 mm, paratypes (MNHN-B28753), 1 male 15.6×27 mm, 1 female 19.5×34.7 mm, paratypes (USNM 1009516). Alvin Dive 3338, 31°09′S–111°55′W, 2334 m, 01/14/1999: 1 male 18.6×31.7 mm, 4 females 19.5×33.2 mm, ca 41.3 mm width, 24.4×43.7 mm, 25.7×47 mm, paratypes (MNHN-B28754). Alvin Dive 3339, 31°09′S– 111°55′W, 2338 m, 01/15/1999: 2 males 16×28.5, 21×37 mm, paratypes (MNHN-B28755). Alvin Dive 3340, 31°51′S–112°02′W, 2334 m, 01/16/1999: 2 males 16.8×29.5 mm, 21.3×37.9 mm, 2 females 17.5×30.5 mm, 19×33.4 mm, paratypes (MNHN-B28756). Alvin Dive 3341, 31°51′S–112°02′W, 2333 m, 01/17/1999: 1 male 24.2×42.4 mm, paratype (MNHN-B28757). Alvin Dive 3342, 31°51′S– 112°02′W, 2338 m, 01/18/1999: 1 male 14.9×25.5 mm, 1 female 21.1×37.8 mm, paratypes (MNHN-B28758).
Etymology. The new species is named to honour Dr Robert Vrijenhoek (Monterey Bay Aquarium Research Institute) in recognition of his important contribution to our knowledge on evolution and population genetics of hydrothermal vent organisms.
Description. Size 42.4 mm (male) and 47 mm width (female). Carapace broad, transversely elliptical, almost flat mid-dorsally, slightly arched from anterior to posterior, near lateral margins. Dorsal surface with regions indistinct, glabrous, smooth, except on anterolateral regions ornamented with flat granules; granular transverse row. Anterolateral, frontal margins of carapace, margins of suborbital plate beaded with granules that are more developed that those of anterolateral parts, practically never red- or brown-tipped. Front broad, weakly deflexed, obscurely bilobed, no external angles. Eyes, antennules, antennae deeply recessed under front. Antennules horizontally folded, basal article hardly exposed. Suborbital plate very narrow, markedly elongated, smooth, subdivided by uncalcified fissure in two parts, superior one narrowest, more tapering externally than inferior one. Orbits almost non-existent. Eyestalks narrow, hardly broadened distally; cornea unpigmented or with a small dark remnant, sexually dimorphic, tip more or less fusiform, laterally fringed with soft hairs. Subhepatic, pterygostomial areas without setose patches; setae only at bases of chelipeds and walking legs. Anterior margin of epistome strongly protruding ventrally into mouth field, divided into six unequal lobes lined with low, generally not red-tipped granules: internal lobes of each side joining together to form wide, advanced protrusion medially subdivided by fissure; internal lobe separated from intermediate lobe by a large notch with straight margin; intermediate lobe small, pointed, less advanced; external lobe more advanced, wider. Floor of endostome with palatal ridge located only posteriorly, not delimitating lateral efferent channel; internal half of endostome covered with velvety pubescence. Lacinia of mxp1 not extended laterally but with developed mesial lobe. Mxp3 filling mouth field except for large gap between anteroexternal part of merus of endognath and epistome; exognath overlapping sides of mouth frame; all surfaces nearly smooth, punctate, except for granules on merus. Endognath: merus produced with marked mesial lobe; palp developed, reaching 2/3 length mesial margin of ischium; propodus asymmetrically ovate in ventral view; dactyl half-ensiform, long, deeply inserted in mesial and distal sides of propodus when flexed. Thoracic sternal sutures 4/5 and 5/6 joining together by their extremities; sutures 6/7 oblique, interrupted; sutures 7/8 reaching the median line. Male abdomen with triangular telson. Prominence of press button thick. Chelipeds dimorphic in both sexes; heterochely and heterodonty in males. Male large cheliped (crusher, on right), with propodus stout but not markedly inflated, rugose, pitted in superior half; fingers slightly gaping, grooved, blunt-tipped; dactyl with very low proximal tooth on occluding margin; fixed finger with pointed proximal tooth, two smaller ones on occluding margin. Male small chela (cutter) weakly broadened, markedly rugose in superior half; fingers not gaping, grooved, with pointed tips; dactyl without teeth on occluding margin; fixed finger with three–four main acute teeth. Female chelipeds asymmetrical, elongated. Superior half of propodus of large cheliped rugose, pitted, with granules on small cheliped; both fingers grooved; on both chelae, occluding edge of dactylus not toothed, occluding edge of fixed finger with four acute main teeth. Male small chela and both female chelae with pilose patches at the base between fingers, becoming narrower along occluding margin; developed setose patch on the internal surface. Both sexes with dactyl of cheliped brown-coloured or completely white; brown-coloured, elongated area at bases of palm and fixed finger, that may be partly depressed; in large chela, another coloured zone (sometimes only traces or absent) oblique along proximal part of palm, bordering fixed finger. Walking legs elongated, slender; meri cylindrical, not proximally broadened; setae of carpus and propod regular, not sparse in scattered tufts. Dactylus with rows of setae. Male first gonopod rather long, narrow, curved but non-twisted, regularly tapering, without setae; second gonopod markedly longer than first. Vulvae forming large, rounded membranous area; oblique lunate slit (Figs. 1–6).
Remarks.Bythograea vrijenhoeki n. sp., collected in hydrothermal vent localities on the southern East Pacific Rise south of the Easter Microplate (31°09′S and 31°51′S), is the fifth species of the genus Bythograea to be described. The most closely related species is B. laubieri Guinot and Segonzac, 1997.
Bythograea vrijenhoeki n. sp. and B. laubieri share similar carapaces, ocular regions, mouthparts, sternal plates, chelipeds, as well as the presence of setose patches and coloured areas on the chelae. The examined material of B. vrijenhoeki n. sp. shows pale brown-coloured areas on the chelae, but several individuals were entirely white. A violaceous area persisted along the inferior border of the palm in the most discoloured individuals of B. laubieri. It is worth noting that the granules on the carapace are practically always white in B. vrijenhoeki n. sp., while in B. laubieri they are red- or brown-tipped, often resembling crescent spots. The morphological differences between the two species are: a narrow, regularly curved, tapering first male gonopod in B. vrijenhoeki n. sp. (stout, not much curved, with a thick subdistal part and a long and pointed apex in B. laubieri); a smooth suborbital plate in B. vrijenhoeki n. sp. (granulous in B. laubieri); a weak granulous ornamentation on the carapace and chelipeds, superior half of palm only rugose on male large cheliped in B. vrijenhoeki n. sp. (granulation more marked in B. laubieri); the shape of chelae (palm stouter in B. laubieri, Fig. 7), walking legs (shorter and thicker in B. laubieri: maximum length of P5 meri 2.2 times mean maximum width in male B. laubieri versus 2.9 in male B. vrijenhoeki; for P5 propodi, 1.3 in male B. laubieri versus 2.3 in male B. vrijenhoeki; for P4 meri 2.5 in male B. laubieri versus 3.2 in male B. vrijenhoeki; for P4 propodi, 1.7 in male B. laubieri versus 2.5 in male B. vrijenhoeki; for P3 meri 2.5 versus 3.3 in male B. vrijenhoeki; for P3 propodi, 1.8 in male B. laubieri versus 2.3 in male B. vrijenhoeki); and a triangular male telson in B. vrijenhoeki n. sp. (ovate in B. laubieri).
The material examined establishes a southern extension to 32°S for Bythograea laubieri, and its cohabitation with B. vrijenhoeki n. sp. Two specimens of B. laubieri were found mixed with B. vrijenhoeki n. sp. in two dives: Alvin Dive 3338, 31°09′S–111°55′W, 2334 m, 01/14/1999: 1 male 29.9×52 mm (MNHN-BB28764), and Alvin Dive 3339, 31°09′S–111°55′W, 2338 m, 01/15/1999: 1 female 27.1×46.7 mm (MNHN-B28765). The chelipeds are strongly dimorphic in that female, the small one is only rugose on the palm, the coloured areas on palm and the fingers are pale brown, and the granules of the carapace are white, as in B. vrijenhoeki n. sp. But in the male (large cheliped absent) the granules of the carapace are coloured at tip, as in the typical B. laubieri, and the small chela shows brown (not violaceous) areas. The largest known specimen of B. laubieri, a female about 60 mm width, collected at 21°26′S–114°16′W (Hector vent, Alvin Dive 3283, 2832 m, 10/13/1998, MNHN-B28622), has markedly stout and inflated chelae, with a brown–violaceous colour largely on the palm (Fig. 7).
Bythograea vrijenhoeki n. sp. also cohabits with Allograea tomentosa Guinot, Hurtado and Vrijenhoek, 2002 [9]. The two species were collected by the same dives 3337 and 3338, 31°09′S–111°55′W, at 2335 m.
In contrast to Bythograea vrijenhoeki n. sp., B. thermydron Williams, 1980 is characterized by a wider suborbital plate, broadened cornea, anterior border of the epistome markedly divided into distinct lobes (without wide, U-shaped notch), triangular male telson, short, S-curved and twisted G1, very long and crossed G2, and more elongated, slender walking legs provided with scattered tufts of stiff, darkened setae.
3.2 Bythograea galapagensis n. sp.
A species different from Bythograea thermydron, B. microps, B. laubieri, and B. vrijenhoeki n. sp. was collected by Alvin on the Galapagos Rift in 1990. It was taken from Rose Garden, one of the three vent areas [11] where B. thermydron was first discovered by Alvin in 1979 and its paratypes were selected [1].
Bythograea intermedia de Saint Laurent, 1988 [3,5,8,10] is the other known species found at Rose Garden on the Galapagos Rift. This species has been reported only once and was described based on early crab stages and megalopa mixed with B. thermydron specimens from the original collection of the Galapagos Rift studied by Williams [1]. Hessler and Martin [5, (p. 645)] commented on the unfortunate situation created in erecting a new species “on the basis of a single megalopa larva and on the assumption that some of the smaller juveniles described by Williams (1980) were of this previously unrecognized species”. In fact, de Saint Laurent [3, (Fig. 3b after Williams)] never saw directly the juvenile individual measuring 4.5×6.8 mm – but indicated as 6.3 mm in the caption of the Fig. 3b by de Saint Laurent – and supposed to be stage II, which she selected as holotype (it keeps the number USNM 180065) of B. intermedia. The holotype was chosen among a lot of six early crab stages, all with detached legs, attributed by Williams [1, (Fig. 11b, e)] to B. thermydron. Only the megalopa (4.3×5.5 mm) figured by Williams [1, (Fig. 11a)] was examined by de Saint Laurent, who selected it as paratype [3, (Figs. 3a and 4b)]. Consequently, the holotype individual was never clearly separated in the USNM Collection: it now receives the number USNM 268862, while the paratype (megalopa) has the number USNM 180065 (this lot was wrongly catalogued as holotype of this species); the non-type megalopae+crab stages receive herein a new number, USNM 1010859 (this lot required a new number because the truthful holotype of B. intermedia was pulled out of the jar with megalopae and crab stages which were identified as B. thermydron by Wiliams [1]). The exact locality of the juvenile material of Williams, which perhaps contains B. thermydron mixed with B. intermedia, is unknown.
Morphological comparisons between the adult crabs collected from Rose Garden on Galapagos Rift in 1990, used here to describe B. galapagensis n. sp., and the juvenile used as holotype of B. intermedia, collected from the same site and examined for the present paper, do not allow to establish whether they correspond to the same species. Similarly, we could not obtain DNA information to resolve this issue, from some of the small individuals assumed to be used in the description of B. intermedia from the collection examined by Williams [1]). For these juvenile individuals, we attempted unsuccessfully to extract DNA and amplify small fragments (less than 400 base-pairs) of two DNA mitochondrial genes (mtCOI and cytochrome b), known to easily amplify in other bythograeid species. Unfortunately, the DNA of these individuals may be severely degraded, and prevented us to establish whether they and the specimens we used here to describe B. galapagensis n. sp. are the same species.
In view of the inconclusive results of the molecular analysis and morphological comparisons with the existent material of B. intermedia, we have chosen to describe the adults of the Galapagos Rift as B. galapagensis n. sp., until a full series of megalopae, juveniles and adults can be found for both species. Bearing in mind that de Saint Laurent's B. intermedia is a valid species (“the availability of a taxon is not affected even if the name is based on one stage in the life cycle”, ICZN, 1999, 17.3) and given that it is not possible to associate our adults to B. intermedia, the only reasonable option seemed to describe the characters of the species which is mixed with B. thermydron in some places, i.e., to establish the new species B. galapagensis n. sp.
Material examined. Galapagos Rift, Alvin Dive 2224, 0°48.2′N–86°13.9′W, Rose Garden, 2461 m, 05/29/90: 1 male 29.6×52.3 mm, holotype (MNHN-B 28725), 1 female 24.8×44.6 mm, allotype (MNHN-B 28744), 1 female juv. 9.5×16.1 mm (MNHN-B 28745).
Description. Size 52 mm (male) and 45 mm width (female). Body and legs white. Carapace broad, transversely elliptical, almost flat mid-dorsally. Dorsal surface with regions indistinct, glabrous; anterolateral regions with low granules; granular transverse row. Anterolateral and frontal margins of carapace, margins of suborbital plate lined with uniform, white granules, more developed than those of anterolateral parts. Front broad, weakly deflexed, only obscurely bilobed, median depression shallow, no external angles. Eyes, antennules and antennae recessed under front. Antennules horizontally folded, basal article hardly exposed. Suborbital plate elongated, narrow, smooth. Orbits almost non-existent. Eyestalks narrow, depressed, only very slightly broadened distally; cornea fringed with soft hairs laterally, sexually dimorphic, the tip more fusiform in adult female; juvenile females with cylindrical eyestalks and pigmented cornea. Subhepatic, pterygostomial areas without setose patches; only setae at bases of chelipeds and walking legs. Anterior margin of epistome strongly protruding ventrally into mouth field, divided into four unequal lobes: internal lobes of each side joining together to form advanced protrusion, separated from wide external lobe by sinuous margin. Floor of endostome with palatal ridge located only posteriorly, not delimiting lateral efferent channel; internal half of endostome covered with velvety pubescence. Lacinia of mxp1 not extended laterally but with developed mesial lobe. Mxp3 filling mouth field except for very large gap between anteroexternal part of merus of endognath and epistome; exognath overlapping sides of mouth frame. Endognath: merus granular, produced with marked mesial lobe; palp reaching 2/3 length mesial margin of ischium; propodus asymmetrically ovate in ventral view; dactyl half-ensiform, long, deeply inserted in mesial, distal sides of propodus. Male abdomen with telson relatively long and triangular. Prominence of press button thick, clearly consisting of two parts (basal part thicker, touching suture 5/6; apex pointed). Male large cheliped (crusher) missing. Male small cheliped (cutter) inflated, rugose in superior half; fingers not gaping, weakly grooved, with pointed tips; dactyl finely denticulate on occluding margin; fixed finger with small teeth. Female chelipeds dimorphic, both inflated, fingers grooved; superior half of propodus rugose; occluding edge of dactylus hardly toothed (large cheliped) or nearly smooth (small cheliped); occluding edge of fixed fingers with low teeth. Male small chela and both female chelae with developed pilose patches covering most of internal surface of palm; no setae visible on external surface and between fingers. Fingers practically colourless. Walking legs rather elongated. G1 relatively long, twisted, tapering in long, pointed apex; many minute spinules in lateral tract on whole distal part, except at tip; dense, long setae on subdistal mesial part. G2 almost straight, not crossed, flagellum not curved. Vulvae forming relatively small, rounded membranous area; lunate slit located almost medially (Figs. 8–12).
Etymology. This species was collected from the Rose Garden site on the Galapagos Rift, hence the specific name galapagensis.
Remarks.Bythograea galapagensis n. sp. is represented by two adult individuals and a juvenile female, so that variations, particularly the coloration and the degree of wear, could not be checked. In the original description [1] B. thermydron was reported from the same Rose Garden site: “Crabs from ‘The Rose Garden’ white and not worn”. Our three B. galapagensis specimens are white, lack dark-tipped granules and coloured areas on chelipeds, which are characteristic of B. thermydron (although entirely white individuals of B. thermydron also exist), and do not seem worn. All B. thermydron specimens deposited in the MNHN collection were examined and belonged to B. thermydron Williams, 1980.
Most morphological characters of Bythograea galapagensis n. sp. are close to those of B. thermydron. Both species have somewhat similar carapaces, mouthparts, sternal plates, and chelipeds. The setation on the walking legs consists of scattered tufts of stiff setae in both species. But the distinction between the two species is clear, particularly in males. B. thermydron principally differs by the more developed suborbital plate, slightly thicker eyestalks, and the broadened cornea in both sexes (in contrast to the fusiform tip of B. galapagensis). The male pleopods are the most distinguishable characters: G1 short, markedly twisted, round-tipped and glabrous in B. thermydron (longer, less twisted, acute-tipped and subdistally setose in B. galapagensis); G2 longer and crossed in B. thermydron. Other differences are on the shape of male telson (longer and forming a more pointed triangle in B. thermydron), and the proportions of the walking legs in both sexes. They are much more elongated and slender in B. thermydron: maximum length of P5 meri 3.0 times mean maximum width in male B. thermydron versus 2.5 in male B. galapagensis; for P5 propodi, 2.4 in male B. thermydron versus 2.1 in male B. galapagensis; for P4 meri, 3.5 in male B. thermydron versus 3 in male B. galapagensis; for P4 propodi, 3.1 in male B. thermydron versus 2.7 in male B. galapagensis; for P3 meri, 3.6 versus 3.1 in male B. galapagensis; for P3 propodi, 2.6 in male B. thermydron and male B. galapagensis as well. The slight differences that we have observed in the shape of the anterior margin of epistome (external lobe entire in B. galapagensis, sinuous in B. thermydron) need to be further examined in the additional material.
Bythograea galapagensis n. sp. shares with B. laubieri narrow eyestalks, small cornea and short, thick walking legs. B. laubieri differs from B. galapagensis n. sp. by the suborbital plate being granular at least on the inferior portion, the red-tipped granules of the carapace, the anterior margin of the epistome with a broad, U-shaped notch, an ovate male telson, chelae with coloured areas, regularly setose walking legs and a thick, glabrous, non-twisted G1.
Bythograea galapagensis n. sp. shares with B. vrijenhoeki n. sp. narrow eyestalks, narrow and smooth suborbital plate (but narrower in B. vrijenhoeki), and short, thick walking legs. B. vrijenhoeki n. sp. differs from B. galapagensis n. sp. by the more fusiform cornea, shorter male telson, wider submedian notch on anterior border of epistome, more regular setation of walking legs, and the non-twisted, regularly tapering, and glabrous G1.
4 Discussion
All bythograeid species resemble each other in gross morphology, particularly carapaces, mouthparts and sternal plates. The most significant differences are in the eyes, antennules and antennae in normal location (Allograea, Cyanagraea) or deeply recessed under front (Bythograea, Segonzacia, Austinograea); orbits which vary from defined pockets (Cyanagraea, Allograea) to absent; eyes from rather large, nevertheless little mobile (Cyanagraea) to being replaced by a suborbital plate (Bythograea) or fused to the orbital wall and invisible (Austinograea); ventral exposure of mxp3 (coxa obscured in Austinograea); mxp3 (without specialized features in Allograea, in contrast with the three other genera); shape of chelae; patches of cuticle with different texture or coloured spots (supposedly sensory) on carapace and chelipeds; male sexual pleopods; vulvae; and spermatozoal ultrastructure [15].
The genus Bythograea is characterized by the presence of a suborbital plate, that is depressed laterally and ventrally in relation to the eyestalks, demarcated by dorsal and ventral rows of granules, and subdivided by an uncalcified fissure. The degree of reduction of the eyes is variable among the species, very conspicuous in B. microps and less marked in B. thermydron. A clear sexual dimorphism was observed, the tip of the eyestalks being more fusiform in the adult females of B. galapagensis n. sp., a phenomenon similarly found in cavernicolous crabs [16]. The megalopa of B. intermedia was observed to have compound eyes and the loss of the visual components already in the early stage crabs. In Bythograea the metamorphosis of the eyes in developing high-sensitivity, naked retinas seems to be a specific adaptation for life at vents [17,18].
Two groups are clearly distinguishable: Bythograea thermydron and B. galapagensis n. sp., which cohabit at Galapagos Rift (Rose Garden), and B. laubieri and B. vrijenhoeki n. sp., which cohabit on the Southern East Pacific Rise vents (31°–32°S), south of the Easter Microplate (also with Allograea tomentosa). The first male pleopods, which appear as the most conservative structure in the Brachyura, support close relationship between the two pairs: B. thermydron and B. galapagensis n. sp. (thermydron group) with twisted and S-shaped G1; and B. laubieri and B. vrijenhoeki n. sp. (laubieri group) with non-twisted G1. The shape of male pleopods provides the most reliable character to separate the two groups, while small but unequivocal differences allow the distinction between the four species. The setation of walking legs is similar in the thermydron group, with scattered tufts of stiff setae, in contrast to short and regular setation in the laubieri group. The proportions of the walking legs in males (length-width relationships for P3 to P5) similarly separate the two groups: B. thermydron, proportionately with the longest and more slender pereopods, while in B. galapagensis n. sp. the pereopods are less elongated; B. laubieri and B. vrijenhoeki n. sp., both with short and stout pereopods, the shortest legs being in B. laubieri males.
Despite the fact that B. thermydron (between 21°N and 18°38′S) and B. laubieri (between 11°S and 32°S) were never found together, it is not completely excluded that they may cohabit. With the discovery of the two new species, all identifications based only on video observations [8] need to be re-appreciated. In both the laubieri group and thermydron group, a more widely distributed species coexists sympatrically with a species that is restricted to one locality (at least for the moment). Results of behavioural experiments showed that B. thermydron megalopae could easily swim ten to even hundreds of kilometres in search of suitable vent sites and that swimming behaviour may be an important component of locating warm vent settlement sites in the otherwise cold waters surrounding a vent field [19].
The morphological comparisons clearly show that Bythograea microps (between 21°N and 9°50′N) is unique among the species of Bythograea. It is the smallest species (24 mm width) of the genus and it shows the most drastic reduction of eyestalks. It is setose (on carapace, and especially on chelae) and more granular (on carapace and pereopods, in particular on merus and carpus; male small chela and both chelae of female are granulated and setose on both external and internal surfaces; male large chela with occluding portion of fixed finger bearing rows of granules). The anterior margin of the epistome is similar to that of B. vrijenhoeki n. sp. B. microps has strongly dimorphic chelipeds, with a violaceous, smooth spot at the base of the palm of the male small chela and of both female chelae. The male telson is comparatively the shortest in the genus Bythograea. B. microps resembles B. thermydron by the long and crossed G2 but differs by the non-twisted and pointed G1.
5 Comparison of DNA sequences
Comparison of DNA nucleotide sequences of a fragment of the mitochondrial Cytochrome c Oxidase Subunit I gene (mtCOI) among four Bythograea species confirms the status of B. vrijenhoeki n. sp. and B. galapagensis n. sp. as new species, and their relationships with other Bythograea species. Although B. thermydron and B. galapagensis n. sp. are morphologically very similar to each other, and B. laubieri and B. vrijenhoeki n. sp. as well, mtCOI sequence divergence clearly revealed that they correspond to four different species. Kimura-2-parameter nucleotide sequence divergence between B. galapagensis n. sp. and B. thermydron is 9.6%, and between B. vrijenhoeki n. sp. and B. laubieri is 7.3%. These values are well in the range of species-level mtCOI sequence divergence reported in marine invertebrate groups [20,21], including Brachyuran species: 7.2 to 17.2% in Cancer species [22], 9.9 to 28.4% in Potamonautes non-hybridising sympatric species, and 0.7 to 16.0% in Potamonautes hybridising sympatric species [23].
Average divergence between the group B. thermydron/B. galapagensis n. sp. and the group B. laubieri/B. vrijenhoeki n. sp. is 14.5%, which is consistent with morphological comparisons that suggest they correspond to two different groups. For each pair group, we found larger intraspecific divergences in the cosmopolitan species than in the species with more restricted distribution. Maximum observed intraspecific divergence was 2% for B. thermydron, 1.7% for B. laubieri, 0.5% for B. vrijenhoeki and 0.3% for B. galapagensis. The phylogenetic tree in Fig. 13 depicts the relationships between the four species using Cyanagraea praedator as outgroup. B. thermydron/B. galapagensis n. sp. form a monophyletic group supported by a 98–99% bootstrap value, and B. laubieri/B. vrijenhoeki n. sp. form another monophyletic group supported by a 100% bootstrap value.
In conclusion, morphological and molecular data support the distinction of B. vrijenhoeki as a new species within the genus Bythograea. In the case of B. galapagensis, the adults used for its description are clearly different from known adults of other Bythograea species. However, we could not compare B. galapagensis with adults of B. intermedia. Therefore, we considered it was most appropriate to erect B. galapagensis as a new species. Hopefully, the collection of a large series consisting of megalopae, juveniles and adults of Bythograea galapagensis n. sp. in future expeditions to the Galapagos Rift should permit their identification by molecular techniques and comparison with the material used to describe B. intermedia. Another possibility is to raise megalopae to adults in the laboratory.
Acknowledgements
Grateful acknowledgements are due to R. Lemaitre (USNM, Washington) for the loan of the type of Bythograea intermedia and kind cooperation during our work, to M. Segonzac (IFREMER, Brest, France) for providing information, documentation and precious comments, to P. Castro (California State Polytechnic University, Pomona, USA), M. Mateos (University of Arizona, Tucson, USA), V. Leignel (‘Laboratoire de biologie et génétique évolutive’, ‘Faculté des sciences et techniques du Mans’, France), and M. Judson (MNHN, Paris) for critical review of the manuscript and valuable suggestions, to D. Geffard, P. Loubry and D. Serette (MNHN, Paris) for assistance in the illustration, and to L. Albenga (MNHN, Paris) for technical assistance. Pilots and crew members of DSV Alvin are owed a special debt of gratitude for collecting the material described here. We thank Dr Robert Vrijenhoek (Monterey Bay Aquarium Research Institute) for facilitating the specimens used in this study.


