Version française abrégée
1 Introduction
La puissance du champ gravitationnel terrestre constitue un caractère du milieu ambiant, qui n'a pas changé au cours du processus de l'Évolution. Du point de vue biologique, il est évident qu'un tel élément a pu influencer de nombreux aspects des systèmes de l'Évolution. De là l'intérêt d'effectuer de nombreuses recherches portant sur les effets de la gravité sur plusieurs systèmes.
Les études effectuées en milieu microgravitationnel suggèrent que les conditions de microgravité exercent leur influence sur de nombreuses fonctions biologiques et biochimiques. Les données que nous possédons sont limitées à l'étude de systèmes biologiques complexes et, étant donné la complexité de la structure et de l'organisation de la cellule vivante, elles pourraient être le résultat d'interactions entre l'effet de la microgravité et les systèmes de régulation et de contrôle de la cellule elle-même.
La biologie des plantes est un secteur de la recherche qui se prête très bien à la recherche dans l'espace, et il a été prouvé que la gravité joue un rôle évident, tant au niveau morphologique (orientation des racines) qu'au niveau métabolique.
Les graines à réserve de lipides ont un temps de germination plus long que celles à réserve amilacée et paraissent être étroitement liées au cycle du glyoxylate qui, avec ses deux enzymes clés (isocitrate lyase et malate synthase), joue un rôle de contrôle fondamental pendant la germination, dans le sens qu'elle permet une synthèse nette des glucides à partir des lipides.
L'activité de l'isocitrate lyase est un indicateur de l'état de germination des graines à réserve lipidique. Depuis environ trente ans, nos études ont démontré qu'au cours de la germination de graines de Pinus pinea, l'activité de l'isocitrate lyase et la déplétion des réserves lipidiques atteignent leur maximum entre le onzième et le quatorzième jour de germination.
Forts de ces connaissances, nous avons voulu vérifier si un choc gravitationnel appliqué aux graines avant le début de la germination produit des altérations morphologiques et moléculaires au cours du processus de germination.
À ce propos, nous avons effectué une recherche sur l'évolution, dans le temps, de la germination des graines de Pinus pinea soumises à un stress gravitationnel (1000 g pendant 72 h à 4 °C), soit en milieu sec, soit en milieu humide, avant d'être déposées sur des plaques de germination. Pendant toute la période de germination, nous avons surveillé l'état de croissance de l'embryon, les niveaux d'activité des enzymes les plus représentatives des principales voies métaboliques (isocitrate lyase pour le cycle du glyoxylate, glucose 6-phosphate déshydrogénase pour le shunt des pentose phosphates, isocitrate déshydrogénase pour le cycle de Krebs et piruvate déshydrogénase pour la glycolyse). Nous avons effectué, en outre, une étude histologique au temps zéro sur des graines de contrôle et des centrifugés (sec et humide), avant qu'elles ne soient déposées sur les plaques de germination.
2 Procédures expérimentales
2.1 Stress hypergravitationnel
Le stress hypergravitationnel a été provoqué en appliquant aux graines de Pinus pinea une force centrifuge sous deux types de conditions expérimentales : graines sèches et graines mouillées. Les deux sortes de graines ont été déposées dans des tubes de centrifugation sèches et contenant de l'eau, respectivement à 1000 g pendant 64 h à 4 °C. En même temps, des graines de Pinus pinea ont été maintenues dans les mêmes conditions (sèches et mouillées) à 1 g.
A la fin de la période de centrifugation, toutes les graines (témoins et graines sèches et mouillées) ont été déposées à l'obscurité dans les plaques de germination.
Un certain nombre de graines de Pinus pinea ont été regroupées en quatre lots, ainsi traités :
- – lot 1, graines conservées dans l'eau à 4 °C pendant 64 h (contrôle graines mouillées) ;
- – lot 2, graines sèches à 4 °C pendant 64 h (contrôle graines sèches) ;
- – lot 3, graines centrifugées à 1000 g, 4 °C pendant 64 h dans l'eau (graines mouillées centrifugées) ;
- – lot 4, graines centrifugées à 1000 g, 4 °C pendant 64 h à sec (graines sèches centrifugées).
Les lots 1 et 2 représentent les contrôles des lots 3 et 4 respectivement.
2.2 Germination des graines
Les graines (lots 1–4) ont été déposées dans quatre plaques différentes de germination (plaques I–IV).
Chaque plaque a été divisée en quatre quadrants (quadrants A, B, C, D), chacun à son tour divisé en huit sous-quadrants. Dans le quadrant A ont été placées 96 graines du lot 3, 12 graines pour chaque sous-quadrant ; dans le quadrant B, 96 graines du lot 1, 12 graines pour chaque sous-quadrant ; dans le quadrant C, 96 graines du lot 4, 12 graines pour chaque sous-quadrant ; dans le quadrant D, 96 graines du lot 2, 12 graines pour chaque sous-quadrant. La germination a été effectuée à l'obscurité et à 20 °C.
2.3 La préparation histologique
Une partie des exemplaires ont été fixés au glutaraldéhyde à 2,5 % et au formaldéhyde 2 % dans un tampon cacodylate 0,1 M, de pH 7,4, puis par une solution de OsO4 1 % dans un tampon phosphate 0,1 M, de pH 7,4, et enrobés dans une résine époxy (Epon 812). Des sections de 1 μm d'épaisseur ont été colorées à l'aide de la toluidine alcaline bleue, et observées au microscope optique. D'autres sections, d'une épaisseur d'environ 70 nm, ont été colorées à l'uranyl acétate, puis au citrate de plomb, et observées au moyen d'un microscope électronique à 80 kV (Joule 1010).
2.4 La préparation des extraits
Les plaques I et II ont été utilisées pour l'échantillonnage des graines à des intervalles de temps variables, afin de les homogénéiser. Les plaques III et IV ont été utilisées pour photographier les graines à des intervalles de temps variables au cours du processus de germination.
À des moments prédéterminés, les graines ont été enlevées d'un des sous-quadrants (12 graines), pour chacun des quadrants A, B, C et D des plaques I et II. L'endosperme de ces graines a été enlevé et homogénéisé à l'ultraturrax dans la triéthanolamine à 150 mM, à pH 7,5, avec 9 mM de MgCl2 et 1,5 mM d'EDTA dans un rapport 1:2 (poids/volume). L'homogénat a été filtré sur une compresse de gaze, centrifugé (à 10 000 g, pendant 30 min) et la partie surnageante a été filtrée sur un filtre à papier.
Dans le même temps, nous avons pris des photos des plaques III et IV, de façon à obtenir un aperçu général de la germination au niveau morphologique.
3 Discussion
La graine de Pinus pinea, très résistante, peut conserver pendant des années une bonne capacité de germination. Son revêtement ligneux, très dur, constitue un excellent milieu, apte à la protéger contre les attaques extérieures. La germination est un processus assez long, qui dure environ vingt jours. Toutes ces propriétés font de la graine de Pinus pinea un excellent modèle pour étudier les effets du stress hypergravitationnel sur la germination.
D'après les données obtenues, il apparaı̂t qu'une période de 64 h de centrifugation à 1000 g avant insertion des graines dans les soucoupes de germination a donné des résultats différents selon que les graines avaient été centrifugées en milieu humide ou sec. Dans le premier cas, comparé au contrôle, on remarque une réduction sensible du nombre de graines germées (2 % par rapport au contrôle). Au niveau moléculaire, l'activité de l'enzyme clé de la germination dans les graines possédant des réserves de lipides (ICL, cycle du glyoxylate) est réduite à moins de 50 %. La même chose se produit, évidemment dans une moindre mesure, pour ICDH (cycle de Krebs) et pour G6PDH (pentose phosphate shunt). PK semble être moins sensible au stress gravitationnel. Il est intéressant de remarquer que l'effet de l'hypergravité ne réduit pas les activités enzymatiques calculées dans des graines sèches centrifugées ; de fait, en ce qui concerne le jour de germination présentant les plus hautes activités enzymatiques, on pourrait parler d'une légère augmentation des niveaux enzymatiques pour ICDH. Les niveaux d'activité de G6PDH et de PK se superposent. Les photographies au microscope électronique mettent en évidence un effet considérable sur les préparations de graines obtenues en milieu humide. Étant donné ce qui précède, on peut conclure que l'application d'un stress gravitationnel aux graines dans la phase qui précède leur germination crée une perturbation moléculaire et macroscopique dans le processus de germination, en comparaison à la situation de contrôle 1 g. La condition de base est qu'un tel stress soit appliqué à des graines ayant séjourné en milieu humide. Dans le cas de graines soumises à l'hypergravité en milieu sec, l'effet de réduction de la germination n'est pas rencontré. Dans le cas de graines centrifugées humides, il est utile de remarquer que, pour les quatre enzymes traités, PK est assez indifférent au stress hypergravitationnel. En tenant compte du fait que PK est un enzyme typique de la phase soluble du cytoplasme (glycolysis), tandis que les enzymes plus sensibles, ICL et ICDH, sont situés au sein de cellules organelles, nous supposons que l'effet du stress hypergravitationnel est plus marqué sur les enzymes liés à des parties structurellement complexes des cellules, telles que les organelles d'une certaine taille.
Enfin, il sera intéressant d'étudier si l'effet observé reste constant dans le temps ou s'il disparaı̂t au fur et à mesure que l'embryon grandit et, par la suite, donne naissance à des plantes plus petites.
Le fait que l'effet de l'hypergravité disparaisse si on applique cette dernière à des graines traitées en milieu humide pourrait expliquer le fait que le maintien de la graine dans ce genre d'ambiance pendant 64 h provoque certainement une imbibition des tissus qui amènera les cellules à renouveler leur activité vitale, abandonnant la phase de quiescence, typique de l'état de graine. Le facteur « imbibition » et le renouvellement d'activité du métabolisme cellulaire pourraient rendre les cellules plus sensibles à l'état de stress. L'effet calculé semble appartenir plutôt au genre quantitatif que qualitatif, dans le sens où les niveaux d'activité des enzymes testés sont plus bas que ceux du contrôle, bien qu'il soit impossible de remarquer la moindre différence dans leur schéma général en ce qui concerne la durée de la germination (le pic de l'activité se situe toujours au 14e jour de germination). Cela pourrait nous amener à interpréter le phénomène comme un effet tendant à endommager les cellules de l'endosperme de la graine, plutôt qu'à en provoquer un fonctionnement différent. Un certain nombre de cellules endommagées amènerait à abaisser le niveau d'activité, mais celles qui resteraient vitales continueraient à fonctionner suivant un sous-schéma métabolique normal. En réalité, cette question pourrait faire l'objet d'investigations ultérieures. Étant donné que cet effet est accentué par certains enzymes, tels que ICL et ICDH, qui sont situés dans des organelles cellulaires (respectivement des glyoxysomes et des mitochondries), plutôt que par GPDH et PK, qui ont été trouvés dans la phase cytoplasmique, on pourrait supposer qu'en réalité le dommage causé par le stress hypergravitationnel ne s'adresse pas, en premier lieu et surtout, à l'ensemble de la cellule, mais plutôt à sa structure sous-cellulaire.
1 Introduction
One environmental factor that has remained unchanged throughout evolution is the force of the Earth's gravitational field. From a biological point of view, it is apparent how this factor has influenced many aspects of living systems. For this reason, many studies were carried out on the effects of both regular and altered gravity on various systems. Yet, these studies seem to be limited to the qualitative aspect of the problem, since they record changes in the behaviour and the biological structure of systems due to the presence or the alteration of gravity.
Studies in a microgravity environment suggest that microgravity conditions affect some biological–biochemical functions. The data are restricted to the complex biological system level: inhibition/stimulation of cell division and growth, increase in the biosynthetic power, decrease in the carbohydrate metabolism, and resistance to antibiotics may be cited as examples of the effects observed [1,2]. Given the complex structure and organization of the living cell, such observations could probably be the result of the interactions between the effect of microgravity (probably different, depending on the separate mechanism and compartment) as well as the control and regulation systems of the cell itself.
Plant biology is a life science area, which is well suited to Space Research. A number of studies have been carried out on the germination of higher plant seeds. An evident role played by gravity has been demonstrated on the orientation of the shoot and root. Although gross morphological differences between plants grown in microgravity and those grown on Earth (at least over a relatively short period) do not seem to exist, a more detailed examination shows that there is a substantial disturbance of metabolic processes at the cellular and subcellular levels [3].
The duration of lipid-rich seed germination is longer than that of amylaceous ones. In the germination of such seeds, the glyoxylate cycle plays a control role in that, through bypassing the two decarboxylative steps of the Krebs cycle, it allows the net synthesis of glucides from lipids [4].
Thus, the higher plant lipid-rich seeds are heavily dependent on two enzymes, which are unique to the glyoxylate cycle, isocitrate lyase and malate synthase, for germination. These enzymes are synthesized by the seed during germination and they are essential for the production of cell material as long as the plant is unable to perform photosynthesis [4,5]. In other words, these two enzymes determine the operation and control of the molecular events that lead to seed germination.
The activity of isocitrate lyase, the key enzyme in the glyoxylate cycle, indicates the state of lipid-rich seed germination: the stage of germination, the growth of the embryo, the activation and progress of protein synthesis, and the depletion of lipidic supplies [5–7].
For the past thirty years approximately, our studies have demonstrated that, during the germination of Pinus pinea seeds, the isocitrate lyase activity and exploitation of lipidic supplies peaked between the 11th and the 15th day, while the 13th and the 14th days were the more representative for marker-enzyme activity [8–20] (Fig. 1).
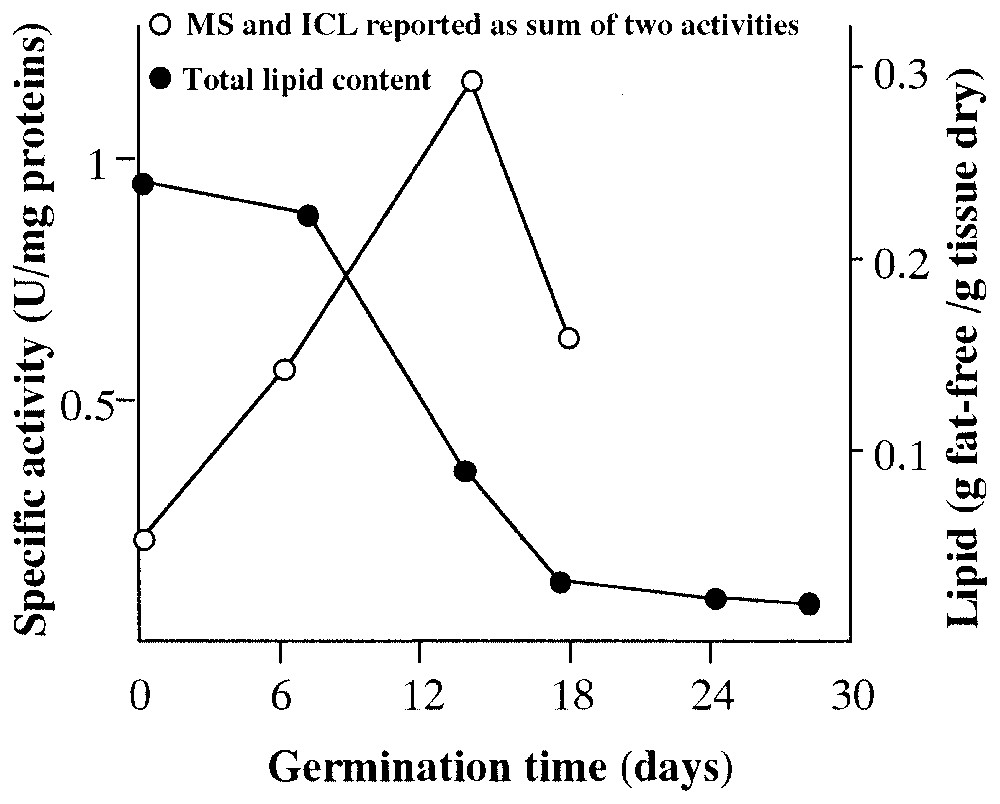
Comparison between level of malate synthase (MS) and isocitrate lyase (ICL) and total lipid content during germination of Pinus pinea seeds.
Based on our above knowledge, a study was undertaken to investigate whether or not a gravitational shock applied to seeds prior to the onset of germination produces morphological and molecular changes during the germination process itself.
In order to investigate the effects of gravity on seed germination, we carried out a study on the time pattern of Pinus pinea seed germination in which the seeds were subjected to a hyper-gravitational stress (1000 g for 64 h at 4 °C), either in a dry or in a wet environment, before being placed in germination plates. During the entire period of germination, we monitored the state of embryo growth, the level of the most representative enzymes of the main metabolic pathways (isocitrate lyase for the glyoxylate cycle, glucose-6-phosphate dehydrogenase for the shunt of pentose phosphates, isocitrate dehydrogenase for the Krebs cycle and pyruvate kinase for glycolyse). A histological study was carried out at time zero (T0) on control seeds as well as on seeds that had undergone centrifugation (wet and dry environment) prior to placing them in the germination plates.
2 Materials and methods
2.1 Hyper-gravitational stress
Gravitational stress was applied by applying centrifugal force to Pinus pinea seeds. The latter were of two types: (i) dry seeds, and (ii) wet seeds which were placed in a centrifugal tube at 1000 g for 64 h at 4 °C immersed in water. At the same time, Pinus pinea seeds were kept in the same conditions (4 °C, and in dry and wet conditions) at 1 g.
When the period of centrifugation was completed, both the controls and the wet and dry seeds, which had undergone centrifugation, were placed, in the dark, in the germination plates.
A total of 1.6 kg of Pinus pinea seeds were divided up into four lots which were treated as follows:
- – lot 1: 400 g of seeds kept immersed in water at 4 °C for 64 h (wet controls);
- – lot 2: 400 g of seeds kept dry at 4 °C for 64 h (dry controls);
- – lot 3: 400 g of seeds that had undergone centrifugation at 1000 g immersed in water at 4 °C for 64 h (wet centrifuged-seeds);
- – lot 4: 400 g of seeds that had undergone centrifugation at 1000 g in dry conditions at 4 °C for 64 h (dry-centrifuged seeds).
Lots 1 and 2 constitute the controls of lots 3 and 4, respectively.
2.2 Seed germination
The Pinus pinea seeds were placed in four different germination tray-containers (trays I–IV).
Each tray was sub-divided into four quadrants (quadrants A, B, C, D), each of which was, in turn, further sub-divided into eight sub-quadrants, as shown in Fig. 2.
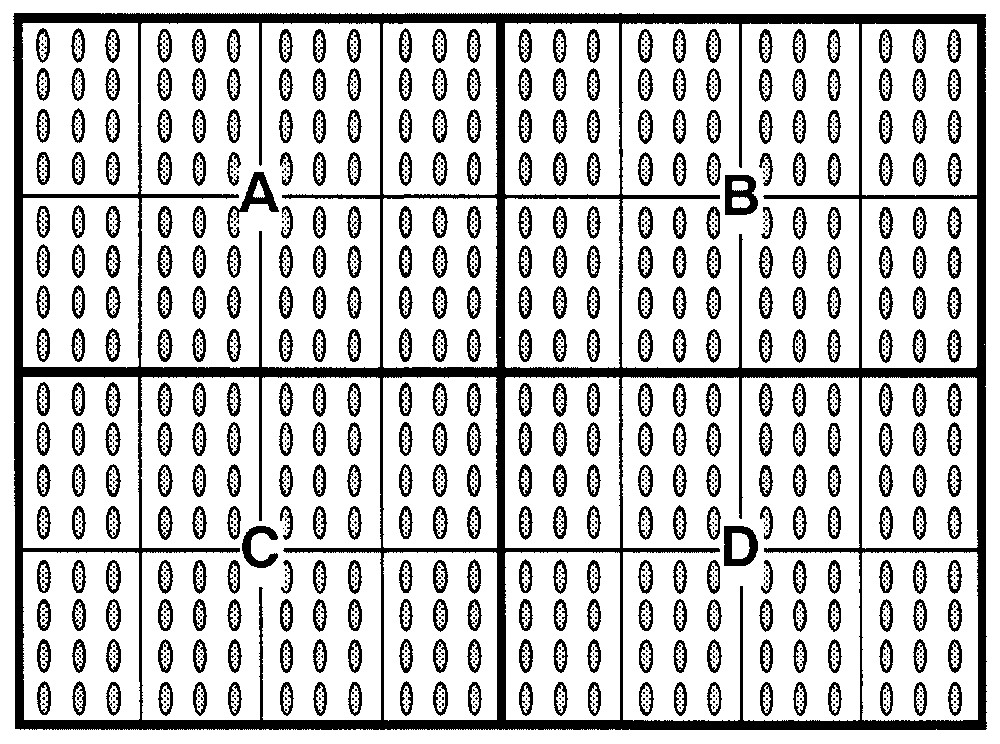
Disposition of Pinus pinea seeds in the germination tray-containers. Each tray was sub-divided into four quadrants (A, B, C, D), each of which was, in turn, further sub-divided into eight sub-quadrants. Twelve seeds were placed in each sub-quadrant to germinate.
In Quadrant A, 96 seeds – i.e. with a total of 12 seeds in each sub-quadrant (12×8) from lot 3 were placed to germinate.
In Quadrant B, 96 seeds – i.e. with a total of 12 seeds in each sub-quadrant (12×8) – from lot 1 were placed to germinate.
In Quadrant C, 96 seeds – i.e. with a total of 12 seeds in each sub-quadrant (12×8) – from lot 4 were placed to germinate.
In Quadrant D, 96 seeds – i.e. with a total of 12 seeds in each sub-quadrant (12×8) – from lot 2 were placed to germinate.
Germination took place in the dark, at 20 °C. The criterion applied to germination is the sprouting of the radicle.
3 Histological preparations
Part of the specimens (endosperm) were fixed in 2.5% glutaraldehyde and 2% formaldehyde in 0.1 M cacodylate buffer, pH 7.4, followed by 1% OsO4 in 0.1 M phosphate buffer, pH 7.4, and embedded in EPON 812. Sections 1-μm thick were stained with alkaline toluidine blue, and observed by light microscopy. About 70 nm thick sections were stained with uranyl acetate followed by lead citrate, and observed using an electron microscope at 80 kV (Joule 1010).
3.1 Preparation of extracts
Trays I and II were used for the sampling, at different time intervals, of seeds to be homogenized.
Trays III and IV were used to photograph the seeds at different time intervals during the germination process.
At previously determined times, seeds were removed from one of the sub-quadrants (12 seeds) of each of the quadrants A, B, C, and D of trays I and II. From said seeds, the endosperm was removed and homogenized using ultraturrax in 150 mM triethanolamine, pH 7.5, 9 mM MgCl2, 1.5 mM EDTA in ratio 1:2 (w/v). The homogenate was filtered using a gauze, then centrifuged (10 000 g, 3 min), and the supernatant was filtered using paper. The enzymatic activities and the total protein content of the filtered supernatant were determined.
At the same time, photographs of trays III and IV were taken so as to obtain an overall picture of the germination at a morphological level.
3.2 Enzymatic assays
3.2.1 Isocitrate lyase (EC 4.1.3.1) (ICL)
Isocitrate lyase activity was determined by the method of Dixon and Kornberg [21], as described in [15].
The assay mixture contained in 1 ml final volume consisted of 80 mM Hepes (pH 7.0), 6 mM MgCl2, 4 mM phenylhydrazine. The reaction was started by adding substrate (4 mM D,L-isocitrate).
Glyoxylate-phenylhydrazone (ε=17 mM−1 cm−1) formation was followed at 30 °C at 324 nm, using an UV-2100 spectrophotometer (Shimadzu, Columbia, MD).
3.2.2 Isocitrate dehydrogenase (NADP) (EC 1.1.1.42) (ICDH)
ICDH (NADP-dependent) activity was determined at 30 °C according to Bernt and Bergmeyer [22], with slight modifications, continuously following the formation of NADPH at 340 nm, using an UV-2100 spectrophotometer (Shimadzu, Columbia, MD). The assay mixture, contained in 1 ml final volume, consisted of 50 mM triethanolamine (pH 7.6), 8 mM MgSO4, 5 mM EDTA, 0.4 mM NADP.
The reaction was started by adding substrate (4 mM D,L-isocitrate).
3.2.3 Glucose6-phosphate dehydrogenase (EC 1.1.1.49) (G6PDH)
G6PDH activity was determined at 30 °C according to Lohr and Waller [23], with slight modifications, continuously following the formation of NADPH at 340 nm, using an UV-2100 spectrophotometer (Shimadzu, Columbia, MD). The assay mixture contained in 1 ml final volume consisted of 50 mM Tris-HCL (pH 7.5), 6 mM MgCl2, 0.4 mM NADP.
The reaction was started by adding substrate (3.3 mM glucose6-phosphate).
3.2.4 Pyruvate kinase (EC 2.7.1.40) (PK)
PK activity was determined at 30 °C according to Hess and Wieker [24], with slight modifications, continuously following the NADPH oxidation at 340 nm, using an UV-2100 spectrophotometer (Shimadzu, Columbia, MD). The assay mixture contained in 1 ml final volume consisted of 50 mM triethanolamine (pH 7.6), 8 mM MgSO4, 5 mM EDTA, 75 mM KCl, 1.5 mM ADP, 0.15 mM NADH, 60 U ml−1 lactate dehydrogenase.
The reaction was started by adding substrate (0.8 mM phosphoenolpyruvate).
The value of 6.22 mM−1 cm−1 is considered to be the NADH (or NADPH) molar extinction coefficient.
One unit of activity is defined as that quantity of enzyme, which transforms 1 μmol of substrate in 1 min, at 30 °C.
3.3 Determination of total protein content
The content of total proteins was determined according to Bradford [25], using a kit produced by BIO-RAD.
3.3.1 Statistical analysis
Two-way Variance Analysis was applied to determine whether the curves obtained of the enzymatic activity were to be considered as being significantly different or not in the seeds centrifuged in a wet environment and in those centrifuged in a dry one, when compared with controls. The results of the investigation are summarized briefly hereafter.
3.3.1.1 Enzymatic activity of Pinus pinea seeds centrifuged in a dry environment compared to controls
ICL: the two curves obtained are not significantly different: P=0.8393.
G6PDH: idem as above: P=0.6725.
ICDH: the two curves are overlaid until day 12, while from day 14, there is a very high activity peak in the centrifuged seeds. Nonetheless, overall, the results are not significantly different: P=0.3033.
PK: the two curves intersect several times and the analysis of the results indicates that they are not significantly different P=0.4057.
3.3.1.2 Enzymatic activity of Pinus pinea seeds centrifuged in a wet environment compared to controls
ICL: The two curves obtained are significantly different: P=0.0002.
G6PDH: The results remain significantly different: P=0.0003.
ICDH: Both curves are significantly different P=0.0001.
PK: the difference in the results obtained over the different time-scale is not always constant, but with statistical analysis, once again both curves are different at the limit of significance P=0.0033.
4 Results
The Pinus pinea seed is an extremely resistant one, which maintains even for years remarkable possibilities for good germination. Its ligneous shell, which is quite hard, is an excellent environment, which is well able to protect it from external attacks. Germination is a lengthy process (about 20 days). We have closely studied this also with regard to the effect of detergents and blockers of protein synthesis. In brief, the pine nut is a coriaceous seed, which withstands factors of perturbation well during its germination process [9–20].
The results obtained are given in the figures and photographs.
Fig. 3 shows the effect of the centrifugation on wet seeds (kept in a wet environment for 64 h at 4 °C before germinating). The photographs of the plates refer to four different germination times (7, 9, 12, and 14 days). It is obvious how the controls germinate in greater number and, calculating the number of seeds germinated per plate, one can say that the centrifuged seeds on average germinate only 20% when compared to controls. This difference in germination is not found for dry seeds (kept in a dry environment for 64 h at 4 °C before germinating).
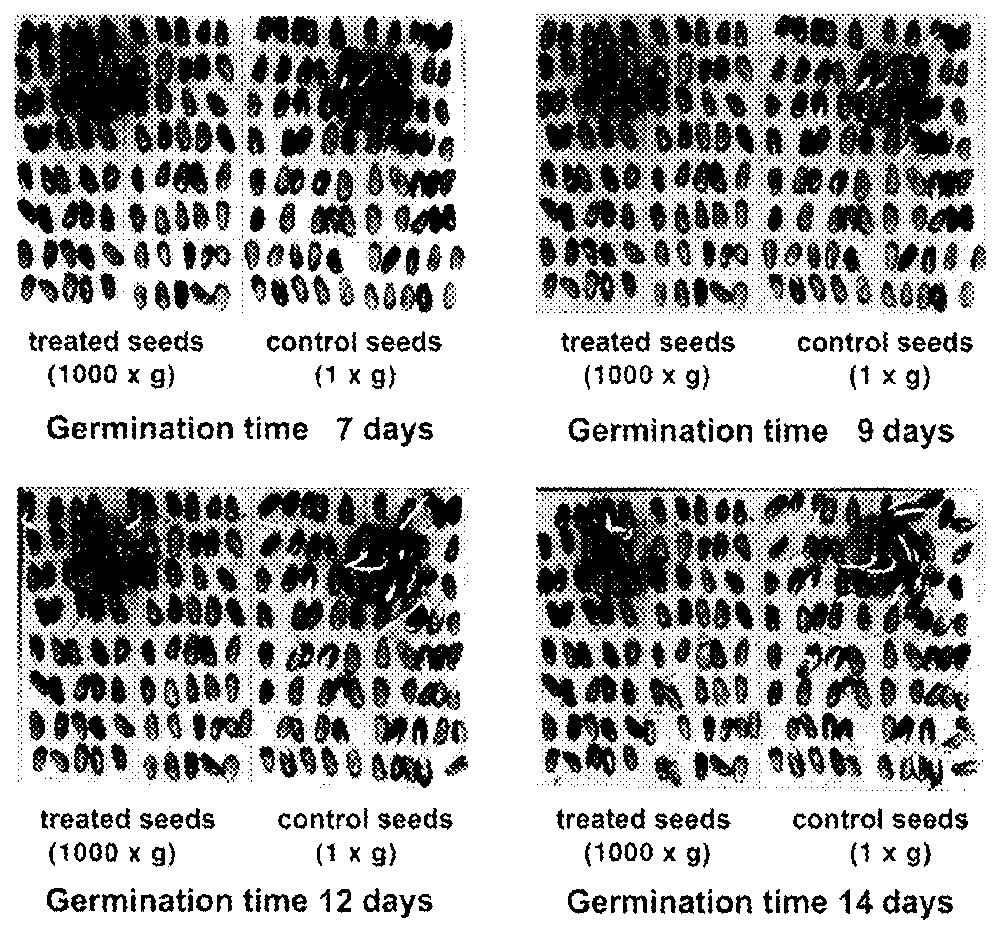
Pinus pinea seeds at different days of germination.
Fig. 4 shows the levels of activity of isocitrate lyase (ICL) in wet (Fig. 4A) and dry (Fig. 4B) seeds at different stages of germination. A marked reduction in isocitrate lyase activity in the wet centrifuged seeds is obvious, while the dry centrifuged seeds seem to show a pattern that almost overlaps that of controls, with the modest exception of the point of maximum activity on the 14th day of germination, in which there seems to be greater activity in the dry centrifuged seeds than in controls.
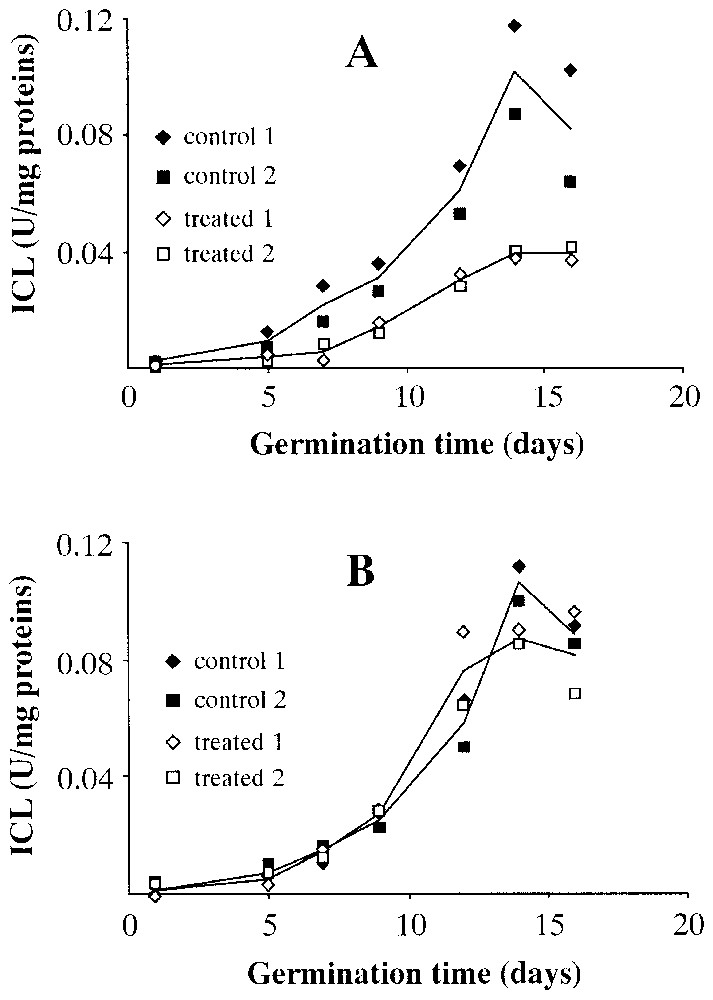
Levels of isocitrate lyase (ICL) activity during Pinus pinea seeds germination. A: Seeds treated in wet environment. B: Seeds treated in dry environment.
Fig. 5 shows the pattern related to the isocitrate dehydrogenase (ICDH) activity in wet (Fig. 5A) and dry (Fig. 5B) seeds. As regards the wet seeds, the levels of enzymatic activity are lower in the centrifuged ones than in controls, whereas as regards dry seeds, what has already been seen in the case of isocitrate lyase is repeated. In other words, the activity curves overlap; they can be fitted with the exception of the point relative to day 14 of germination, in which the centrifuged seeds have a higher activity when compared to the controls.

Levels of isocitrate dehydrogenase (ICDH) activity during Pinus pinea seeds' germination. A: Seeds treated in wet environment. B: Seeds treated in dry environment.
Fig. 6 shows the levels of glucose6-phosphate dehydrogenase (G6PDH) activity in wet (Fig. 6A) and dry (Fig. 6B) seeds. The enzymatic activity measured in the wet centrifuged seeds is lower than that of the controls, while the effect is non-existent in dry centrifuged seeds; indeed, one can say that the pattern almost overlaps that of the controls.
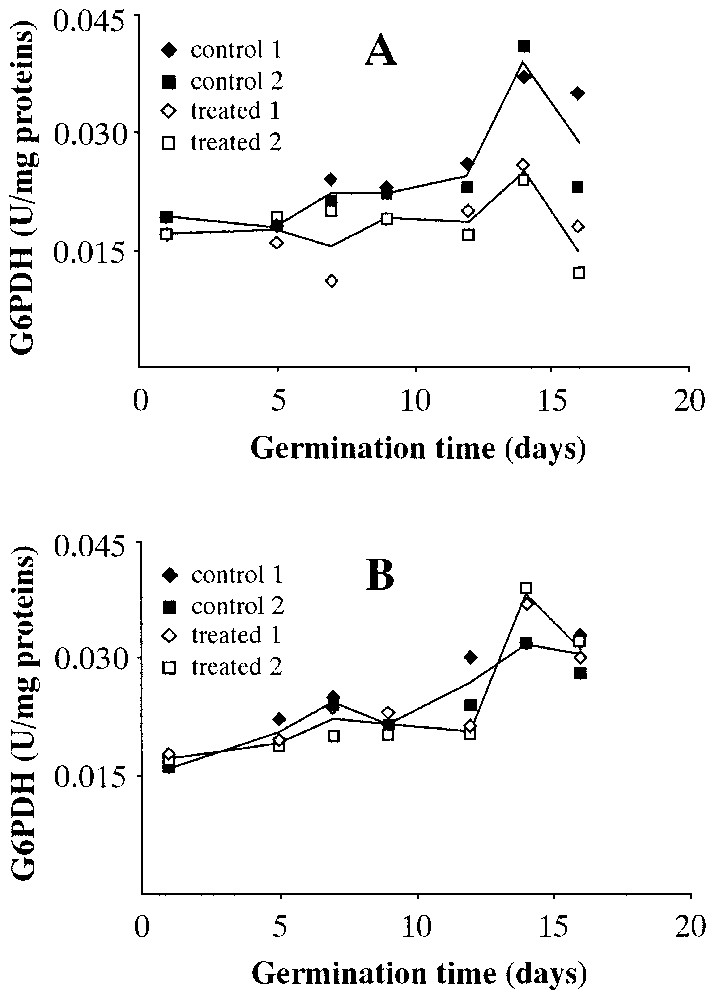
Levels of glucose6-P-dehydrogenase (G6PDH) activity during Pinus pinea seeds germination. A: Seeds treated in wet environment. B: Seeds treated in dry environment.
In Fig. 7, the activity levels of pyruvate kinase (PK) in wet (Fig. 7A) and dry (Fig. 7B) seeds are given. In this case, the pattern of the curve relative to both the wet and dry centrifuged seeds seems to be quite similar to that of the controls. Pyruvate kinase in wet centrifuged seeds shows levels of activity that are only slightly lower than controls, while it can be stated that, in dry centrifuged seeds, the levels almost completely overlap those of the controls, even if there is a greater variability in the maximum activity results.

Levels of pyruvate kinase (PK) activity during Pinus pinea seeds germination. A: Seeds treated in wet environment. B: Seeds treated in dry environment.
Fig. 8 presents the content of total proteins in both wet (Fig. 8A) and dry (Fig. 8B) seeds. This concentration is expressed in mg of proteins per g of fresh tissue. In both cases, there seems to be no obvious difference between wet and dry centrifuged seeds and controls.
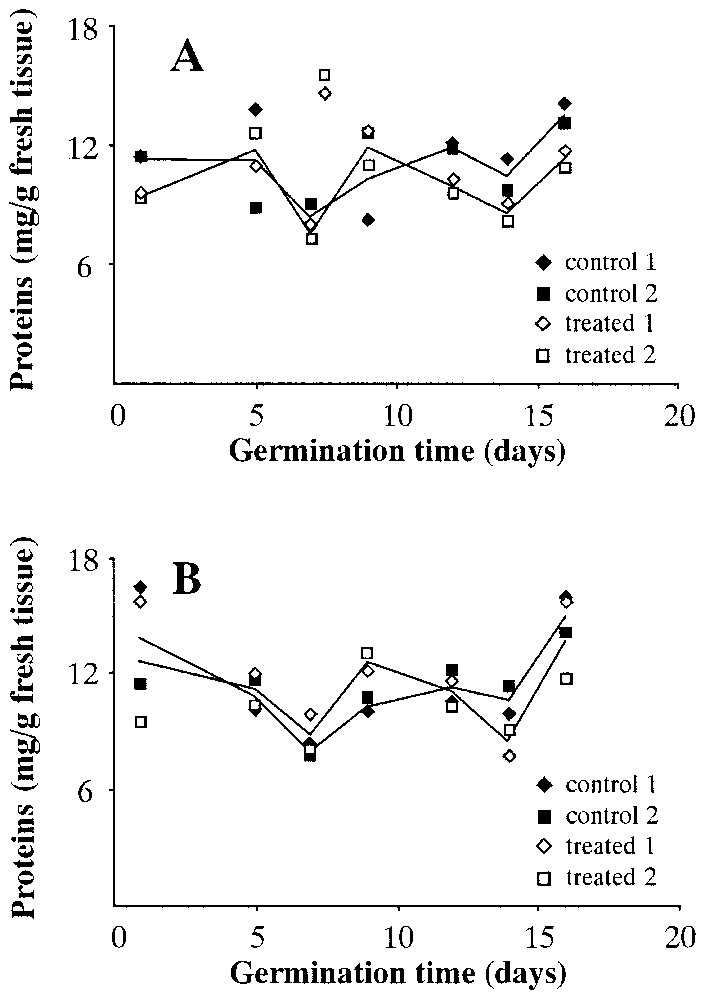
Total protein content during Pinus pinea seeds germination. A: Seeds treated in wet environment. B: Seeds treated in dry environment.
Fig. 9 presents a comparative study, using the electron microscope, of wet and dry centrifuged seeds prior to germination (time zero). It seems possible to state that the micro-photographs obtained do not show particular differences between seeds centrifuged in a dry environment (Fig. 9B) and their respective controls (Fig. 9A), whereas in the micro-photographs of the seeds centrifuged in a wet environment (Fig. 9D), noticeable markings that could be interpreted as lipidic reserves' degradation due to applied gravitational stress can be seen.
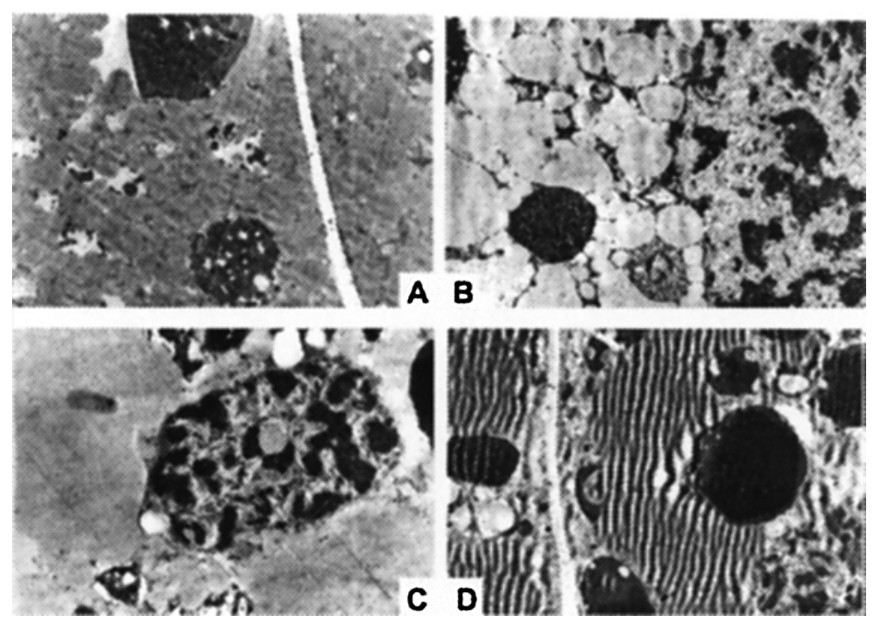
Morphologic effects of gravitational stress (1000 g for 64 h at 4 °C) on the endosperm of Pinus pinea seeds analyzed by electron microscopy. Control and treated seeds in dry (A, B: and wet environment (C, D . Note in the microphotograph D the folding of lipidic reserves due to gravitational stress.
5 Discussion
From the data obtained, it seems that a period of 64 h of centrifugation at 1000 g prior to placing the seeds in the germination trays has produced different results depending on whether or not the seeds have been centrifuged in a wet or dry environment. In the former, compared to controls, one witnesses a marked reduction in the number of seeds that germinate. At a molecular level, the activity of the key germination enzyme in seeds with lipid reserves (ICL) is reduced to less than 50%. The same occurs, even if less obviously, in ICDH (Krebs Cycle) and in G6PDH (pentose-phosphate shunt). PK seems to be less sensitive to gravitational stress.
It is interesting to note that the effect of hyper-gravity does not reduce the enzymatic activities measured in dry centrifuged seeds; indeed, with reference to the day of germination of maximum enzymatic activity, one can perhaps speak of a slight increase in enzymatic levels for ICDH. The activity levels of G6PDH and PK almost overlap.
The electron microscope photographs have highlighted noticeable markings in the microscopic preparations obtained from seeds centrifuged in a wet environment.
Given the above, it seems possible to conclude that the application of a gravitational stress on the seeds in a phase that precedes germination creates a macroscopic and molecular perturbation in the germination process, when compared to the control situation 1 g. The basic condition is that such a stress be applied to seeds that are in a wet environment. In the case of seeds subjected to hypergravity in a dry environment, the effect of the reduction in germination does not exist. In the context of wet centrifuged seeds, it is worth noting that of the four enzymes studied, PK is slightly unaffected by hypergravitational stress. Considering the fact that PK is a typical enzyme of the soluble phase of the cytoplasm (glycolysis), while the two most sensitive enzymes, ICL and ICDH, are situated in cellular organelles, we suggest that the effect of hyper-gravitational stress is greater on enzymes that are linked to structurally complex parts of the cell such as organelles of a certain size.
Lastly, it will be interesting to study whether the effect observed remains constant in time or disappears with the growth of the embryo and, later, of the plant. A long-term effect may, at a macroscopic level, lead to plants, which are reduced in size.
The fact that the effect of hypergravity is felt when applied to seeds treated in a wet environment could be explained by the fact that the 64 h of permanence by the seed in such an environment certainly provoke an imbibition of the tissues, which leads the cells to renew their vital activity, abandoning the quiescent phase which is typical of the seed-state. The imbibition factor and the renewed activity of the cellular metabolism could make the cells more sensitive to the stress state. The effect measured seems to be more of a quantitative than qualitative type, in the sense that the activity levels of the enzymes tested are lower than those of the controls, but one does not notice any differences in their overall pattern as far as the time of germination is concerned (the maximum activity peaks are always on the 14th day of germination and are not shifted). This could lead to the phenomenon being interpreted as an effect aimed at damaging the cells of the endosperm of the seed, rather than at their different way of functioning. A certain quantity of damaged cells would lead to a lowering of activity levels, but those which remain vital would continue to function according to a normal metabolic pattern.
In reality, the question could even be further analysed. Given that the effect is felt to a greater extent by enzymes such as ICL and ICDH, which are enzymes that are situated in cellular organelles (glyoxysomes and mitochondria, respectively), compared to G6PDH and PK, which are found in the cytoplasmic phase, one could suppose that in reality the damage provoked by the hyper-gravitational stress is not, first and foremost, to the overall cell, but rather, to its sub-cellular structures.
Acknowledgements
The authors wish to thank the following for the financial support provided for this work: ASI (Agenzia Spaziale Italiana), ESA (European Space Agency), Ente Cassa di Risparmio di Firenze and Banca Toscana. Special thanks are due to Prof. Proto Pippia for his constant support to our work. In addition, we wish to express our thanks to Prof. Paolo Romagnoli and Mr. Daniele Guasti for the Histological Preparations.



