1 Introduction
Soil is a dense, opaque and complex medium that presents special challenges for ecologists. There has been relative neglect of soil ecosystems, ‘ecology's subterranean blind spot’ [1], and particularly of the role of vertebrate predators. Many non-mammalian subterranean predators are elongate and have reduced or absent limbs, a condition that has evolved independently in several lineages of fossorial amphibians and reptiles. Studies of diet of such predators are rare, but recent publications concerning some representatives have suggested specialist strategies toward abundant soil fauna [2–4], particularly the social insects that can occur locally in very large quantities.
In a recent review, O'Reilly [5] highlighted the dearth of information about diet in the largely subterranean caecilian amphibians (Order Gymnophiona) compared to other tetrapods. Indeed, nothing is known of any aspect of ecology of the vast majority of caecilian species, and even for those investigated there is only the sketchiest of natural history information. Most general texts [6,7] concur that the diet of terrestrial caecilians consists largely of earthworms (Oligochaeta) and termites (Isoptera), two of the three soil macrofauna groups, which, with ants (Hymenoptera: Formicidae), are considered to be soil-ecosystem engineers (SEE) [8]. Most previous references to caecilian diet occur in publications addressing other aspects of caecilian biology [7,9–12]. Some of the more detailed considerations of caecilian diet have concentrated on species that have been occasionally collected in relatively large numbers [13,14]. For terrestrial caecilians, reports of diet often dwell on isolated or unusual observations, rather than data from approaches that might gain a fuller understanding of overall diet. For example, some authors have reported the occasional occurrence of vertebrate prey items in caecilian diets, even though this is seemingly infrequent and possibly unusual (see [15] and references therein). Published observations largely point to a generalist and opportunistic pattern of predation for some caecilian species; something they share with many members of the other amphibian orders, Anura and Caudata [6,16]. However, there are also suggestions of dietary specialisation in some terrestrial species [13] and genera [5], as well as claims that at least one species may be partially detritivorous [13].
In the very few species of caecilians for which natural dietary items have been precisely reported, these were recorded from dissections of preserved specimens from mostly opportunistic collections. Most studies of caecilian ecology have paid little attention to sampling regimes, and the few that have explicitly described collection methods have sometimes assumed sample randomness based on attempts to collect all sizes of caecilian from all habitat types within a study site (e.g., [17]). From our broad collective fieldwork experience with caecilians, we disagree with this assumption because non-randomised collections of caecilians may be biased toward most probable and accessible areas such as under rotting logs, in soft loose soil, and adjacent to streams. Given the lack of current knowledge on caecilian ecology, dietary studies based on opportunistic collections are undoubtedly useful. However, unbiased sampling is an important factor in the design of ecological investigations [18]. To date, data on diet of caecilians using randomised sampling methods is completely absent from the literature.
Gegeneophis ramaswamii Taylor is a direct-developing, oviparous caecilian from southern India [19,20]. Various recent studies have promoted use of this species as a model for investigating caecilian ecology [20–23]; they have also allowed a tentative profile of its natural history to be established, such that this and other ecological studies may generate preliminary data and test basic hypotheses. The only previously published information on diet in this species indicates that G. ramaswamii occasionally takes relatively rare scolecophidian snakes as prey [15], suggesting that this species is perhaps an opportunist predator within its soil environment. Oommen et al. [20] found G. ramaswamii to be abundant in a broad range of agricultural environments (from near-coastal lowland to hilly regions of several hundred metres in altitude), highlighting its apparently readily adaptable nature. High densities (up to 1.87 individuals per m2 per survey) have been found in some surveys, but low density patches also occur within even highly populated localities [23]. One hypothesis to explain this is that it is caused by a patchy distribution of possibly preferred prey items. Densities from surveys at the beginning and mid-monsoon were found to vary greatly at some localities (e.g., from 0.27 to 1.87, [23]) while remaining relatively stable at others, and it is possible that this is also linked with prey availability. Personal observations, anecdotal reports from local people, and inferences from morphology affirm that G. ramaswamii are dedicated subterranean organisms that very rarely venture above ground. This suggests that these carnivores have the potential to significantly affect the population dynamics of their probable SEE prey, and Oommen et al. [20] and Measey et al. [23] have stressed that this is particularly the case at the very high densities in which they sometimes occur.
Here we use data obtained from dissections of animals collected in the randomised surveys reported by Measey et al. [23] to test several hypotheses that stem from a consideration of our previous studies. We test the hypotheses that G. ramaswamii is a dietary generalist (i.e., a species with a broad niche), that prey are exclusively of a subterranean origin, and that diet is dominated by SEE. We also test the hypothesis that dietary composition may, as might be expected, vary among sites and/or sampling times in relation to spatial and temporal heterogeneity of prey species. We further use these samples to test more exploratory hypotheses that may be informative about G. ramaswamii natural history, such as that there is no difference in diet (size, number and type of prey) between sexes or life history ‘stages’.
2 Methods
Detailed information on localities, sites, and collection methods are presented by Measey et al. [23]. All localities surveyed are in the southernmost part of the Western Ghats, Kerala, India. Although originally covered in forest, much of this area is now under cultivation. The climate is monsoonal, and can be divided into a wet monsoon season (June to November) and a drier season (December to May). G. ramaswamii was collected from three localities using a simple and repeatable survey method by digging randomised 1-m2 quadrats to investigate density of this species. Specimens were collected in surveys conducted in early (end of June and early July 2000) and mid-monsoon (August 2000) periods.
Three sites were surveyed. The site at the locality of Bonaccord, a tea (Camellia sinensis (L.) Kuntze) plantation, was a flat, largely grassy clearing at the bottom of tea-planted slopes. A small stream coursed through the site, and this connected to a loose grid of drainage ditches. Makki is dominated by rubber (Hevea brasiliensis (A. Juss.) Müll.-Arg.) cultivation, and although only 4.5 km South of, is 350 m below Bonaccord, separated, at least in part, by naturally forested steep slopes. The survey site was on flat ground with regularly planted rubber trees providing near-total shade, and there was a grid of drainage ditches. Finally, the site at the locality near Punalur was a flat area in a low altitude rubber plantation, at the bottom of, and between, two terraced slopes and crossed by a regular grid of drainage ditches.
Animals used in this study were euthanised (using the anaesthetic MS 222) and fixed (with c. 4% formalin) within two hours of capture, and later stored in 70% ethanol in the collection of the Zoology Department of the University of Kerala. They were dissected in the laboratory under a stereo-zoom microscope. The body cavity was opened with a midventral incision, and the alimentary canal removed from immediately posterior to the heart to the anterior of the cloaca. The alimentary canal (hereafter gut) was weighed to the nearest 0.0001 g. The gut was emptied and reweighed. Gut contents were identified to a taxonomic level that was dependant on state of digestion.
2.1 Data analysis
Initial investigation of total mass-length data for each specimen (not reported here) revealed three discernible groups of animals: first, the smallest and clearly juvenile animals of less than 90-mm total length, second, intermediate sized animals (approximately 90 to 170 mm) that could be sexed (by examination of gonads) that are here termed ‘subadult’, and finally, large (>170 mm) animals termed ‘adults’. Analyses on ontogeny of diet were carried out using these three groups.
Mass of gut contents used in analyses were calculated from the mass of the empty digestive tract subtracted from the entire gut mass. Total gut-content mass was calculated from the sum of all individually weighed food items, i.e., not including detritus. Values given as means (x̄) are ±SD.
Two-tailed () t-tests were used to test for differences between groups of different sexes, ontogeny and sampling times. Diet features were analysed using taxonomic richness (number of taxa identified per gut), and Simpson's diversity index (S); computed using proportions p for each gut i and prey j, as S=1−∑j=1ppj/i2, which is scaled from 0 (no diversity) to 1.
Although a specialist predator is typified by individuals in a population with a narrow niche, a population of generalist predators may consist of individuals with wide or narrow niches, or both [24]: intra- and inter-individual diet variation, respectively. To take account of this, a multivariate analysis specifically designed for analysis of stomach contents (in fish) was used [25] in combination with the software ADE-4 [26]. Individuals without identifiable gut contents were not used in this analysis. Discriminant analyses (between-group eigenanalysis) were carried out on the results of the multivariate analysis, for differences between sexes, ontogenic stages, sites and sampling times, and tested by random permutation tests (9999 runs [26]).
3 Results
A total of 70 Gegeneophis ramaswamii were collected [23] and the numbers found at each site are shown in Table 1. Thirteen percent of G. ramaswamii were damaged during collection [23]. Most of the damaged animals were dissected and data collected without loss, and only three had to be excluded from the analyses.
Numbers of animals captured in surveys (with numbers containing no recognisable food items in guts, excluding nematodes, in parentheses) for juvenile, subadult (TL<170 mm) and adult Gegeneophis ramaswamii. ∗ indicates one damaged animal known to occur in the group but not included in these data or in analyses: see methods. T, total, J, juvenile, s-a, subadult, M, male, F, female
| Locality | Beginning of monsoon | Mid-monsoon | Total | ||||||||||
| T | J | s-a (M) | s-a (F) | M | F | T | J | s-a (M) | s-a (F) | M | F | ||
| Bonaccord | 13 (8)∗ | 0 | 1 (1) | 3 (2) | 3 (1) | 6 (4) | 15 (1) | 0 | 4 (1) | 1 (0) | 5 (0) | 5 (0) | 28 (9) |
| Punalur | 4 (2) | 1 (1) | 0 | 1 (1) | 1 (0) | 1 (0) | 27 (7) | 3 (0) | 12 (2) | 8 (3) | 3 (2) | 1 (0)∗ | 31 (9) |
| Makki | 4 (1) | 0 | 1 (1) | 0 (0)∗ | 1 (0) | 2 (0) | 4 (0) | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 0 | 8 (1) |
| Total | 21 (11) | 1 (1) | 2 (2) | 4 (3) | 5 (1) | 9 (4) | 46 (8) | 4 (0) | 17 (3) | 10 (3) | 9 (2) | 6 (0) | 67 (19) |
Parasitic nematodes (Cosmocercidae) were found in animals from both the early (50% occurrence) and mid- (40%) monsoon samples from Bonaccord, and from the mid-monsoon sample taken at Makki (2 of 4 specimens). None was found in guts of G. ramaswamii collected at Punalur. For the Bonaccord samples, nematodes were found in both sexes, and across all sizes of individuals. Occurrences were from 1 to 11 nematodes, with higher frequencies occurring in both smaller (118 mm) and larger (239 mm) individuals. Nematodes were most often found in the posterior gut, in association with aggregations of food items and other particles. Nematodes have been reported in other terrestrial caecilian species [27], but seemingly not previously for G. ramaswamii. Nematodes were not included in any of the statistical analyses of diet.
Guts devoid of recognisable prey represented 28% of all animals analysed for diet, 52% in the early monsoon sample and 17% in the mid-monsoon sample (Table 1). Ranks of dietary items by frequency show that SEE have the highest total frequencies with soil dwelling social insects (termites and ants) ranking higher than earthworms. SEE account for 92% of all gut content items. All items found in guts were of terrestrial origin, all being largely soil dwelling (see Discussion). The most numerous dietary items were workers of a termite (Odontotermes sp.), with other social insects generally ranking high (Table 2). Worker termites consistently outnumber soldier casts, as do ant brood to adult ants. Ranks by mass again show a dominance of SEE (84% of total), but with earthworms dominating, followed by termite workers (Odontotermes sp.) and ant brood (Table 3). In comparison to social insects, individuals of dietary items, such as earthworms and beetles (Coleoptera), often occurred singly in the dissected guts and were generally of a greater individual mass. Overall total taxonomic richness is 18 morphospecies with a Simpson index of 0.57 (Table 4).
Taxa found in the guts of Gegeneophis ramaswamii collected from three sites in southern India. Prey items are ordered by total frequency of occurrence. Ants (Formicidae) are lumped together but include mostly workers and brood of Pachycondola sp., but also workers of Tetramorium bicarinatum and T. smithi, and minor workers of at least two species of Pheidole. Soil ecosystem engineers (SEE) are shown in bold
| Sex | Locality | Total | |||||||||||||
| juveniles | subadult | adult | early monsoon | mid-monsoon | |||||||||||
| females | males | females | males | Bonaccord | Makki | Punalur | Bonaccord | Makki | Punalur | ||||||
| n | 5 | 15 | 20 | 15 | 12 | 13 | 4 | 4 | 15 | 4 | 27 | ||||
| Odontotermes | workers | 2 | 88 | 106 | 147 | 218 | 6 | 4 | 0 | 383 | 11 | 157 | 561 | ||
| Ant | brood | 0 | 0 | 5 | 100 | 273 | 0 | 0 | 0 | 373 | 0 | 5 | 378 | ||
| Nematodes | 0 | 17 | 23 | 14 | 14 | 39 | 0 | 0 | 23 | 6 | 0 | 68 | |||
| Odontotermes | soldiers | 3 | 10 | 26 | 9 | 14 | 0 | 0 | 0 | 16 | 2 | 44 | 62 | ||
| Oligochaeta | 1 | 0 | 6 | 8 | 5 | 3 | 3 | 2 | 5 | 2 | 5 | 20 | |||
| Ant | workers | 0 | 4 | 2 | 8 | 3 | 0 | 0 | 0 | 11 | 4 | 2 | 17 | ||
| Discuspiditermes | workers | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 8 | 0 | 0 | 8 | ||
| Coleoptera | adult | 0 | 0 | 2 | 3 | 3 | 1 | 0 | 0 | 5 | 0 | 2 | 8 | ||
| other | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | |||
| Discuspiditermes | soldiers | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | ||
| Hermiptera | larvae | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | ||
| Spider | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | |||
| Microstermes | worker | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Orthoptera | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |||
| Dermaptera | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |||
| Coleoptera | larvae | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Diptera | larvae | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | ||
| Total | 6 | 121 | 172 | 291 | 546 | 53 | 9 | 3 | 828 | 25 | 218 | 1136 | |||
| SEE | 6 | 103 | 145 | 272 | 523 | 10 | 7 | 2 | 798 | 19 | 213 | 1049 | |||
| Others excluding parasites | 0 | 1 | 4 | 5 | 9 | 4 | 2 | 1 | 7 | 0 | 5 | 19 |
Mass of dietary items in the guts of Gegeneophis ramaswamii collected from three sites in southern India. Prey items are ordered by their total mass. Missing values represent no items of that taxon found, while 0.00 signifies that the total mass was less than 0.005 g. Ants (Formicidae) are lumped together but include mostly workers and brood of Pachycondola sp., but also workers of Tetramorium bicarinatum and T. smithi, and minor workers of at least two species of Pheidole. Soil ecosystem engineers (SEE) are shown in bold
| Sex | Locality | Total | ||||||||||||
| juveniles | subadult | adult | early monsoon | mid-monsoon | ||||||||||
| females | males | females | males | Bonaccord | Makki | Punalur | Bonaccord | Makki | Punalur | |||||
| n | 5 | 15 | 20 | 15 | 12 | 13 | 4 | 4 | 15 | 4 | 27 | |||
| Oligochaeta | 0.01 | 0.16 | 0.46 | 1.14 | 0.15 | 0.18 | 0.01 | 1.29 | 0.03 | 0.11 | 1.77 | |||
| Odontotermes | workers | 0.00 | 0.05 | 0.09 | 0.14 | 0.34 | 0.00 | 0.00 | 0.51 | 0.00 | 0.10 | 0.62 | ||
| Ant | brood | 0.01 | 0.19 | 0.40 | 0.40 | 0.01 | 0.41 | |||||||
| Coleoptera | adult | 0.02 | 0.14 | 0.02 | 0.01 | 0.15 | 0.02 | 0.18 | ||||||
| Orthroptera | 0.17 | 0.17 | 0.17 | |||||||||||
| Dermaptera | 0.15 | 0.15 | 0.15 | |||||||||||
| Discuspiditermes | workers | 0.03 | 0.01 | 0.03 | 0.06 | 0.06 | ||||||||
| Odontotermes | soldiers | 0.00 | 0.01 | 0.02 | 0.01 | 0.02 | 0.03 | 0.00 | 0.03 | 0.05 | ||||
| Nematodes | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.00 | 0.03 | ||||||
| Dipteran | larvae | 0.02 | 0.02 | 0.02 | ||||||||||
| Spider | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |||||||||
| Ant | workers | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | |||||
| Discuspiditermes | soldiers | 0.01 | 0.01 | 0.01 | ||||||||||
| Hermiptera | larvae | 0.01 | 0.01 | 0.01 | ||||||||||
| Coleoptera | larvae | 0.01 | 0.01 | 0.01 | ||||||||||
| other | 0.00 | 0.01 | 0.01 | 0.01 | ||||||||||
| Microstermes | worker | 0.00 | 0.00 | 0.00 | ||||||||||
| Total | 0.01 | 0.11 | 0.34 | 0.95 | 2.29 | 0.18 | 0.19 | 0.02 | 2.79 | 0.04 | 0.30 | 3.51 | ||
| SEE | 0.01 | 0.09 | 0.29 | 0.79 | 1.94 | 0.15 | 0.18 | 0.01 | 2.31 | 0.04 | 0.25 | 2.94 | ||
| others | 0.00 | 0.02 | 0.05 | 0.16 | 0.35 | 0.02 | 0.01 | 0.01 | 0.49 | 0.00 | 0.05 | 0.58 |
Simpson Index and taxonomic richness for prey of Gegeneophis ramaswamii collected in Kerala, southern India. NB: Care should be taken when comparing Simpson Index values calculated from different numbers of individuals
| Sex | Locality | Total | ||||||||||
| juveniles | subadult | adult | early monsoon | mid-monsoon | ||||||||
| Taxonomic Richness | 2 | 10 | 13 | 7 | 15 | 18 | ||||||
| Simpson Diversity Index | 0.2778 | 0.1723 | 0.5786 | 0.7337 | 0.5601 | 0.5702 | ||||||
| females | males | females | males | Bonaccord | Makki | Punalur | Bonaccord | Makki | Punalur | |||
| Taxonomic Richness | 6 | 6 | 7 | 11 | 5 | 3 | 2 | 10 | 3 | 8 | ||
| Simpson Diversity Index | 0.1616 | 0.1797 | 0.5464 | 0.5971 | 0.7143 | 0.642 | 0.4444 | 0.5635 | 0.4765 | 0.1563 |
Eigenvalues of the first two axes of %PCA analysis (47.3% and 16.1% of the total variation, respectively), were sufficient to illustrate the main structure of diet composition (Fig. 1a). Four of the 19 prey taxa determined dominated the gut contents: termites (Odontotermes sp.), oligochaetes, coleopterans (adults) and ants (Pachycondyla sp.). The other prey taxa (less than 1% of the total gut contents) were found to cluster at the centre of the axis (Fig. 1b).

Biplot of prey items and individual Gegeneophis ramaswamii obtained from a %PCA for all data. (a) Histogram of eigenvalues, the first two values shown with solid bars. (b) Distribution of the contents of individual guts (blocks) on the first factorial plane (n values are given where blocks overlie each other), according to their prey items (arrows). Prey representing <1% of the total gut contents were omitted.
Subadults are, by the operational definitions employed here, smaller than adults. Correspondingly, the mass of gut contents of subadults is unsurprisingly less ( g) than in adults ( g). The null hypothesis that there is no difference in prey taxa between subadults and adults could not be rejected (but almost, P=0.0575) using discriminant analysis, although subadults had a considerably lower than average Simpson diversity index (Table 4). There is no indication that subadults feed on a greater frequency of smaller prey items compared to adults, because subadults were found to have significantly fewer numbers of prey items () than adults (; , P=0.042). Insufficient (five) juveniles were collected to test hypotheses about any possible earlier ontogenetic differences.
As a group, adult males were found to have a greater mean mass of total gut contents than adult females (0.141 g versus 0.106 g), although this difference is not significant (; P=0.400). Males and females had very similar Simpson diversity indices for prey items (0.5821 and 0.5520 respectively), but adult males had a higher taxonomic richness than adult females (Table 4). Although males had a higher mean number of prey items (males ; females ), this difference is also not significant (, P=0.652). Discriminant analysis failed to find significant differences in diet composition between males and females (P=0.3826). For all animals dissected, there is no consistent relationship between total mass and gut contents, although the upper bound for males (approximately equal to 10% of their total body mass) is slightly greater than for females (7% of total body mass, Fig. 2).
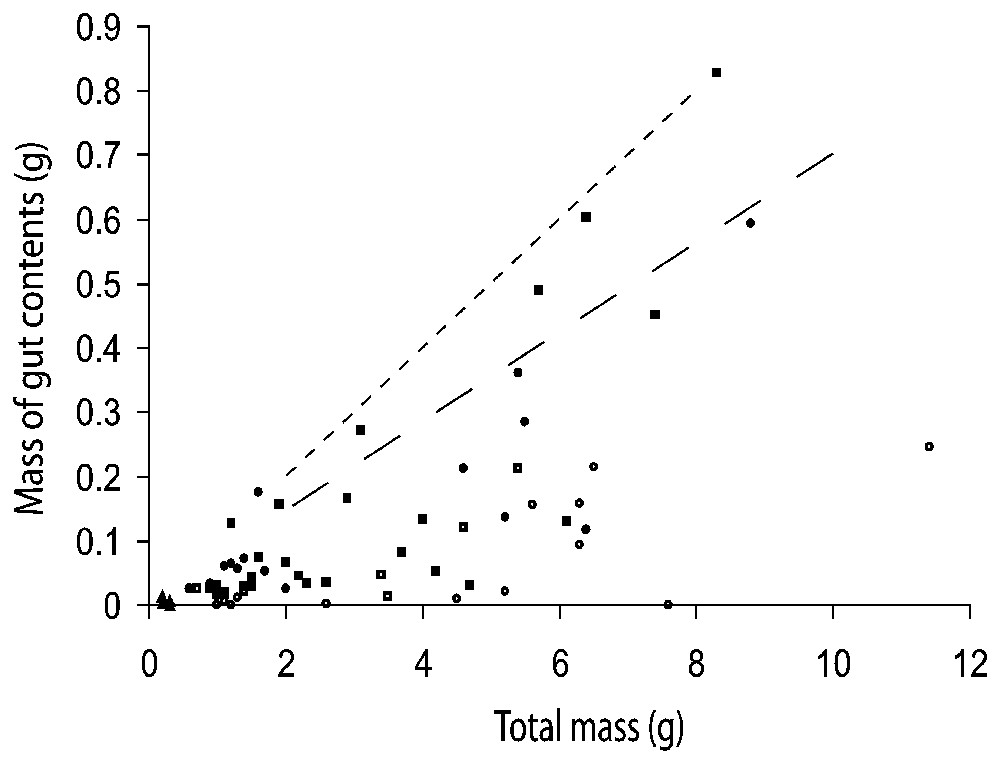
Scattergram of mass of gut contents versus total mass for Gegeneophis ramaswamii from surveys in Kerala, India. Males (squares) have gut contents less than 10% of their total mass (dotted line), while females (circles) have gut contents less than 7% (dashed line), juveniles (triangles) were not numerous enough to interpret. Collections were made at the beginning of the monsoon (open symbols), and mid-monsoon (solid symbols).
Discriminant analysis found no significant effect when sites were analysed as groups (P=0.0768), although Simpson diversity index scores were much lower for Punalur (0.1784), than for Bonaccord (0.5710). Despite each site having a different species composition, SEE dominated the gut contents for each, both in frequency and mass (Tables 2 and 3).
Data grouped by sampling time gave the only significant result for discriminant analyses (P=0.0067), accounting for 8.4% of the total variation in the dietary data (ratio of total and between class inertia). This variation is mostly explained by a shift from earthworms, which dominate in the early monsoon period to termites (primarily Odontotermes sp.) in the mid-monsoon sampling (Table 2). Simpson diversity index scores were consistently lower for the mid-monsoon samples, although fewer G. ramaswamii had empty guts (Table 1) and the taxonomic richness was higher (Table 4). There is a positive correlation (0.94) between the number of guts with recognisable prey items and the total number of prey species for each survey sample.
4 Discussion
The raw data on gut contents suggest that Gegeneophis ramaswamii is a generalist predator, but a more complete understanding can be obtained from the multivariate analysis and biplot presentation of diet composition. This enables interpretations to be made from, and give meaningful insight into, feeding patterns of individual animals, and hence possible resource partitioning within and between populations [25]. The %PCA analysis confirms the dominant prey items of G. ramaswamii to be soil-ecosystem engineers (termites, earthworms and ants), and adult beetles. The distribution of individuals in Fig. 1 shows groups of animals toward the ends of the major arrows that have gut contents dominated by a single prey taxon. Theoretically, this pattern might result from either a specialist predator, or from a generalist foraging within a patch of prey items. For G. ramaswamii, we prefer the latter explanation, and support for this is found in the significant differences in gut content composition between the pooled early monsoon sample and the mid-monsoon sample.
Individuals toward the centre of the biplot (Fig. 1) have guts containing either only rare prey taxa or a mixture of more common prey; an inspection of the raw data (Tables 2 and 3) shows the former (one or several rare prey taxa) to dominate this group. The remaining G. ramaswamii (12.5%) are distributed between those with gut contents dominated by earthworms and those containing rare prey. The low number of points occurring between the tips of the arrows indicates that the vast majority of G. ramaswamii had not fed on combinations of the dominant prey taxa. This is interpreted as evidence of opportunistic foraging on patchily distributed prey. Clear support for this is found in animals occurring in the same sampling quadrat that have the same dominant prey taxon in their guts (see below).
Both earthworms and termites are known to exhibit seasonal rhythms in their abundance in similar sites in the Western Ghats (J.-P. Rossi, pers. comm.) [28]. However, it is not known whether the differences in the composition and frequency of dietary items between the early and mid-monsoon samples results solely from temporal changes in the soil invertebrate macrofauna, or dietary requirements/behaviour of G. ramaswamii, or something else, but this is open to future investigation with greater sampling of predators and prey. Similarly, future simultaneous quantitative sampling of the invertebrate macrofauna will allow hypotheses of prey selection to be tested. The non-significant differences in diet among sites may be a reflection of the low numbers of G. ramaswamii collected at some sites early in the monsoon period. Subadults prey on fewer individuals of the same taxa that are preyed upon by adults. There is some evidence that subadult diet is less diverse, but this is confounded by the inherent size difference between the groups. Too few juveniles were collected to examine possible differences in the diet of earlier ontogenetic stages. The data do not support sexual resource partitioning.
Workers and soldiers of the termites Odontotermes sp. and Discupiditermes sp. were found in guts (Table 2) in approximately the same proportions as they occur in nests (D. Jones, pers. comm.), perhaps indicating that G. ramaswamii did not select against soldiers, or that they did select against soldiers while under attack. There is no direct evidence to suggest that caecilians were feeding on termites within their nests. Some fossorial, limbless squamate reptiles are known to specialise on termites, including stages only available in nests [2,3].
Arthropods, including social insects, may be prone to overestimation in analyses of gut contents, because of their relatively indigestible chitinised exoskeletons ([28] but see below). This may have happened in this study because arthropods (especially termite head capsules) but not earthworms were recognised in the more posterior parts of the gut. Earthworms were the only dominant prey item that were found both on their own, and together with rarer prey items. Although earthworms are known to occur in patchy distributions [29] these patches are likely to be far larger (10–30 m) than for dense social aggregations of termites and ants. Thus, G. ramaswamii foraging on earthworms are likely to come across and feed on rarer prey items opportunistically. This is consistent with Presswell et al.'s [15] conclusion that the scolecophidian snake Ramphotyphlops braminus (Daudin) is a relatively rare and unusual component of the diet of G. ramaswamii.
The presence of pieces of large earthworms and part of a cricket in the guts of G. ramaswamii suggest that some items are broken up prior to ingestion, either accidentally during prey capture by autotomy (as is known for earthworms [30]) or deliberately to facilitate ingestion. However, G. ramaswamii can also ingest reasonably large, but elongate prey such as scoelcophidian snakes [15] and some larger earthworms.
To the best of our knowledge, this is the first report of ants in the diet of any caecilian. The highest frequencies of G. ramaswamii feeding on ants were of ant brood, with relatively few workers being ingested (Table 2). It may be that ants, in the form of brood, are underrepresented by examination of gut contents that is biased toward undigested parts of food items. At least one of the ingested ant taxa, Pachycondyla sp., relies on a powerful sting for defence (B. Bolton pers. comm.). Selection of brood over worker ants is known for fossorial scolecophidian snakes, some of which specialise in feeding on ant brood [3,31,32, and references therein]. Webb and Shine [31] provided evidence that some specialist scolecophidians feeding on ants find their nests by following ant trails, and that they may avoid damage from attacking adults by very rapid binge feeding within nests. Olfactory powers of caecilians are known to be substantial, including sensitivity to prey species [33], but nothing is known about how caecilians may withstand attacks from adult ants. Some frogs that feed on ants use them to augment the defensive toxicity of their skin [34]. Caecilians are also known to have toxic skin secretions [11,35,36], and the relation between these and diet is worthy of future investigation.
In measuring the mass of dietary items in G. ramaswamii, we did not consider soil inside the guts of earthworms, which may account for between 30–50% of their mass (P. Lavelle, pers. comm.). Many of the specimens recorded as having no recognisable gut contents did contain soil or detritus, especially in the posterior of the gut. Contrary to Hebrard et al.'s [13] study of Boulengerula taitanus Loveridge, we consider this to originate from gut contents of prey items and/or as accidental ingestion, and not as evidence of the deliberate ingestion of soil and detritus. Largen et al. [10] and Nussbaum and Pfrender [12] interpreted presence of soil in caecilian guts (Sylvacaecilia grandisonae [Taylor] and Schistometopum thomense Barboza du Bocage, respectively) as the residue from digestion of earthworms, while Breckenridge et al. [37] interpreted soil and detritus in the faeces of Ichthyophis glutinosus (L.) as originating from incidental ingestion while feeding. Mineral and vegetable matter have been reported from the guts of the semiaquatic Chthonerpeton indistinctum (Reinhardt and Lütken) [38] and the aquatic Atretochoana eiselti Nussbaum and Wilkinson [39] and Typhlonectes compressicauda (Duméril and Bibron) [40,41], but claims have apparently not been made for omnivory in these South American typhlonectids.
The vast majority of identified prey taxa are known to be largely or entirely soil dwelling, and this supports our hypothesis that G. ramaswamii forage within soil. While no effort was made to further identify the coleopterans, many species occur in at least the surface layers of soil and their presence is not considered to be inconsistent with the hypothesis that G. ramaswamii is a wholly subterranean predator. Similar conclusions can be reached for the rarer prey taxa. For example, the hemipteran nymphs were identified as spittle bugs (Auchenorrhyncha: Cercopoidea), which often feed on roots (M. Webb, pers. comm.). Earthworms and insects have been reported in the diet of many terrestrial caecilians [10,12,17], and dietary specialism (on termites or arthropods [5,9]) has thus far been hypothesised only for species of the East African caeciliid Boulengerula.
Our combination of randomised sampling techniques over different sampling periods is pivotal to our conclusion that G. ramaswamii is a generalist. For example, in one of the surveys from which specimens were collected for this study [23], 16 G. ramaswamii were found in 5 m2 of randomly sampled habitat at Punalur. Under non-random collecting circumstances, continued searching in this area would probably have yielded a large number of individuals that might consequently have been deemed an excellent sample for analyses of diet. If such an analysis was conducted, this would have led to the conclusion that G. ramaswamii is largely a specialist predator, with less than 5% of gut content items being non-termite. Some of the other results of this study emphasise the importance of a randomised sampling strategy. The two G. ramaswamii with large quantities of ant brood in their guts were collected in a single quadrat far from the irrigation ditches at Bonaccord, on higher, harder and drier ground than where the majority of specimens were found. This is a microhabitat that we would not normally favour for maximising the efficiency of G. ramaswamii collection [20], so that it might not have been sampled if the aim had been only to collect enough animals for gut contents analyses. Caecilians have a reputation as a rare component of the tropical soil fauna [42], and the vast majority of the few large collections have been made non-randomly. However, making generalisations about diet from these animals may be misleading.
This study has examined diet of G. ramaswamii in agricultural settings during the wet season. It will be of interest to obtain comparable data for their presumably original forest habitat, and throughout the year. The distribution of G. ramaswamii overlaps with at least three other species of caecilian from two families [20,23], and nothing is yet known of their niche partitioning. There are no data on occurrence or movement in the soil of G. ramaswamii or the vast majority of other caecilian species. Vertical migration in caecilians and its relation to prey type could be better studied with a more comprehensive depth sampling strategy.
5 Conclusions
The broad range of taxa, high diversity indices, and the %PCA analysis all support the hypothesis that Gegeneophis ramaswamii is a generalist predator within the soil, feeding particularly on termites, earthworms, ants, and beetles. The guts of some individuals collected in the mid-monsoon contained only termites, and this is interpreted as a consequence of generalist foraging on patchily distributed prey. Differences between sites were not found to be significant, but differences in the frequency and composition of diet between early and mid-monsoon samples are. Gut content data do not suggest ontogenetic resource partitioning, but instead indicate that sub-adults prey on the same but fewer items as adults.
G. ramaswamii occurring at high densities in some agricultural habitats [20,22,23] and feeding mostly on soil-ecosystem engineers could potentially, as postulated by Oommen et al. [20], have a substantial influence on soil ecology. This might have applied implications. For example, in agricultural areas, species of Odontodermes and Microtermes can also become pests, and have been recorded attacking tea [43]. It is possible therefore that predation by G. ramaswamii occurring in high densities may be advantageous to tea production. The potential influence on soil ecology of terrestrial caecilians and other subterranean lower vertebrates remains an open question, and much further research is clearly needed.
Acknowledgements
We thank Johnson Baby, Joseph Mathai, Smita and George Mathew, Biju Thomas and family, S. Vishvambaran and R. Janardhanan for their assistance in the field, the landowners for generously granting us permission to conduct surveys, and many people in Kerala for their generosity, hospitality, advice and practical help. GJM would like to thank Alison and Ian O'Connell for logistical help. Other assistance, advice, information, equipment, and taxonomic expertise was generously provided by Roger Bamber, Barry Bolton, Flo Dubs, Paul Eggleton, Tim Ferrero, Sam Gluck, Eileen Harris, David Jones, Patrick Lavelle, John Martin, Jean Mariaux, Gill Sparrow, Marvalee Wake and Mick Webb. This work was supported in part by NERC grant GST/02/832 to MW, and by an MRF award to MW and DJG. GJM is supported by an EU Marie-Curie fellowship: HPMF-CT-2001-01407.


