Natural killer cells are specialized effector cells that within the immune system play a major role in host defence against tumour or pathogen-infected cells [1,2]. In spite of their relevance in defence mechanisms, major questions concerning their mode of action and their precise nature were only recently clarified. Indeed it has been well established that NK cell activity is regulated by a complex balance between inhibitory and activating signals that are mediated by an array of different cell-surface receptors upon engagement by specific cellular ligands. In human NK cells, the inhibitory receptors include KIRs (Killer–Cell Immunoglobulin-like receptors) specific for groups of alleles of MHC class I, and the CD94/NKG2A heterodimer specific for non-classical HLA-E molecules [3–5]. In particular, the three KIRs that bind different subgroups of HLA-C alleles (KIR2DL1–KIR2DL3) [6–9] and KIR3DL1 [10–13], which is specific for HLA-BW4 alleles, seem to play a major role in the inhibitory control of NK-cell-mediated cytotoxicity against HLA class I+ normal target cells. On the other hand, activating signals take place only in the case of partial or total loss of HLA class I molecules, which occurs for example during tumour transformation or viral infections [5,14,15]. A remarkable exception to this rule is represented by the ability of NK cells to kill normal autologous HLA class-I+ dendritic cells (DC). In this context, it has recently been shown that the cognate interaction between these two cellular types results in a potent cross signalling [16–21], which results in priming, proliferation and activation of NK cells. Moreover, once activated, NK cells acquire the ability to kill immature DC (iDC) and may also induce DC maturation (Fig. 1). The observation that the cytolytic activity of polyclonal NK cell populations against autologous iDC could be incremented in the presence of anti-HLA class I mAb prompted us to investigate whether heterogeneity existed among NK cells in their capability to kill monocyte-derived iDC. Indeed, consistent with our hypothesis, only a fraction of NK cell clones tested showed spontaneous cytotoxicity against autologous iDC. On the basis of this cytolitic propriety and considering the phenotype in terms of expression of KIR2DL, KIR3DL1 and CD94/NKG2A, we grouped these clones into three different functional categories. A first group (group A) of NK clones was characterized by high spontaneous cytolytic activity against iDC. These clones expressed the CD94/NKG2A complex but lacked KIR2DL and KIR3DL1 molecules reactive with self-HLA class I alleles. NK cell clones belonging to a second group (group B) were able to spontaneously kill iDC. However, at variance with group-A clones, their cytotoxicity could be increased by masking HLA class I or NKG2A molecules thus indicating that the partial inhibition of cytotoxicity was mediated by CD94/NKG2A. A third group (group C) of clones did not display cytotoxicity against autologous iDC and expressed KIR2DL or KIR3DL1 receptors. In line with this data, reconstitution of their cytolytic activity against iDC could be obtained with anti-KIR mAbs. Finally a minor fraction of group C NK cell clones was KIR− CD94/NKG2A+ and their cytotoxicity could be reconstituted by mAb-mediated blocking of CD94 molecules.
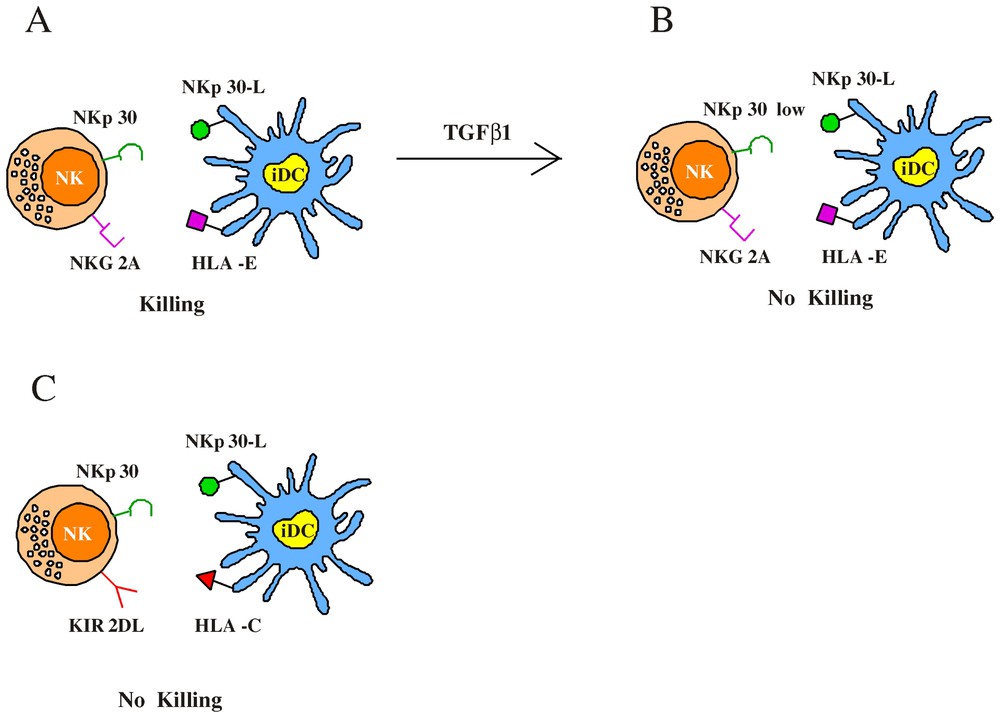
Regulation of NK-mediated lysis of immature DC. (A) The ability to kill iDC is restricted to an NK subset characterized by the CD94/NKG2A+ surface phenotype; indeed the amount of HLA-E molecules expressed by iDC is not sufficient to efficiently engage NKG2A. This results in NKp30-induced iDC lysis by NK cells. (B) TGFβ1 treatment induces a strong downregulation of NKp30 molecules and dramatically reduces the NK-mediated cytotoxicity against iDC. (C) Optimal interactions between classical HLA-I alleles and KIR prevent iDC lysis by KIR+ NK cells.
Then we tested the cytolitic activity of the various NK cell clones against iDC derived from allogeneic (KIR-mismatched) individuals [22]. In this case, KIR+, NKG2A− cells were alloreactive because their KIRs receptors failed to recognize HLA class I alleles on allogeneic DC. Otherwise, as expected, in the case of NKG2A+, KIR− clones no substantial difference existed in the ability to kill autologous or allogeneic iDC. Altogether this analysis indicated that among different NK cells, the ability to kill autologous iDC is restricted to an NK subset characterized by the CD94/NKG2A+ KIR− surface phenotype (although all NK cells could lyse iDC in the presence of anti-HLA class I mAb). This would suggest that sufficient interactions between classical HLA-I molecules and KIR could occur at the iDC surface while on the contrary the amount of HLA-E would be inadequate to efficiently engage NKG2A molecules on effector NK cells. Indeed we could verify that HLA-E (detected by the HLA-E-specific 3D12 mAb) [23] was almost undetectable in iDC (downregulated as compared to monocytes), while it was only partially re-expressed on mDC. Based on these results, we considered that a better understanding of the role played by HLA-E molecules in preventing DC lysis could derive from the analysis of mDC. Thus these cells were assessed for susceptibility to the same panel of NK clones tested against iDC. Our data indicate that most NK clones that lysed iDC did not kill mDC. In particular, in the case of group-A clones, lysis of autologous mDC was lower as compared to that of iDC, but could be increased by mAb-mediated masking of HLA class-I molecules. Regarding group B NK clones, they lysed mDC only in the presence of anti-HLA class I or anti-CD94 mAbs. Finally clones belonging to group C (in most instances KIR+), unable to kill iDC, also failed to kill mDC and cytotoxicity against mDC could restored upon mAb-mediated disruption of the HLA class I/KIR interactions.
The different ability to lyse iDC and mDC displayed by NK clones was likely due to the higher expression of HLA-E in mDC as compared to iDC. Since the cytolytic activity of a given NK cell clone is the result of a balance between inhibitory and triggering receptors, we analysed the levels of expression of the inhibitory and activating molecules particularly involved in DC interactions. Therefore we focused our attention on the expression of NKG2A (i.e. the receptor for HLA-E) and of NKp30, the triggering receptor belonging to NCR family [24], which plays a predominant role in the induction of NK-mediated lysis of iDC and mDC. [16] This analysis revealed that KIR-NKG2A+ clones belonging to group C, able to lyse iDC only upon masking of NKG2A molecules, expressed very high levels of NKG2A as compared to group-A and -B clones. Moreover, group-A NK clones, able to kill spontaneously iDC, were characterized by a lower expression of NKG2A as compared to group-B clones. These data indicate the existence of an inverse correlation between the levels of NKG2A expression and the ability to kill DC. The low amounts of HLA-E molecules expressed in iDC may be differentially sensed by NK cells expressing high or low levels of NKG2A, while mDCs (expressing higher levels of HLA-E) are susceptible to lysis only by NK clones characterized by very low NKG2A surface density. At variance with NKG2A, the expression of NKp30 was comparable in most NKG2A+ clones analysed.
The finding that NKp30 represents the major NK receptor involved in killing of DC [16] led us to investigate whether certain soluble factors (such as cytokines) could modulate the NKp30 surface expression impairing the ability of effector NK cells to lyse DC. To this end NK cells were cultured in the presence of different immunomodulatory cytokines, including IL4 and IL10 [25–27]. The only cytokine that exerted a significative effect was represented by Transforming Growth Factor beta 1 (TGFβ1) [28]. Indeed TGFβ1-conditioned fresh NK cells showed a sharp down-regulation of NKp30 surface expression, while the surface density of other receptors including NKp46 [24] or co-receptors such as 2B4 [29], NTBA [30] and NKp80 [31] was comparable to that detected in NK cells cultured with rIL-2-alone. This effect was observed also on established polyclonal and clonal NK cells, cultured in the presence of rIL-2 for over one month. Further studies revealed that the TGFβ1-mediated downregulation of NKp30 was a consequence of gene regulation at the transcriptional level. The finding that TGFβ1 selectively inhibited the surface expression of NKp30 suggested a possible effect on the NK-mediated killing of DC. So iDC were analysed for their susceptibility to lysis by polyclonal NK cell populations cultured either in rIL-2 alone or in the presence of TGFβ1. In agreement with previous data [16], iDC were efficiently lysed by rIL-2-cultured NK cells and, as demonstrated by mAb-mediated interruption of NKp30/NKp30L interactions, the lysis was almost completely dependent on the engagement of NKp30 molecules. Remarkably the TGFβ1-treated NK cells displayed a dramatic reduction of cytotoxicity against iDC. The residual lysis was mostly dependent on NKp30, since a further reduction was detected upon the addition of anti-NKp30 mAb. We also analysed the effect of TGFβ1-conditioning on the ability of NK cells to kill mature allogeneic KIR-mismatched DC (mDC) susceptible to NK-mediated killing. Unlike NK-mediated lysis of iDC the cytotoxicity against mDC was only partially inhibited by anti-NKp30 mAb, thus suggesting that additional activating receptors may be involved in triggering of NK cells upon interaction with mDC. Indeed, different from iDC, lysis of mDC could be inhibited, at least in part, also by mAb-mediated masking of NKp46. According to this observation, TGFβ1-conditioning only partially affected the NK-mediated lysis of mDC. Moreover, a significant reduction of mDC lysis was obtained by mAb-mediated masking of both residual NKp30 and NKp46 molecules. Taken together, these data provide evidence that the NK-mediated killing of iDC, that is mostly dependent upon the engagement of NKp30, is strongly affected by TGFβ1-treatment of NK cells, while killing of mDC is less affected, due to the involvement of additional triggering receptors. Similar to mDC, it is well known that multiple triggering NK receptors contribute to the lysis of most tumour target cells. Indeed, in line with these observations, killing of these targets by TGFβ1-conditioned NK cells was only partially reduced. Moreover, lysis of LCL 721.221 (EBV-transformed cell line) target cells, which is mostly NKp46-dependent [32], was not significantly reduced in TGFβ1-conditioned NK cells. These data indicate that the overall cytolytic potential of TGFβ1-conditioned NK cells is not significantly reduced as compared to untreated cells.
So here we deepened some aspects regarding NK cell-DC interaction. First we demonstrated that the cytotoxicity against iDC is restricted to a subset of NKG2A+KIR− NK cells. These cells are believed to mediate a crucial quality control of DC undergoing maturation in peripheral tissues keeping in check the expression of HLA-E at the DC surface [19]. Moreover we showed that TGFβ1, by inducing a dramatic downregulation of NKp30 molecules, could limit the NK-mediated attack preserving and regulating the size of DC pool. In this context, it has been shown that TGFβ1 is produced by iDC in response to the uptake of apoptotic bodies generated during physiological tissue regeneration [33].


