1 Introduction
In humans, nasal detection of volatile chemicals present in the external environment is mediated by two separate, but interrelated sensory systems: the olfactory and trigeminal systems. The first is served by cranial nerve I (the olfactory nerve), the second by cranial nerve V (the trigeminal nerve). Stimulation of the olfactory nerve results in sensations of smell, whereas stimulation of the trigeminal nerve gives rise to various sensations such as irritation, freshness, stinging, prickliness, burning and tingling that can be generically termed pungent sensations [1,2]. Two major fibre systems, C-fibres (unmyelinated) and Adelta-fibres (myelinated) participate in the afferent chemosensitive innervation of the nasal respiratory epithelium [3]. Stinging sensations are likely to be mediated by Adelta-fibres, whereas burning sensations are largely mediated by C-fibres [4]. The receptors for intranasal sensory irritation in the nose are located close to those of the olfactory system and often both are stimulated at the same time by the same stimulus [5]. Because most chemosensory stimulants, at sufficient concentration, elicit both olfactory and trigeminal activation [6–10], it is relevant to determine how these anatomically distinct systems interact. For a long time, many studies have already dealt with the interrelationships between odours and pungency in order to assess their role in odour perception [11–14]. Interestingly, it has been demonstrated since three decades that the trigeminal nerve may potentiate or have a synergistic effect on olfaction [15]. However, theses published works were often based on the simultaneous presentation of two stimuli, one producing little apparent irritation and the other odourless one inducing nasal irritation. In these conditions, the interaction between odour and pungency has been described, when different stimuli were used for eliciting odour and irritation, as a mutual inhibition [13] that may represent an important determinant of odorous sensations. Thus, it would be relevant to see whether sequential presentation of irritant before odour alters the inhibitory response or not.
The aim of this study was to investigate the influence of a previous nasal trigeminal stimulus on human olfactory sensitivity. More precisely, the experiment was designed to evaluate by psychophysical measurements the modification of olfactory thresholds for two different odours, phenyl ethyl alcohol (PEA) and butanol (BUT), after a previously trigeminal activation by allyl isothiocyanate, i.e., mustard oil (AIC). Thresholds were determined using a two-alternative forced-choice procedure with a classical ascending concentrations commonly employed in olfactory research [7,14,16–19].
PEA and BUT odour stimuli, frequently used in olfactory studies, were selected in relation to their different levels of hedonic valence and trigeminal activation. PEA is a pleasant rose-like smelling compound [16,20] considered as a pure odorant, meaning that it only stimulates the olfactory nerve [6,19]. On the contrary, butanol has neither a highly pleasant nor a highly unpleasant character [18] and stimulates both olfactory and trigeminal nerve [6].
AIC trigeminal stimulus was chosen because it is not toxic, widely used as a flavouring agent in a variety of foods in many countries and because it has been employed in previous studies [8,21,22]. AIC applied on the skin leads to a clear burning sensation [23] and has been found to activate all cutaneous receptors and predominantly excite C-fibre afferents in the upper skin layers [24].
2 Materials and methods
2.1 Subjects
The subjects were 20 volunteer students (13 women and 7 men, mean age 26 years 7 months) enrolled in master's degree course of biology at the University of Franche-Comté (Besançon, France). All participants were non-smokers, healthy and free of head colds or nasal allergies at the time of the tests. They were informed about the general purpose of the study and gave their consent prior to their inclusion in the experiment. The study was conducted in accordance with the Helsinki/Hong Kong Declaration.
2.2 Nasal stimuli
Olfactory thresholds were determined for two specific odorants in relation to their trigeminal properties. The first was phenyl ethyl alcohol (PEA) [C8H10O; molecular weight=122.2] without intranasal trigeminal properties, and the second was butanol (BUT) [C4H10O; molecular weight=74.12] with middle trigeminal properties. A dilution series was prepared in deionized water for each stimulus. Starting from a stock solution 8% for PEA (step 1 or P1) and 4% for butanol (step 1 or B1), dilutions by a factor of 2 and 3, respectively, were prepared (Tables 1 and 2). After successive dilutions, the full series include steps 1 to 20 for PEA and steps 1 to 15 for butanol. 4 ml of each concentration were placed, for both odorants, into glass bottles (7.5 cm high, 1 cm in diameter at the opening). Another bottle was filled with only 4 ml of deionized water (blank).
Concentrations of phenyl ethyl alcohol
| Concentration (% v/v) | logC (v/v) | (g cm−3) | (mol cm−3) | |
| Pure liquid | 100 | 0 | 1.0202 | 8.35×10−3 |
| Dilution step | ||||
| 1 | 8 | −1.097 | 8.16×10−2 | 6.68×10−4 |
| 2 | 4 | −1.398 | 4.08×10−2 | 3.34×10−4 |
| 3 | 2 | −1.699 | 2.04×10−2 | 1.67×10−4 |
| ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| 20 | 1.526×10−5 | −6.816 | 0.17×10−6 | 1.19×10−9 |
Concentrations of butanol
| Concentration (% v/v) | logC (v/v) | (g cm−3) | (mol cm−3) | |
| Pure liquid | 100 | 0 | 0.8 | 1.08×10−2 |
| Dilution step | ||||
| 1 | 4 | −1.397 | 3.2×10−2 | 4.4×10−4 |
| 2 | 1.33 | −1.875 | 1.07×10−2 | 1.47×10−4 |
| 3 | 0.44 | −2.352 | 3.556×10−3 | 4.89×10−5 |
| ⋯ | ⋯ | ⋯ | ⋯ | ⋯ |
| 15 | 8.36×10−7 | −8.078 | 6.69×10−9 | 9.2×10−11 |
The nasal stimulus used to elicit a trigeminal activation was allyl isothiocyanate (AIC) [C4H5NS; molecular weight=99.15]. A stock solution (95%) of AIC was diluted in mineral oil by a factor of 4. The nasal stimulus was presented in a glass bottle filled with 4 ml of liquid.
2.3 Procedure
2.3.1 Olfactory thresholds measurements without previous trigeminal stimulation
A trial consisted in the presentation of two bottles at a time, one being the blank (deionized water) and the other containing the dilution of the chemical studied. The bottles were opened and immediately placed under the subject's nose. The subject's task was to indicate which one of the two randomly presented stimuli contained the odorant. Even if no sensations were perceived or if no difference was apparent between the bottles, the participant was required to choose one bottle or the other. No feedback was given concerning the correctness of the responses. For each odorant, testing began at the weaker concentration so as to avoid olfactory receptor's saturation. For each concentration level, the test was performed five times. Concentration increased in steps until the subject achieved four correct and consecutive responses in a row at some concentration and the next higher. Threshold was defined as the lowest odorant concentration of the two successive concentration levels. The determination of the olfactory thresholds was based on criteria widely employed in studies dealing with olfactory sensitivity [16–19,25].
2.3.2 Olfactory thresholds measurements after previous trigeminal stimulation
The same procedure as previously described was followed and, moreover, just before the successive trials of each concentration level, subjects were asked to sniff the bottle containing AIC during a limited period of 2 s (one inspiration). AIC was inhaled only one time at the beginning of a new concentration level, because it is well known that the effect of a trigeminal stimulation runs during a few minutes [26].
For each odorant tested, all subjects participated separately in both sessions described above.
2.4 Data analysis
Olfactory thresholds were statistically evaluated using Student t-tests (paired and independent). An alpha level of 0.05 was considered as statistically reliable. The standard deviations were reported and not significant results were noted as NS.
3 Results
The results are reported in Fig. 1. Mean thresholds given were based on the dilution steps. For PEA, the statistical analysis showed a significant difference between the thresholds without (mean threshold=13.55; SD=1.5) and with (mean threshold=15.15; SD=2.75) previous AIC stimulation (t=2.387; p<0.027). In the same way, for BUT the statistical analysis showed a significant difference between thresholds without (mean threshold=7.85; SD=2.36) and with (mean threshold = 9.95; SD = 3.33) previous AIC stimulation (t=2.372; p<0.05). For both PEA and BUT, the thresholds appeared lower (i.e. obtained at lower concentration steps) when the nasal AIC trigeminal stimulus was previously delivered, meaning that olfactory sensitivity was increased.
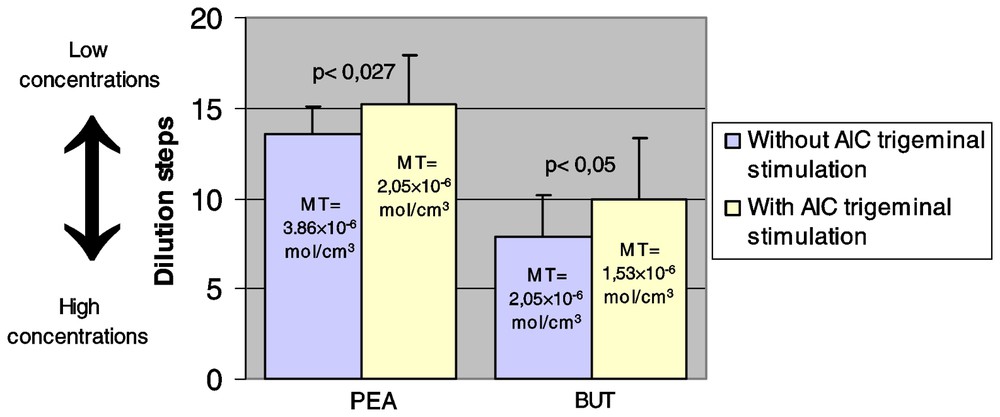
Phenyl ethyl alcohol (PEA) and butanol (BUT) thresholds∗ with or without previous allyl isothiocyanate (AIC) trigeminal stimulation (N=20). Starting from a stock solution 8% for PEA (step 1) and 4% for butanol (step 1), dilutions by a factor of two and three respectively were prepared. The full series include steps 1 to 20 for PEA and steps 1 to 15 for BUT. ∗ Thresholds were based on the dilution steps. The corresponding mean thresholds (in mol cm−3) were noted as MT.
For PEA (r=0.106) and for BUT (r=0.06), the correlation coefficients between the thresholds with or without previous AIC stimulation were very low. Thus, the rise in olfactory sensitivity to PEA and BUT following AIC stimulation would be independent of the subjects' original sensitivity.
A correlation analysis between the PEA and BUT thresholds without AIC previous stimulation (r=0.345) or after AIC previous stimulation (r=0.058) showed no correlation. It meant that a subject with a high sensitivity to PEA for example did not display systematically a high sensitivity to BUT. Moreover, this low correlation pointed out the great variability of the trigeminal stimulation impact among subjects.
A comparative analysis of the male (N=7) and female (N=13) subjects showed that, whatever the odour used, the thresholds were not depending on gender without (for PEA t=1.215 NS; for BUT t=0.979 NS) as well as with previous AIC trigeminal stimulation (for PEA t=0.174 NS; for BUT t=0.226 NS).
4 Discussion
The results of the present study indicate that a previous AIC trigeminal stimulation significantly decreased the olfactory PEA and BUT thresholds. In other words, these findings reveal that a short activation of the trigeminal system preceding an olfactory stimulation produced an increase in sensitivity to odorants. Moreover, the fact that the results concerned both PEA and BUT showed that this rise was independent of the odorant quality (i.e., hedonic valence and trigeminal activation levels).
Several studies that have previously explored the interaction between odour and pungency have also shown that trigeminal activation influenced the perception of single odorants [14,27], suggesting that the trigeminal system was involved in olfactory responses to odour stimuli. Contrary to the present findings, the psychophysical observations of these works argued rather for an inhibitory influence of trigeminal over olfactory activity. However, the experimental procedures used were not similar to the one we used: they consisted in presenting a chemical stimulus with both odorant and pungent properties and/or a strong irritant molecule. As early as the 19th century, the philosopher Alexander Bain (1868) noted that concentrated carbon dioxide (carbonic acid) evoked pungency and remarked: “If a current of carbonic acid accompanies an odour, the effect (odour) is arrested” [28]. In the same way, Katz and Talbert [29] observed that a vapour with both odour and pungency might lose odour at high concentrations, irritation masking odour. A similar effect was seen by Cain [27] in an experiment in which both the odour and the pungency of butanol were estimated by subjects. One subject, who generally found the stimulus to be more irritating that did any of the other subjects, reported that the irritation produced by the highest concentration masked odour. In a later experiment, the interaction between odour and pungency was described to be a mutual inhibition when different stimuli for eliciting odour and irritation were used [13]. Participants received four concentrations each of CO2 and amyl butyrate (a mixed olfactory and trigeminal stimulus) and their 16 binary mixtures. They were required to rate overall intensity, the intensity of odour and that of irritation. It was found that the odour of amyl butyrate was suppressed by CO2, which confirmed that pungency could diminish odour. Cain and Murphy [13] also presented CO2 (two seconds) before amyl butyrate (two seconds on the same inhalation) in order to see whether sequential presentation of irritant before odour would alter the pattern of inhibitory response or not. It was discovered that irritation inhibited odours, but only by about one-fourth the amount noted with simultaneous presentation. These findings suggested that the timing of olfactory and trigeminal activation might be involved in the decrease or increase in olfactory sensitivity. Moreover, the results observed were probably dependent on the nature of the odorant and/or trigeminal substances used.
Several investigations have described several aspects and characteristics of chemical irritation [3,9,26] that could explain the possible mechanisms by which trigeminal activity may influence olfactory processing [10]. In the field of the intranasal trigeminal chemosensory modality, the most frequent molecule used is capsaicin, the pungent ingredient of red peppers [30–32]. This chemical irritant is known to activate the afferent chemosensitive C-fibres and to induce a local and central release of substance P (SP) and of other neuropeptides [33]. Electrophysiological studies indicated that spontaneous activity of olfactory receptors cells can be modified via a local axon reflex triggered by odours and inducing the release of SP and other peptides [34–36] from trigeminal fibres innervating the olfactory epithelium [37]. This modulation capacity of olfactory receptor responses to chemical stimuli could be related to the rise in olfactory sensitivity obtained after trigeminal activation. Otherwise, it has been shown that an application of capsaicin could induce an increased nasal vascular permeability and irritating nasal symptoms, such as sneezing [38]. Thus, trigeminal activation may influence olfactory perception indirectly via nasal trigeminal reflexes designed to minimize potentially damaging exposure to noxious substances. Therefore, in addition to direct alteration of receptor cell activity, the release of peptides from trigeminal fibres in the epithelium may influence receptor responses to odorants by changing the physical conditions in the receptor environment [39]. Otherwise, results of a recent study raised the possibility that the trigeminal and olfactory systems could also interact at central level [40]. The findings showed that some trigeminal ganglion cells with sensory endings in the nasal epithelium also had branches reaching directly into both the spinal trigeminal complex and olfactory bulb. Thus, the collateral innervation of the epithelium and bulb may provide an avenue whereby nasal irritants could affect processing of olfactory stimuli and consequently olfactory sensitivity.
The present work shows that a previous trigeminal stimulation with AIC has the capacity to enhance olfactory sensitivity, a fact that underlines the powerful influence of the interrelationships between the olfactory and trigeminal systems on odour perception. Further research could precise these interactions by using for the previous trigeminal activation other nasal irritants differing in terms of their chemical characteristics. It would also be of interest to verify that the results are not the same when using a pure odorant as previous stimulation. Another way to describe in great detail this phenomenon would be to change the moment and the duration of trigeminal stimulation as well as the chemical irritant concentration.


