1 Introduction
Posidonia oceanica (Linnaeus) Delile, a Mediterranean endemic marine Magnoliophyta, grows between the surface and 30–40-m depth and forms extensive meadows [1]. These meadows constitute a key ecosystem that plays a major ecological role [2,3]. Extensive meadows are restricted to warm waters, to the exclusion of colder areas such as the Gulf of Lions and the northern Adriatic Sea. The Provence Coast (France) is localized near this range limit [1].
During the year 1999, exceptionally high seawater temperatures occurred between the end of August and the beginning of October from Liguria (Italy) to Provence (France). They were consistently above 24 °C at 20–30-m depth for almost two months [4]. These high temperatures were associated with a mass mortality event of sessile benthic invertebrates [5–10]. The western geographical limit of mass mortality was located between Marseilles and the Camargue (Provence).
From in situ measurements, the upper lethal temperature limit of Posidonia oceanica is 29 °C [11]. In addition, it develops healthy and extensive meadows in areas where summer temperatures usually reach 24–25 °C (e.g., Thyrrenian Sea, Sardinia, Corsica [12–14]), i.e. nearly the same temperature as that observed in Provence during the 1999 warm-water episode. According to Short and Neckles [15], an increase in temperature, associated with global climate change, should have a positive effect on warm water seagrasses. Furthermore, Zupo et al. [16] established a production model, in which the production rate of Posidonia oceanica is directly linked to the temperature prevailing 3–4 months earlier. For these reasons, a negative impact of the 1999 warm episode was unlikely. In order to test this hypothesis, deep Posidonia oceanica overlapping the geographic limit of the invertebrate mortality event [5–10] were analysed using the lepidochronological methods [17–19].
2 Materials and methods
Lepidochronological analysis is based upon the long term (up to centuries) persistence of leaf bases (‘scales’), after leaf shedding, along the Posidonia oceanica rhizomes. Cyclic variations of scale thickness along the rhizomes provide an annual signal that makes it possible to backdate biological parameters, e.g., the number of leaves produced per year and the annual rhizome growth [17–22]. Study sites were located between the east of Marseilles and the Camargue (Provence, France) at the lower depth range of Posidonia oceanica, at (east to west) En-Vau
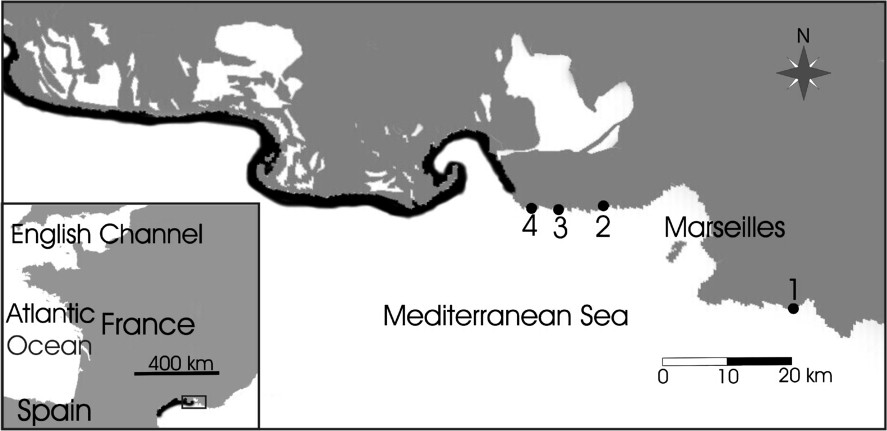
Location of study sites: En-Vau (1), Niolon (2), Sausset-les-Pins (3), Carro (4). Black areas show coasts where extensive meadows of Posidonia oceanica are absent [1].
At each site, 15 orthotropic (erect) and plagiotropic (creeping) shoots, with the underlying rhizomes, were collected by SCUBA diving on 6 February 2003 for Niolon, Sausset-les-Pins and Carro and on 1 June 2004 for En-Vau. According to Pergent [22], 15 shoots are sufficient for lepidochronological analysis. For the shoot sampling, a plastic bar (1 m) was laid flat on the meadow. The shoot nearest to the extremity was collected. The bar was then relocated with the near end at the sampling point, the direction of the bar being determined from a random table. Again, the shoot nearest to the farthest extremity of the bar was collected. And so forth up to the last shoot. For each past year (1998–1999 to 2000–2001), the number of leaves produced and the rhizome growth were noted. The years considered in this study, based upon the lepidochronological analysis are the years of leaf production (i.e. biological cycle; from October to October of the next year). Statistical analysis involved ANOVA and post-hoc analysis (Newman–Keuls test) using Statistica 6.0 (Statsoft®).
3 Results
For three out of the four sites (En-Vau, Niolon, Sausset-les-Pins), a significant decline of number of leaves and growth rate of both rhizome types was observed for the year of leaf production 1999–2000 in comparison with the previous (1998–1999) and the following years (2000–2001) (Fig. 2, Table 1). In contrast, no significant variation between the year 1999–2000, the previous year and the following year was observed at Carro. We compared these three years since records of the water column temperature were available [4], together with the geographical range of invertebrate mass mortality [6–8]. In addition, at the three sites concerned by the warm-water episode, the growth and leaf production values for the year of leaf production year 1999–2000 were among the lowest values for the 6-year study period (data not presented).

Lepidochronological results for the orthotropic (erect) rhizomes at En-Vau, Niolon, Sausset-les-Pins and Carro. (●) Mean number of leaves produced and (■) mean growth of rhizomes (mm) per year of leaf production. Error bars are 95% confidence intervals.
Analysis of variance (ANOVA) between the year of leaf production 1999–2000, the previous and the following years, for the number of leaves and the rhizome growth (mm), for orthotropic (erect) and plagiotropic (creeping) rhizomes, at each site. Significant values are in bold. (F: ANOVA factor, p: probability)
| Number of leaves | ||||
| Rhizome type | Orthotropic | Plagiotropic | ||
| F | p | F | p | |
| En-Vau | 7.731 | 0.011 | 7.000 | 0.007 |
| Niolon | 14.189 | 0.000 | 8.000 | 0.003 |
| Sausset-les-Pins | 5.087 | 0.013 | 15.187 | 0.001 |
| Carro | 1.713 | 0.200 | 0.118 | 0.890 |
| Growth of rhizomes | ||||
| Rhizome type | Orthotropic | Plagiotropic | ||
| F | p | F | p | |
| En-Vau | 6.612 | 0.017 | 3.933 | 0.042 |
| Niolon | 10.581 | 0.001 | 3.909 | 0.039 |
| Sausset-les-Pins | 5.128 | 0.013 | 2.160 | 0.162 |
| Carro | 1.553 | 0.231 | 0.847 | 0.453 |
4 Discussion
At all sites concerned by the 1999 invertebrates mass mortality [6], Posidonia oceanica growth (number of leaves produced and rhizome growth) was significantly altered during the 1999–2000 year, i.e. the lepidochronological year following the warm-water episode. This decline could not be linked to a flowering event inducing a reduction of the number of leaves on the flowering shoots [23,24] since no floral stalks (evidence of past flowering according to [22]) were observed, a feature confirmed by divers. If we hypothesize that high temperature is responsible for this weak growth, the reason could be the impact of the warm-water episode on carbohydrate storage within rhizomes. The warm-water episode took place during the period (only during summer months) when Posidonia oceanica stocks carbohydrates (starch) produced by photosynthesis for further utilization, i.e. for the next biological cycle. During the rest of the year, the carbon balance between production and consumption is negative [25].
The possibility that moderately high temperatures could negatively affect Posidonia oceanica near its cold limit of distribution is a rather unexpected result. It is interesting to note that, according to Hartog [26], the optimum temperature for Posidonia oceanica growth lies between 17 and 20 °C, but this assumption is not supported by experimental data. The fact that Posidonia oceanica could be sensitive to high temperatures, at least at its deep limit, could explain its relatively restricted distribution at depth in oligotrophic and transparent waters of some eastern Mediterranean regions [27] (e.g., 25 m at El Dabaa (Egypt) in a region not influenced by turbidity from the Nile river [28]; 34 m at Ischia (Italy) [29]; 32 m at Salina Island (Tyrrhenian Sea) [30]; see also [31,32]). In contrast, in colder and less transparent waters of the northern Mediterranean Sea, Posidonia oceanica reaches deeper bottoms (e.g., 38 m at Port-Cros (France, [33]).
This result should be interpreted in the context of the general regression of the lower depth limits of Posidonia oceanica meadows [34]. Interestingly, the regression of the lower limit of Posidonia oceanica meadows affects a large part of the northwestern Mediterranean, including regions where the decline in seawater transparency does not seem to be a likely explanation. Contrarily to the invertebrate mass mortality [9], our results do not allow us to state that the 1999 high temperatures were the direct cause of a decline in Posidonia oceanica growth. Clearly, a sporadic reduction of leaf and rhizome growths does not mean that it induces a regression of the lower limit. The possible negative effect of high temperatures could be indirect. This would be consistent with the steady upward regression of the lower limit [34] and the present-day warming trend of Mediterranean waters [10,15,35–38]. Our results thus open up a new and unexpected line of research to shed light on the current regression of Posidonia oceanica meadows, and their possible fate in the context of the warming up of the Mediterranean Sea.
Acknowledgments
The authors thank L. Laubier for his valuable comments and the both anonymous referees for theirs corrections, F. Bachet, B. Baniel, G. Bernard, P. Bonhomme, G. Cadiou, E. Charbonnel and A. Gantaume for diving assistance, F. Garcia and J. Patrone for their support, I. Taupier-Letage, J. Garrabou and T. Perez for bibliographical information and M. Perret-Boudouresque for bibliographical assistance. This work was supported by a research contract between CQEL 13 and GIS Posidonie.


