1 Introduction
Since the pioneering work of Monod, Novick and Szilard [1,2], continuous cultures of micro-organisms in the chemostat became a very popular way to study the growth of populations of micro-organisms. The basic assumption about the chemostat – an assumption that is questioned in this paper – is that it is perfectly stirred, and, as a consequence, that each individual has an equal access to the nutrients. Under this assumption, the basic mathematical model for the growth of one species of micro-organism on a single substrate S is given by the following set of two differential equations:
| (1) |
| (2) |
Now, consider the chemostat model with two competitors for the same substrate which is written below:
| (3) |
This is easily generalized to more than one population and one substrate. In fact, in the chemostat model, it turns out that coexistence of p species is not possible if the number of nutrients is strictly smaller than p. If this theoretical prediction has been corroborated by the experiences of Hansen and Huben [5] where two species competing for one nutrient grow in a chemostat, there are many examples of continuous cultures where a large number of species are competing for comparatively few substrates and where no species seems to be eliminated. In aquatic ecosystems, for instance, only a few resources are potentially limiting (it is recognized that this number is around 10), while dozens of phytoplankton species coexist. This has led to the well-known ‘paradox of the plankton’ (cf. [6]) and has generated a great amount of literature trying to find an explanation to this paradox. Other examples of this paradox can be observed in continuous well-mixed wastewater treatment plants where very complex ecosystems involving hundreds of species seem not to be simplified over the time in the presence of a small number of limiting substrates (cf. [7]). Different possible explanations of this persistence have been advanced.
Obviously, the first seminal observation can be attributed to Armstrong and McGehee, who claimed that ‘coexistence’ is not synonymous of ‘coexistence at equilibrium’ and proposed examples of model ecosystems with coexistence of two species via self-sustained oscillations (cf. [8,9]). This work can be considered as the starting point of many mathematical investigations about the existence of self-sustained oscillations systems (via limit cycles or chaos). A very good survey about this question has been written by Scheffer et al. [10]. The question whether these considerations provide a solution to the ‘paradox of the plankton’, or not is still a matter of controversy (cf. [11,12]). However, we do not discuss further this point since our explanation is based on a quite different basis.
In this note we propose a possible explanation based only on physical (abiotic) reasons. We consider a mathematical model for the biomass growth in the chemostat in which the kinetics function does not only depend on the concentration of substrate but also on the density of the biomass of each species and decrease with it. Using this new model, it is shown that the number of coexisting species can be arbitrarily large. To support our proposal, a number of physical evidences are pointed out in Section 4, which can be read independently of the rest of the paper as a motivation of the present work.
2 A ‘density-dependent’ chemostat model
We consider the following model for competition of n species for one substrate in a chemostat:
| (4) |
- (H1) , , is an increasing function.
- (H2) For each i and j the mapping is decreasing.
The first set of hypotheses expresses that for a given size of the various populations, the growth rate of each population increases with the concentration of substrate. The second set expresses that a competition is exerted by each j species on the i species: the bigger the concentration of the jth species, the smaller the growth rate of the ith species. In fact, this simply expresses that there is a competition of all species for the substrate. Notice that this model complies with the concept of ‘mass conservation’ of the classical chemostat. A general mathematical theory of this model is out of the scope of the present paper and is being currently developed (cf. [13]). From this theory, it turns out that if the ‘intra species’ competition is greater than the ‘inter species’ one, in a sense to be specified, then all species can coexist. We make it precise in a particular case, which will be sufficient for our argumentation.
Consider the system:
| (5) |
- (H3) , , is a increasing function.
- (H4) For each i the mapping is decreasing and tends to 0 at infinity.
- (H5) For every i, there exists a such that . This hypothesis simply expresses that, in the absence of the other species, none of the species is washed out. This is clearly a necessary condition for the coexistence of all species.
- (H6) From H4 it follows that for every , there exists a unique such that . Denoting , we assume that the inequality holds.
Consider the system(5)under assumptions H3–H6. Then, there exists a unique equilibrium of (5) noted such that for every i one has and it is globally asymptotically stable. This means that the system converges towards this equilibrium whatever the initial conditions satisfying .Proposition 1 cf. proof in [13]
In Proposition 1, only ‘intra species’ competition is taken into account, since the functions only depend on . Since a system like the one used in Proposition 1 is ‘robust’ against a small perturbation, this system is still stable. This means that Proposition 1 applies also for a general growth rates provided that assumptions H3–H6 are valid for the system with and the differences are small enough in a sense to be specified.Remark
In order to further investigate this model, we performed computer simulations for the specific case of the model:
| (6) |
The constant parameter values (which define a completely artificial system) reported in Table 1 were used.
Parameter values used in the simulations
| Index | 1 | 2 | 3 | 4 |
| Colour of the trajectory (colour version of the article) | Green | Red | Blue | Black |
| 0.83 | 1.00 | 1.20 | 1.60 | |
| 0.20 | 0.20 | 0.30 | 0.40 |
The trajectories of the dynamical system (6) over 400 time units are plotted in Fig. 1 for and in Fig. 2 for , while and in all simulations. We see that species 2 wins the competition when , while, for , there is no winner to the competition.
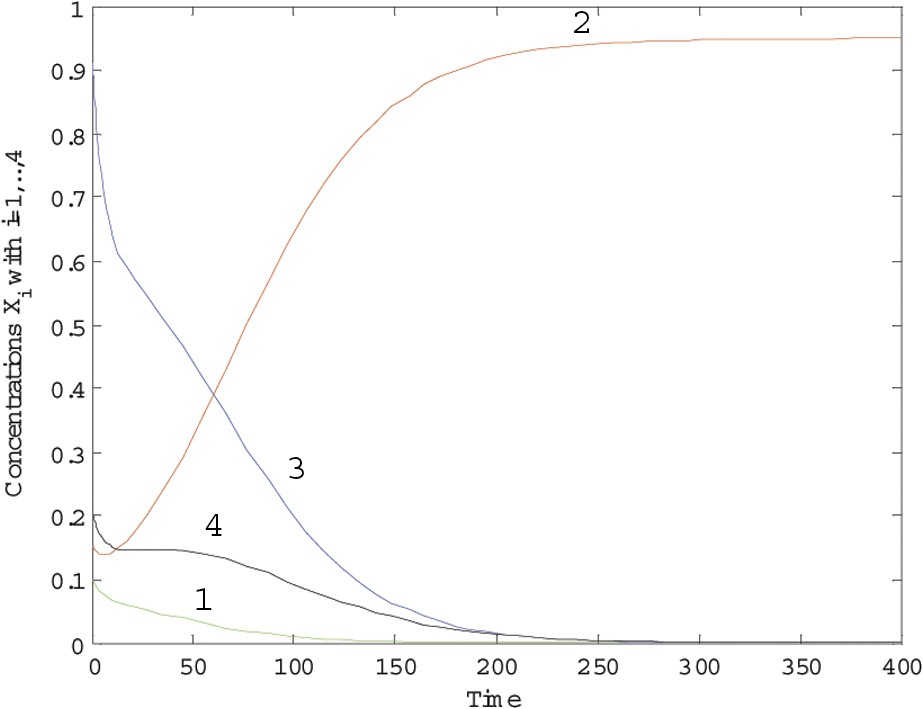
Trajectories of system (6) with c=0.
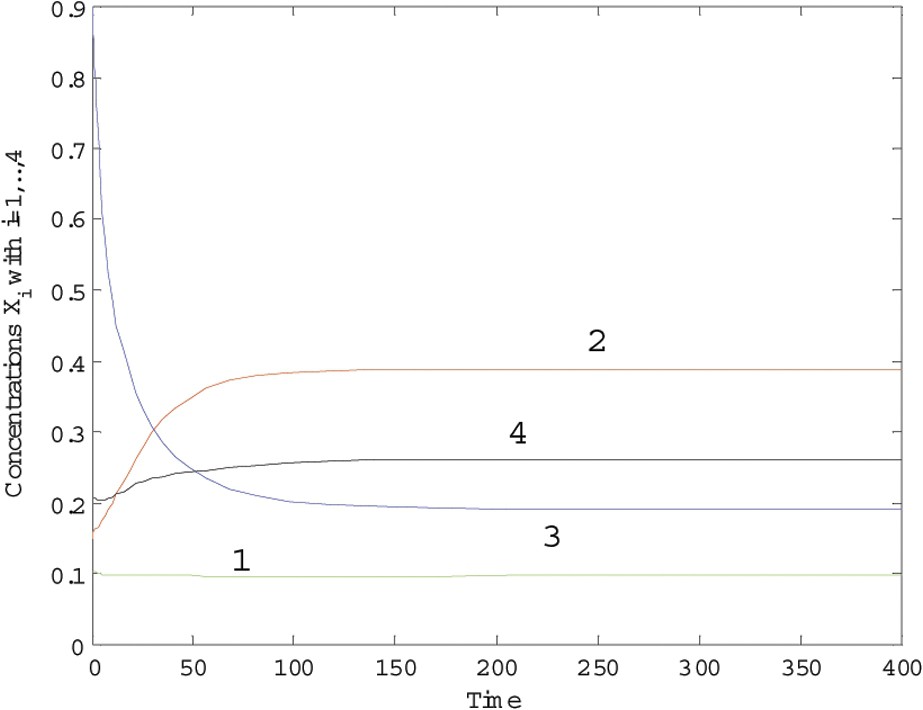
Trajectories of system (6) with c=1.
We also performed simulations when the 's do depend on all species, namely with the model:
| (7) |
The results are plotted in Figs. 3 and 4, with and and over periods of 400 and 1000 time units, respectively. This model highlights some competition of each species with the others. From the remark on Proposition 1, it is expected that, for λ small enough, there will be coexistence. When , the coexistence is still a property of the model, but for , it is not longer the case and one species, at least, disappears. It seems to us particularly important to notice that this remark provides an interesting insight into what is called the ‘barrier effect’ of ecosystems against invaders.
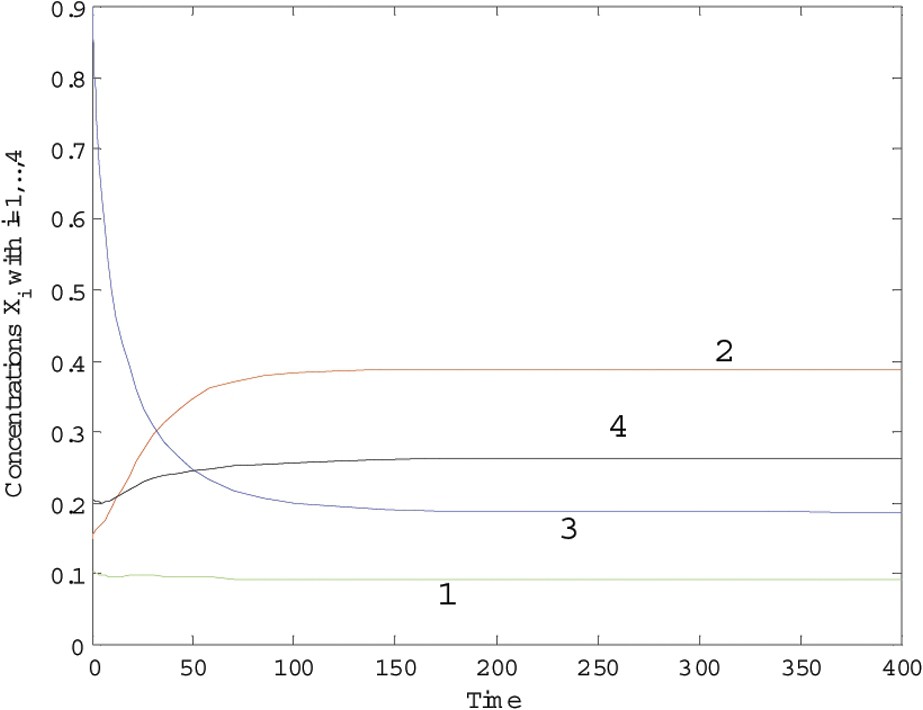
Trajectories of system (7) with λ=0.1.
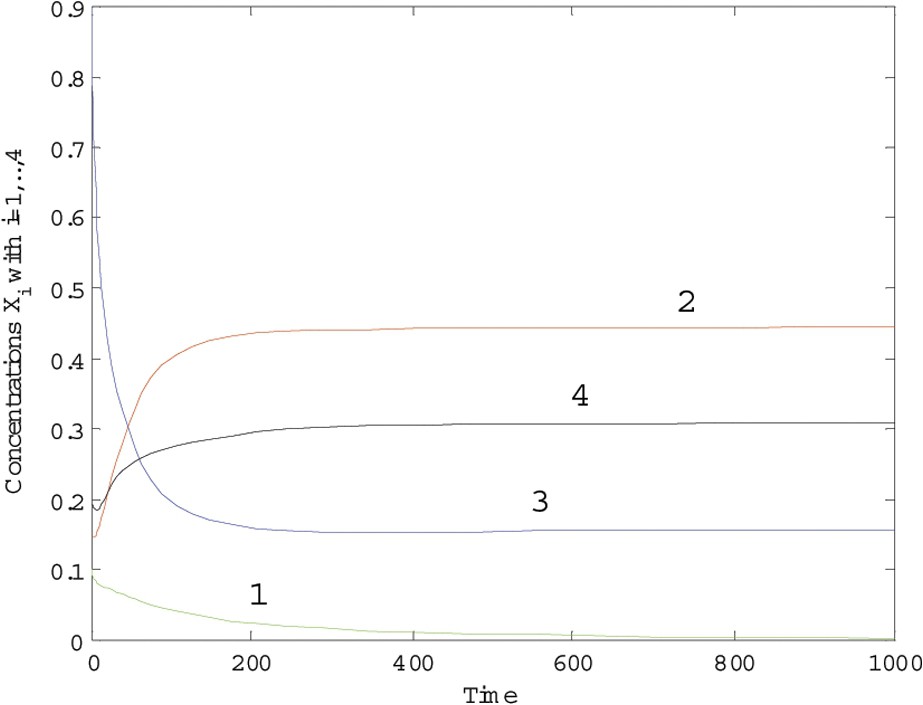
Trajectories of system (7) with λ=0.55.
3 Comparison with other models
Notice that the actual chemostat model with competition is more specific than the following general competition model:
| (8) |
However, thanks to the mass conservation principle, it is known that the sum in the system (4) tends towards and thanks to the fact that the system trajectories are bounded, the asymptotic behaviour of system (4) can be approximated by the asymptotic behaviour of the system:
| (9) |
Since it is well known that general competitive systems like (8) can present any complex behaviour, we conclude that our result relies definitively on our specific hypothesis associated to the competition for the substrate in the chemostat. There are many other models of competition in the chemostat where coexistence can be proved, but these models rely on assumptions of different nature than ours. Some models assume that the flow rate or the concentration of the incoming substrate is not constant (cf. [4] or [14]), while others assume that the flow rates (assimilated to mortality rates) are different for each species. In particular, in a recent paper [15], coexistence was proved from a density-dependence hypothesis, namely for the model:
| (10) |
| (11) |
Models of this kind are called ‘ratio dependent’. They originate from research on theoretical ecology, but they have been shown to be equivalent to a class of kinetics functions used in microbial ecology and known as the Contois model [16]. A ratio-dependent competition model in the chemostat could be:
| (12) |
4 Access to substrate as a limiting factor
In the chemostat model, the concentration of substrate is assumed to be the same at each point of the reactor or, at least, due to the mixing, in average, it is assumed that each individual has an equal access to the substrate. This is probably true for low concentrations of micro-organisms, but it may become questionable at high concentrations.
Consider the scheme in Fig. 5. We have represented a one-dimensional profile of biomass (in red or dark grey) and substrate in black. Since the biomass is absorbing the substrate, if the diffusion is low compared to the rate of absorption a gradient of concentration is established. The concentration is low at the centre of the biomass since the incoming substrate is absorbed by the biomass that is at the boundary. In a stationary mode we see, on this one-dimensional scheme, that the growth of the population is not proportional to the total population but just on those that are at the two boundaries. The thickness of the layer where the concentration of substrate is positive does not depend on the size of the colony (if it is large enough), but only on the diffusion coefficient of the substrate. This means that, after some transient, the growth of the colony, instead of being exponential, is linear.
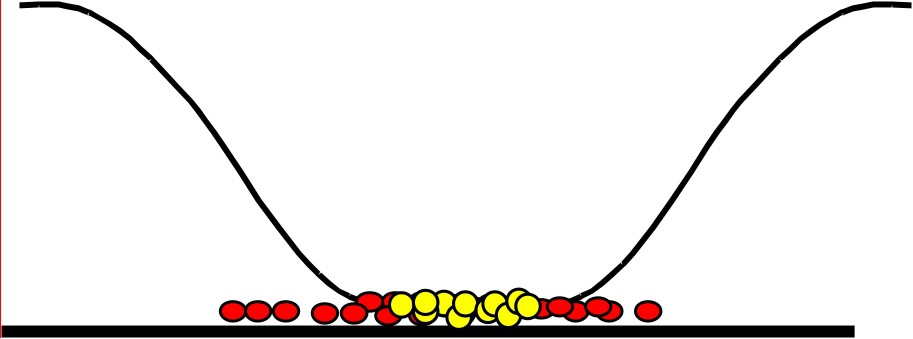
Growth of a species in a 1D scheme.
For a two-dimensional scheme (Fig. 6) the total population (red or dark grey + yellow or light grey) is proportional to the radius of the ‘colony’, while the active population (the one that has access to the substrate) in red (or dark grey) is contained in the corona, whose thickness only depends on the parameter of diffusion of the substrate, but not on the size of the population. Since the surface of the circle is proportional to the square of the radius and the surface of the corona is proportional to the radius, it turns out that the growth equation is given by:
| (13) |
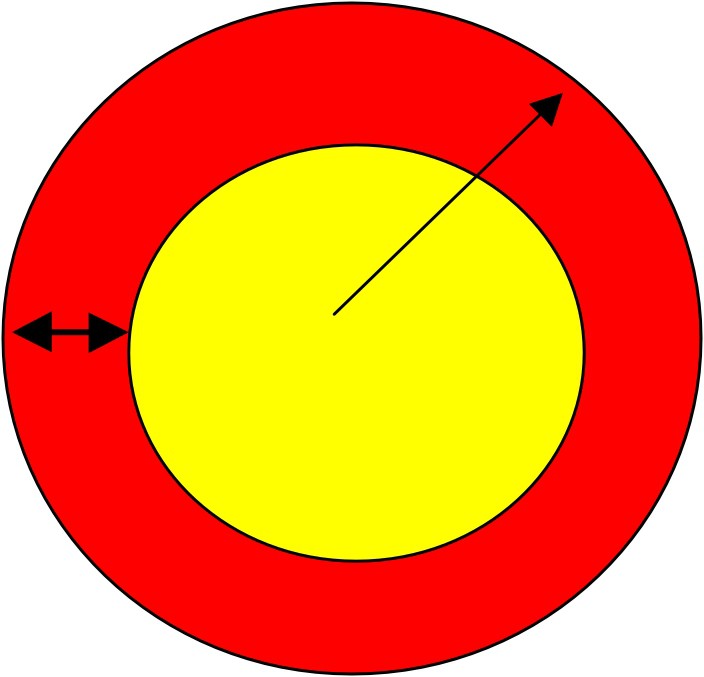
Growth of a species in a 2D scheme.
In the case of a three-dimensional scheme (Fig. 7), using the same argumentation, we obtain a growth equation like , which can be rewritten as with a function g that is decreasing with X. This explains our choice for the simulations. Now, let us assume that we have two species growing on the same substrate. Due to the fact that each population is growing through cellular division, it is likely that the majority of cells of one species are surrounded by individuals of the same species, as we show on the scheme below where colours red (or dark grey) and yellow (or light grey) stand for the two species.

Isolated cells before (top) and after the growth (bottom).
These arguments are in favour of the model (7) where we used a function g which behaves like
5 Discussion
For a long time now, ecology and microbiology have continued to develop independently of each other. Yet these two scientific fields have in common the same problems. One of the key processes studied in both disciplines is the way a microorganism consumes a resource. In ecology, this problem is equivalent to studying the functional response of a population, while in microbiology the question is related to the characterization of the growth rate of microorganisms. About ten years ago, Arditi et al. (cf. for instance [16]) have pointed out the major role of the density of preys in the growth rate process of a predator population. In particular, they have shown that, in a number of cases, it is more appropriate to model the functional response of a predator population in incorporating the ratio of the predator and prey densities instead of considering only a function of the density of preys in the growth process. As underlined in a recent paper in which he considers what ecologists can earn from microbiology, Jost (cf. [17]) pointed out that the same fundamental problems arise in microbial growth processes and that the Contois function is precisely a ratio-dependent model. In the present paper, we show that a kinetics function in which both the substrate and micro-organisms intervene can be seen as a way to model problems related to the accessibility of the biomass to its substrate. In particular, while the ratio-dependence was shown to be very important when studying a predation process, we show that it seems to be even more important when put in the light of competition phenomena. Apart from the fact that it could be a way to model the substrate diffusion, it is particularly useful to explain the coexistence of an arbitrarily large number of species on a single substrate. However, at the present time, it should be noticed that “abiotic conditions due to substrate limitation phenomena are at the roots of the coexistence of several species on a single resource” is only a work hypothesis that needs additional theoretical studies and conception of experimental tests to be validated. Furthermore, it should be noticed that we do not claim that coexistence in microbial ecosystems through an equilibrium in models with density-dependent functional responses is the only explanation of coexistence. In fact, we do believe that a large number of mechanisms already proposed in the literature (like variations of the environment – periodic external forcing –, sustained self oscillations, density dependence and other mechanisms to be discovered) are responsible all together for the coexistence of numerous species on comparatively few resources.
6 Conclusion
We have presented a possible mathematical model for the growth of n species in a chemostat in the presence of a single substrate. The model is general and does not presuppose any particular form for growth-rate functions of the various species. It only requires general natural assumptions (positivity, monotony...) and assumes that the growth rates are dependent on the density of the populations in a way that can be interpreted as ‘intra-species’ and ‘inter-species’ competition. It is shown by means of simulations (and as theoretically proved in [13]) that, in this model, if ‘intra-specific’ competition is large enough compared to the ‘inter-specific’ one, then there exists a stable equilibrium in which every species is present. We tried to explain this kind of competition on a purely abiotic basis by a non-uniform repartition of the substrate due to diffusion processes and the growing mechanism by the division of the cells. For sure, at this date, it remains a quite questionable hypothesis that needs experimental and further theoretical investigations.
Acknowledgements
This work presents part of ideas and results obtained in close collaboration with ecologists, mathematicians, and microbiologists through ongoing active common projects. This note is simply a preliminary one. In particular, we wish to thank Roger Arditi, INAPG, Paris, Jean-Jacques Godon, INRA–LBE, Narbonne, Antoine Sciandra, CNRA, Villefranche-sur-Mer, Alain Rapaport, INRA–LASB, Montpellier, and Frédéric Mazenc, INRA–INRIA MERE, Montpellier.


