Gamma irradiation may interact with biological medium to cause damage, either directly by disrupting critical molecules (such as enzymes, DNA or RNA), or indirectly by producing free radicals such as hydroxyl radicals [1]. The design of compounds that can protect efficiently against γ-irradiation is a great challenge. 2-(1-Naphthylmethyl)-2-imidazoline (naphazoline, NP), a drug belonging to the vasoregulator class, is a very interesting compound used in therapeutics as a peripheric -adrenergic agonist [2]; see Fig. 1 for its structure. Several years ago, NP was reported to have a good radioprotective effect even at low dose [3]. Further, NP appears to be, in vivo, an efficient protector agent against ionizing radiation with a Dose Reduction Factor (DRF) equal to 1.5, when it is injected at 30 mg/kg, 15 min before irradiation [4].

2-(1-Naphthylmethyl)-2-imidazoline hydrochloride (naphazoline).
Laval et al. have demonstrated that the association of NP with amifostine (WR-2721, S-2-(3-aminopropylaminoethyl)-phosphorothioic acid), the most effective radioprotector currently known, enhanced the radioprotective activity on γ-irradiated mice without increasing the toxicity of each compound [4]. Phosphorothioates are known to protect tissues or cells by free-radical trapping, hydrogen-atom donation and induction of hypoxia [5]. The mechanism of radioprotection of NP is still unknown. The aim of this work was to get a further insight into this unexpected activity of NP against γ-irradiation via in vitro experiments. In order to highlight the NP radioprotective activity, a comparison of its ability to inhibit DNA strand breaks produced, either by γ-irradiation or Fenton's reaction [6], was performed.
First, in order to observe the protective effect of NP against γ-ray-induced DNA damage, solutions of 0.5 μg of ΦX 174 plasmidic DNA (4361 bp, Amersham Pharmacia Biotech Inc) in 30 μl phosphate buffer 5 mM (pH 7.4, NaCl 10 mM) were submitted to various γ-radiation doses ( γ-rays at 25 °C; 50 Gy/h) aerobically in the absence or in the presence of different concentrations of 2-(1-naphthylmethyl)-2-imidazoline hydrochloride (Sigma Aldrich) (0.5, 1, 5, 10 mM). The doses applied in these experiments were similar to those involved in cellular tests (4, 5, 7 Gy) [7]. The supercoiled (Form I) and open circular forms (Form II) of DNA were separated by electrophoresis on an 0.8% agarose gel containing 25 μl ethidium bromide (10 mg/ml) after addition of 10 μl of bromophenol blue (75% glycerol, 24.95% Tris buffer, 0.05% bromophenol blue) in each sample. The number of single-strand breaks per mole of DNA generated by γ-irradiation was calculated from the relative percentage of forms I and II. The Student's t-test was used to determine effect of NP on the protection of DNA.
As shown Fig. 2A, γ-rays induced the formation of single-strand breaks (SSB), which increased with the dose. The yield of single-strand breaks (SSB) was evaluated around , and for 4, 5 and 7 Gy irradiation respectively (significantly different, drug + irradiation vs untreated control).

(A) Agarose electrophoresis gel pattern of ΦX 174 DNA exposed to γ-rays (7 Gy) in the presence or in the absence of NP. Lane 1: Untreated DNA, lane 2 γ-irradiated DNA (7 Gy), lanes 3, 4, 5, 6: γ-irradiated DNA (7 Gy) in the presence of NP (0.5; 1; 5; 10 mM). SC: supercoiled form; RF: relaxed form. (B) Percentage of DNA SSB generated by different doses (□ 0, ▪ 4, 5, 7 Gy) of γ-rays in the presence or in the absence of NP (0.5; 1; 5; 10 mM). All the samples are taken in triplicates and values are expressed as mean ± S.D. Drug + irradiation vs. untreated control; ; .
From Fig. 2B, we could observe that the control ΦX 174 contained mostly supercoiled DNA and only a small amount of the relaxed form evaluated to . The addition of NP in the reaction medium induced a decrease of the amount of single-strand breaks after γ-irradiation (Fig. 2). This effect increased with the concentration of NP. At a concentration of 5 mM, NP inhibited almost of the SSB formation. NP protects efficiently DNA against γ-rays.
The ability of NP to inhibit the deleterious effect of OH radical on DNA was investigated. For this purpose, Fenton reactions were performed. Indeed, the Fenton reaction allows us to simulate the indirect effect of γ-radiation called radiolysis of water, which leads to the production of . Hydrogen peroxide and Fe(II) can produce hydroxyl radicals according to the following reaction:
As shown in Fig. 3, radical generated induced the formation of SSB whereas 10 mM NP does not. In these conditions of supercoiled DNA is converted into the relaxed form by the radicals attack, whereas of relaxed form is present in untreated DNA (Fig. 3). This difference is significant. Addition of NP in the reaction medium induced a drastic decrease of the yield of SSB starting at 0.5 mM. At this concentration, around 46% of the formation of SSB was inhibited. This inhibitory effect increased with the concentration of NP. The maximum effect was reached at 5 mM, when DNA was almost completely protected. At this concentration, the amount of single-strand breaks corresponds to of the whole plasmidic DNA as in non-treated DNA (difference not significant). This concentration of NP is fairly realistic and is similar to that used for other radioprotectors such as verbascoside [8] to protect DNA against hydroxyl radical attack.

(A) Agarose electrophoresis gel pattern of ΦX 174 DNA exposed to generated by Fenton reaction in the presence and absence of NP (0.5; 1; 5; 10 mM). Lane 1: untreated DNA, lane 2: DNA exposed to , lane 3: DNA treated with NP 10 mM. DNA exposed to and treated by NP: lane 4: 0.5 mM, lane 5: 1 mM, lane 6: 5 mM, lane 7: 10 mM. SC: supercoiled; RF: relaxed form. (B) Percentage of DNA SSB generated by Fenton's reaction in the presence or in the absence of NP at various concentrations (0.5; 1; 5; 10 mM). All the samples are taken in triplicates and values are expressed as mean ± S.D. **Drug + Fenton reaction vs. untreated control; p<0.01.
This result accords with the radioprotective effect of NP observed on γ-rays irradiated DNA and mice. Moreover, all of these experiments strongly suggest that NP acts as an radical scavenger and so this property may partly contribute to the inhibition of the indirect effect of γ-rays. To confirm this last assumption and to evaluate the capability of NP to scavenge radicals, electron-spin resonance spectrometry studies were performed by using 5,5-dimethyl-2-pyrolidine-1-oxide (DMPO, 150 mM, Sigma Aldrich) as a spin trap [9]. Hydroxyl radicals were generated by Fenton's reaction as described above. All reactions were carried out in phosphate buffer 5 mM (pH 7.4, NaCl 10 mM) in a final volume of 100 μl. In each experiment, reactants were added in the same order: EDTA (100 μM), hydrogen peroxide (100 μM), NP (0; 0.5; 1; 5; 10 mM), ferrous sulphate solution (100 μM) and DMPO (150 mM). ESR measurements were performed on a Bruker EFP 300e spectrometer at room temperature. The intensity of the signal obtained at different concentrations of NP, was compared with the signal of DMPO/OH.
In the absence of NP, an ESR signal corresponding to (, ) was detected (Fig. 4). Addition of NP at various concentrations (0.5, 1, 5, 10 mM) induced a drastic decrease of the ESR signal attributed to OH radicals. This effect increased with the concentration of NP to raise a plateau at 5 mM (Fig. 4) as in DNA radioprotection experiments. This result confirms that NP is a potential antioxidant.
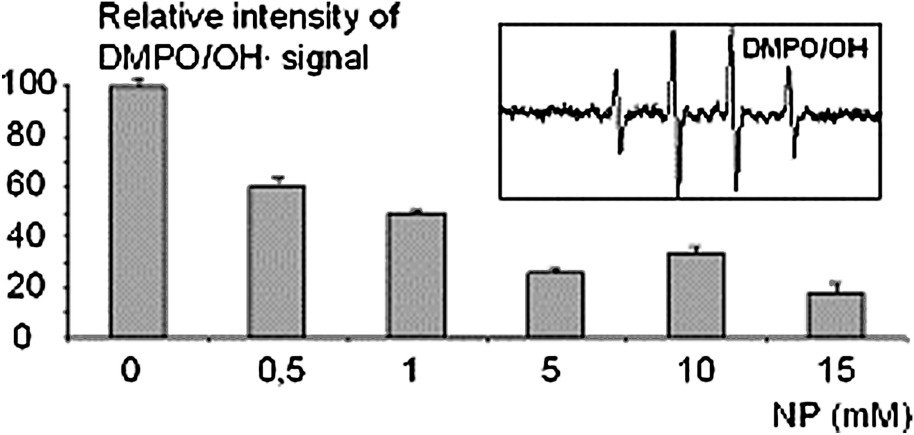
EPR investigation of the OH radical scavenging by NP at various concentrations. Percentage of the signal intensity of adduct produced in the presence of NP by comparison with a control without NP. Insert: signal of the adduct.
Radical trapping is one of the most-known mechanisms of chemical radioprotection. However, one physiological mechanism of radioprotectors known is the induction of hypoxia in different tissues. For example, the 5-hydroxytryptamine is described as a radioprotector, one of the mechanisms of action of which is tissue hypoxia as a consequence of vasoconstriction [10]. The reduction of oxygen tension of the blood-forming organs decreases the sensitivity of these organs towards radiation. NP possesses peripheric α-adrenergic property that induces hypertension. This pharmacological activity is effective when NP is administrated at high doses as in in vivo tests of radioprotection [3,4]. Our study shows clearly that NP has also antioxidant properties at high concentration. The activity of NP as radioprotector may result from a combination of both radical scavenging efficiency and vaso-constrictive property.
Conclusion
In conclusion, this work points out for the first time that NP may act as an antioxidant, thus preventing the deleterious effects of γ-radiation. Moreover, this effect can be increased by the cationic charge of NP at physiological pH, which can facilitate interactions with cell membranes and anionic proteins in the serum.
Acknowledgements
The authors wish to thank the ‘Délégation générale pour l'armement’ (DGA), the ‘Ministère de la Défense nationale’, France, and the ‘Office national d’études et de recherches aérospatiales', Centre de Toulouse (ONERA), France.


