1 Introduction
To validate a model is a tough issue because of the difficulty to link the modelled and the experimental variables. The most frequently met example is that of phytoplankton chlorophyll a (
As a consequence, adding a variable
This study aims at testing and comparing the inclusion of different formulations of
2 Materials and methods
2.1 Study site
New Caledonia is located in the Pacific Ocean at 22°S and 165°E and is subject to tropical or subtropical meteorological conditions. Winds are characterized by two main directions [14]. Trade winds blowing from 60°to 160°at speed higher than 4 m s−1 represent 69% of yearly wind occurrence (
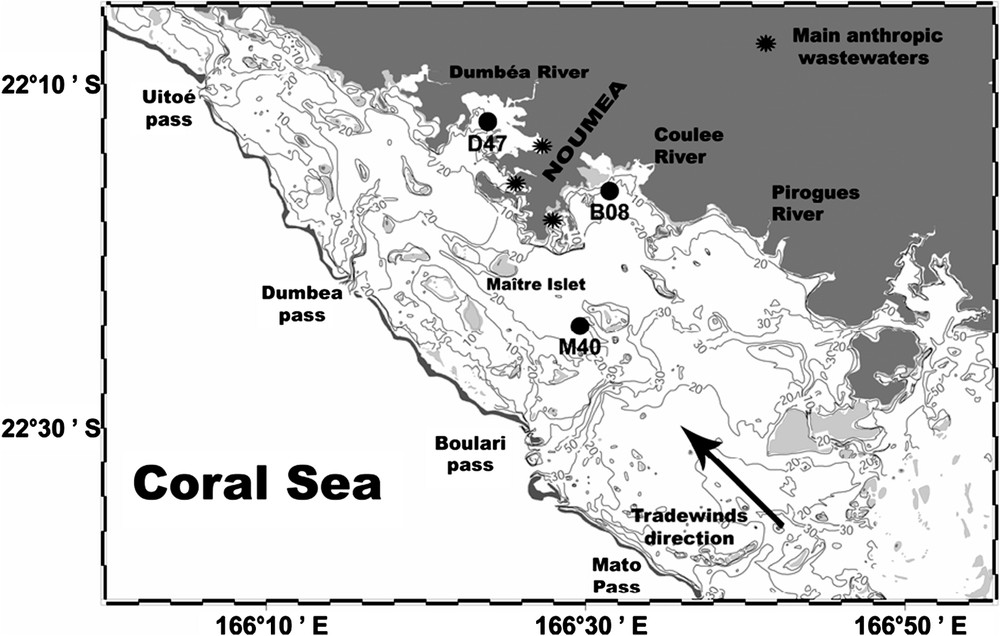
Map of the Southwest lagoon of New Caledonia. Black dots represent the studied stations (M40, B08, D47).
2.2 Hydrodynamic model
Hydrodynamic studies have been conducted in New Caledonia for over 25 years [18,19]. Recently, a model of the water circulation in the Southwest lagoon was developed and validated [20–23]. In these papers, model description, circulation analysis, vertical structure of the currents, and the initial and boundary conditions were presented in details. In this study, we use a
2.3 Biogeochemical model
The first biogeochemical model was developed by Bujan et al. [24] and Pinazo et al. [13]. An improved version was implemented by Faure [25] by adding an explicit microbial loop. The structure of the whole model is described in Fig. 2. The structure is typical of NPZD models (nutrients–phytoplankton–zooplankton–detritus). However, the zooplankton is considered as a theoretical population, undifferentiated and static, and no state variable is associated to it. Thus, the model is closed by a function of predation. Each compartment is expressed in carbon and nitrogen units, leading to 11 state variables (Table 1). One particularity of the model is the expression of the growth rate of phytoplankton based on the formulation of cell quota [26,27]. The variable stoichiometry allows taking into account the nutritional status of the cells, the nutrient limitation of phytoplankton growth, and the computation of nutrients uptake. Primary production is limited by temperature, light and nutrients (ammonium (NH4) and nitrate (NO3)). Bacterial production (BP) is also modelled with the cell quota concept [28]: BP is limited by the cell internal quota in carbon and nitrogen. All state variables equations could be found in Pinazo et al. [13] and Faure [25]. Here, we present only the pertinent equations (Table 2).
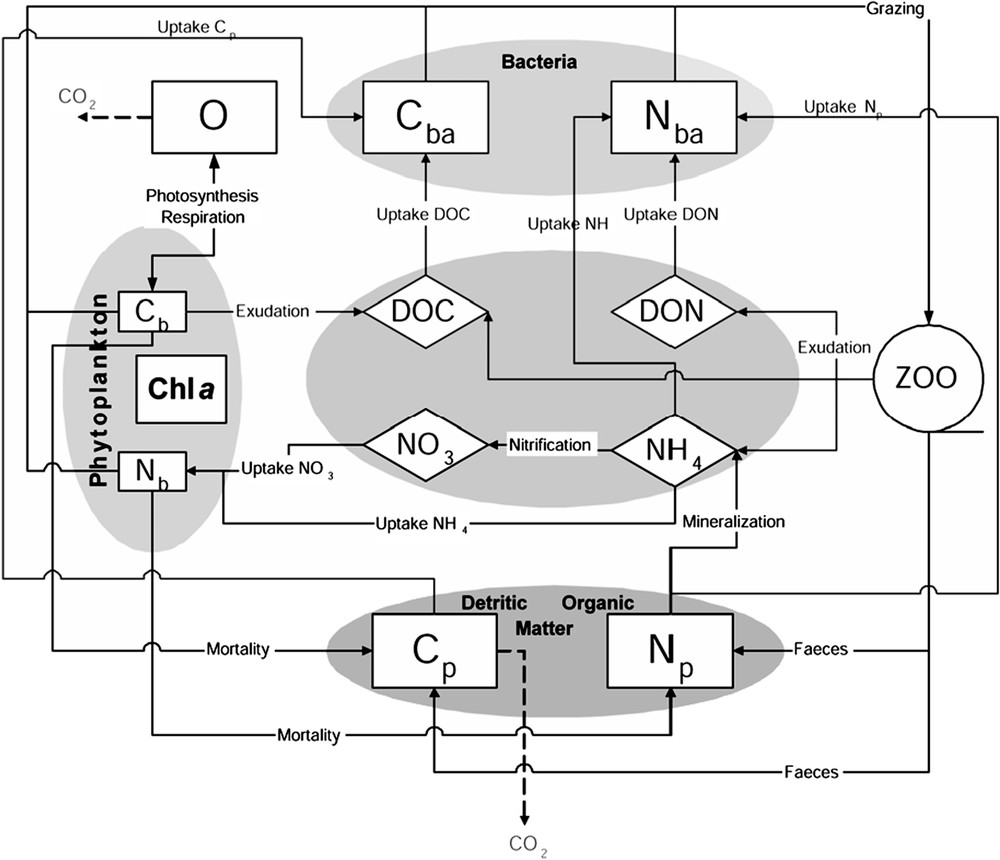
The pelagic biogeochemical model.
State variables in μmol l−1, except Chla in μg l−1
| CB | phytoplankton carbon |
| NB | phytoplankton nitrogen |
| Chla | phytoplankton chlorophyll a |
| CBA | bacterial carbon |
| NBA | bacterial nitrogen |
| CP | particulate detritic carbon |
| NP | particulate detritic nitrogen |
| DOC | dissolved organic carbon |
| DON | dissolved organic nitrogen |
| NH4 | ammonium |
| NO3 | nitrate |
| O | oxygen |
Three different formulations were tested in order to model the
In CHL_CST, modelled
As the model simulated a variable phytoplankton N:C ratio, the second formulation chosen (CHL_ST) was a stationary equation from Smith et al. [7] (Table 2, Eq. (6)). This equation calculated the
In CHL_DYN,
2.4 Coupled model
Eq. (1) gives the standard three-dimensional equation for each of the biogeochemical concentration (Conc). BIO_TEND represents the gain and losses of the considered variable:
The standard structure of three-dimensional biogeochemical equations is presented in Eq. (1). The left-hand side of the equation accounts for temporal variability, vertical and horizontal advection, and settling velocity of particles. The right-hand side of the equation accounts for biogeochemical gain and loss (BIO_TEND), and turbulent diffusivities. The current velocities (
The hydrodynamic model was forced by tide and by realistic wind with a time step of 10 min. It was dynamically coupled with the biogeochemical model [25]: the scheme of advection–diffusion calculated the evolution of biogeochemical variables in parallel to the execution of the physical code, with the same time step of 50 s. The biogeochemical model was forced by irradiance measured each hour, and calculated the trends of biogeochemical variables, with a time step of 20 min.
2.5 Simulation strategy
Initialization conditions were established thanks to a campaign during which 90 stations were sampled over the lagoon (Camecal 5, 19–27 June 2003). Boundary conditions were adjusted to field measurements and published data (Table 5). Freshwater inputs from the three main rivers were introduced in the model. The strong trophic gradient between the bays around Nouméa and the central part of the lagoon led us to consider the inputs in nitrogen from human sewages in three bays of Nouméa (Sainte-Marie, Koutio and Grande Rade, see Fig. 1). Moreover, coupling between pelagic processes and benthic mineralization could represent a source of nitrogen in coastal areas of the lagoon (C. Grenz, unpublished data). We added a constant positive flux of ammonium and nitrate into the pelagic water column along the coastline (NH4: 15 μmol m−2 h−1; NO3: 5 μmol m−2 h−1; Grenz, unpublished data).
Boundary conditions of state variables
| Chla (μg l−1) | CBA (μmol l−1) | NBA (μmol l−1) | CP (μmol l−1) | NP (μmol l−1) | DOC (μmol l−1) | DON (μmol l−1) | NH4 (μmol l−1) | NO3 (μmol l−1) | O (μmol l−1) | |
| Boundary conditions | 0.2 | 0.7 | 0.11 | 2.1 | 0.5 | 3 | 0.62 | 0.02 | 0.001 | 180 |
A 34-day realistic simulation of the model was run in order to examine the biogeochemical response of the Southwest lagoon to the transient meteorological conditions measured during June and July 2003 (Fig. 3). The simulation began on 19 June and ended on 23 July; it permitted to illustrate the model behaviour under different meteorological events. Firstly, a low irradiance (lower than 600 J cm−2 d−1) occurred around 27 June and 16 July, whereas the mean irradiance for this period is 1257 J cm−2 d−1; strong rains came with the second decrease of irradiance, leading to river flows in each river, up to 300 m3 s−1 for the Dumbéa River. Secondly, a shift from a low northwest to a strong southeast wind took place around the 7 July. The duration of the simulation was chosen in order to compare model outputs with field data.
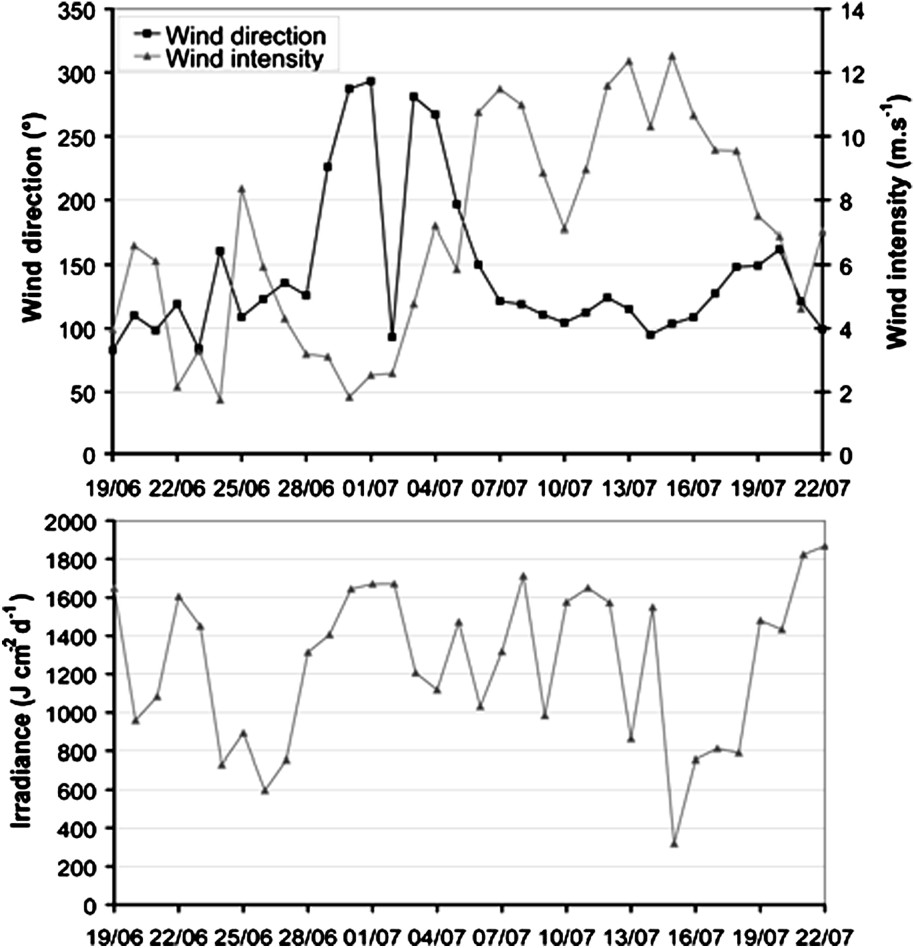
Upper panel: wind intensity (m s−1) and direction (○). Lower panel: irradiance (J cm−2 d−1). Data measured on Maître Islet from 19 June 2003 to 22 July 2003. Wind: mean values over 24 h of data measured every 10 min. Irradiance: daily sum of data measured every hour.
2.6 Measured data
Several lagoon surveys demonstrated that, on average, the water column is nearly homogeneous and that 3-m-depth samples are well representative of the whole water column [13,15]. For an easier presentation, results will be discussed after distinguishing three stations with increasing trophic status (Fig. 1): M40 represents the central part of the lagoon, B08 an open bay and D47 a semi-closed bay around Nouméa.
Experimental data were collected on 8 and 22 July at 3-m-depth on M40, B08 and on 10 and 23 July on D47. Chlorophyll
3 Results
Like experimental studies, the model outputs showed the same homogeneity for the vertical distribution of state variables and we chose therefore to present 3-m-depth model outputs, corresponding to the sampling depth. Fig. 4 presents for each station and each model formulation (CHL_CST, CHL_ST, CHL_DYN) the
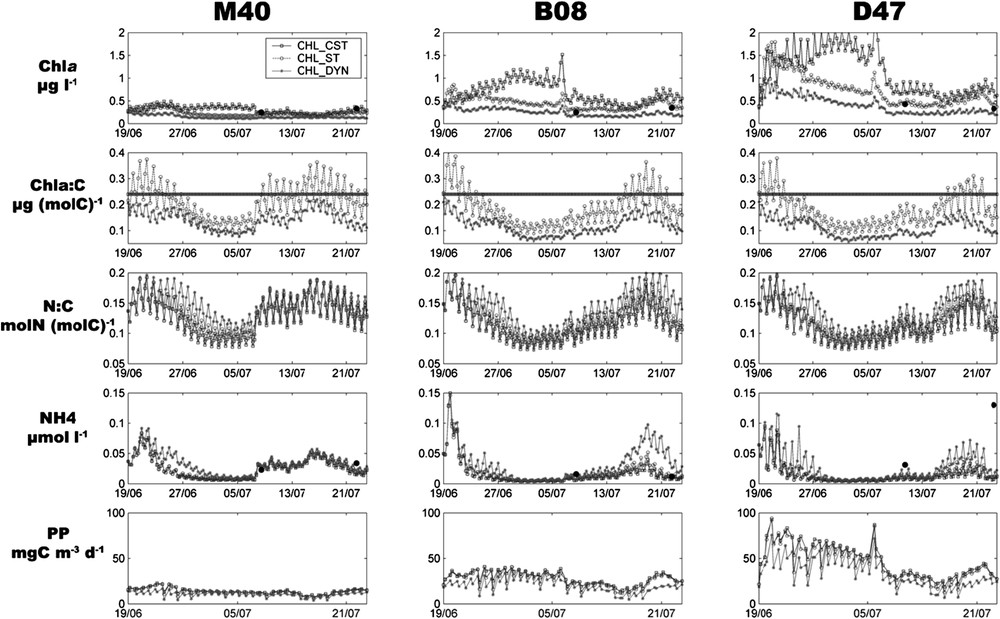
Time evolution of simulated Chla (μg l−1), Chla:C ratio (μg (mol C)−1), N:C ratio (mol N (mol C)−1), NH4 (μmol l−1) and primary production (mg C m−3 d−1), at stations M40, B08 and D47. Different curves represent results from each model formulation. Square: CHL_CST; circle: CHL_ST; star: CHL_DYN. Filled circles represent the measured values for Chla and NH4. Remark: the line for Chla:C ratio curves represents the constant ratio value in CHL_CST.
The impact of physical forcings was evident and explained the main variations of the biogeochemical variables. The two low-irradiance events led to a decrease of PP, and river inputs in the bay (B08 and D47) caused an increase of NH4 from 16 July. The wind shift of 7 July produced a decrease of
Simulated
Primary Production (PP, in mg C m−3 s−1), Chla:C ratio (g (mol C)−1) and Chla (μg l−1), in M40, B08 and D47, averaged on the 34-day simulation. Values are presented for each model formulation: CHL_CST, CHL_STA, and CHL_DYN
| M40 | B08 | D47 | |||||||
| CHL_CST | CHL_ST | CHL_DYN | CHL_CST | CHL_ST | CHL_DYN | CHL_CST | CHL_ST | CHL_DYN | |
| Average PP | 14.09 | 13.26 | 10.43 | 28.35 | 26.43 | 20.17 | 47.88 | 44.33 | 34.09 |
| Average Chla:C | 0.24 | 0.21 | 0.14 | 0.24 | 0.18 | 0.19 | 0.24 | 0.18 | 0.1 |
| Average Chla | 0.29 | 0.24 | 0.15 | 0.67 | 0.45 | 0.25 | 1.1 | 0.74 | 0.4 |
Values of
Model outputs for
4 Discussion
4.1 Validity of simulated variables
The simulated variables appeared to be consistent with usual observations in the Southwest lagoon of New Caledonia [15–17,19]. The gradient of primary production between the central part of the lagoon and the bays was well reproduced. The average simulated values (Table 4) appeared to be coherent with the measured data, with a yearly averaged PP of 8.1 and 45.3 mg C m−3 d−1 at M33 (close to M40) and N12 (in a semi-closed bay of Nouméa), respectively [15]. The measured value of
4.2 Behaviour of each model
As shown by a previous modelling study [13,23], the lagoon channel is deeply influenced by hydrodynamics resulting from trade winds. Oceanic waters enter the lagoon by the southern sector end, and very short flushing times of water are computed in the central part of the lagoon [23]. In our simulation, the different model formulations did not show large differences in M40, therefore under strong hydrodynamical forcings (strong trade winds), the complexity of a biogeochemical model does not significantly change the outputs [33].
However, flushing times of waters in areas like bays around Nouméa are longer [23], and therefore these zones are more controlled by biogeochemical processes. In B08 and D47, each model formulation clearly produced different outputs. During the first part of the simulation (19 June to 7 July), using CHL_CST,
Using the CHL_DYN simulation, the
List of parameters
| Parameter | Definition | Value | Refs. |
|
|
Grazing rate |
|
assumed |
|
|
Respiration |
|
[29] |
|
|
Chlorophyll-specific light-absorption coefficient | assumed | |
| ζ | Cost of nitrogen assimilation | 2.3 mol C (mol N)−1 | [29] |
|
|
Maximum growth rate |
|
[13] |
| b=0.063321 | |||
|
|
Maximum uptake rate for NH4 | 2.1×10−5 mol N (mol C)−1 s−1 | [13] |
|
|
Maximum uptake rate for NO3 | 8.5×10−6 mol N (mol C)−1 s−1 | [13] |
|
|
Half saturation constant for NH4 uptake | 0.2 mol N | [13] |
|
|
Half saturation constant for NO3 uptake | 0.3 mol N | [13] |
|
|
Minimum phytoplankton N:C ratio | 0.05 mol N (mol C)−1 | [11] |
|
|
Maximum Chla:N ratio | 3.8 g Chla (mol N)−1 | assumed |
|
|
Minimum Chla:N ratio | 1 g Chla (mol N)−1 | [7] |
|
|
Maximum phytoplankton N:C ratio | 0.2 mol N (mol C)−1 | [13] |
|
|
Constant phytoplankton Chla:C ratio | 0.24 g Chla (mol C)−1 | [13] |
5 Conclusion – perspectives
Few model studies focused on including a dynamic model of phytoplankton
A comparison between a Redfieldian and a dynamic model [34] showed that fluxes and stocks can be equally well predicted by both models. Both Lefèvre et al. [29] and Faugeras et al. [34] found a good agreement between dynamic model outputs and field data, suggesting that such model was relevant. And indeed, this first attempt of comparing different formulations in the SW lagoon of New Caledonia showed that the three different formulations displayed overall the same pattern.
In Faugeras et al.'s study [34], the variability of the simulated variables was much higher in the mechanistic model thanks to a faster response of this model to its environment. Our results in the Southwest lagoon of New Caledonia showed equivalent conclusions, with strong variations occurring during transient events such as modifications of the nutrients status or of the hydrodynamical forcings. For example, using CHL_DYN and CHL_ST simulations, the impact of nutrients availability on the
In parallel, this work underlines the importance of short-term sampling and the obvious difficulty to interpret field data in such a highly variable environment. Moreover, such modelling approaches could allow investigating the implication of high variability fluxes and variables on the carbon and nitrogen cycles of marine ecosystems.
In this comparative study of the SW lagoon of New Caledonia, the CHL_ST simulation appeared the most appropriate, since it provided outputs that were the closest to measured values, with a minimum parameterization and computation time. We can expect in the future that a dynamic model of the
Acknowledgements
This work was supported by the ‘Institut de recherche pour le développement’ (IRD), and grants from the ‘Programme national Environnement côtier’ (PNEC) and the ZoNéCo programme. Part of the meteorological data was kindly provided by Météo France and DAVAR.


