1 Introduction
The members of the family Kofoidiniaceae F.J.R. Taylor undergo an exceptional morphological transformation during their life cycle. This is responsible for the confusion on the identification of the species of the kofoidiniaceans. In one of the earlier works on marine dinoflagellates, Pouchet described Gymnodinium pseudonoctiluca from the coast of Brittany, southeastern English Channel [1]. Pouchet found intermediate characteristics between gymnodiniaceans and Noctiluca scintillans (Macartney) Kofoid. The two flagella, the occurrence of the cingulum and the sulcus is reminiscent of the gymnodiniaceans, especially the immature stage with an anterior cingulum that resembles a large Amphidinium. The surface of the cell and the radiating fibrils from the perinuclear region resembles N. scintillans [1]. Pouchet illustrated G. pseudonoctiluca as a single species with two different forms. One form corresponded to a large Amphidinium-like pigmented and the other form, the mature stage, was a round or oval non-pigmented laterally compressed cell.
Off Plymouth (southwestern English Channel), near the type locality of G. pseudonoctiluca, Lebour found three specimens of the immature stage of G. pseudonoctiluca [2]. From a single highly pigmented specimen, she described Gymnodinium viridis Lebour [2]. Kofoid and Swezy realized that the species name G. viridis was already occupied and they proposed G. conicum Kofoid & Swezy [3]. They considered that Pouchet had mistaken two species for a single one [3 (p. 244)]. They considered that one of the immature stages of G. pseudonoctiluca in Lebour [2] was a separate species. They proposed Gymnodinium fulgens Kofoid & Swezy based on the Lebour's figure. Pavillard observed immature stages of kofoidiniaceans in the northwestern Mediterranean Sea [4]. He considered that these forms, similar to the records by Pouchet and Lebour of G. pseudonoctiluca, corresponded to a separate species, and Pavillard proposed Gymnodinium lebouriae [4] (originally described as ‘lebourii’ and commonly misspelled as ‘lebourae’; ICBN: Art. 60.11; Ex. 24.; Recom. 60C.1.b; Art. 23.5 and 32.5; [5]). Later, Pavillard described the type species of Kofoidinium that at a first sight mainly differed from mature stage of G. pseudonoctiluca in the lack of the tentacle [6].
Cachon and Cachon investigated the life cycle of the kofoidiniaceans based on live specimens collected from the coastal Ligurian Sea (NW Mediterranean) [7]. They recognized six stages labelled from ‘A’ to ‘F’. The morphology of the immature stages of the kofoidiniaceans was too similar to distinguish and the species could only be identified at the sporont stage. In stages ‘A’ and ‘B’, the spores first develop into Gymnodinium-like ovoid motile cells (<35 μm long), which resemble non-pigmented gymnodiniaceans. As this stage, the cells ingest small particles, such as bacteria. In stage ‘C’, the hyposome has a globular shape, and the cells develop a structure of fibrils radiating from the perinuclear region resembles N. scintillans. This stage was illustrated as G. pseudonoctiluca in Pouchet [1] and Pavillard [4]. Stage ‘D’ has received the names Gymnodinium lebouriae (= G. fulgens) and G. conicum (= G. viridis). In this stage, the globular hyposome transforms into a cylindrical shape that resembles a large Amphidinium-like cell. The shape of the episome is highly variable and sometimes a peduncle is observed. The cell develops a marked net of fibrils towards the cystostome and is able to ingest larger particles such as diatoms and silicoflagellates. At this stage, the cells reach the higher degree of pigmentation. According to Cachon and Cachon, the pigmentation is due to small pigmented lipid droplets and chloroplasts were not observed in any of the life stages [7]. In stage ‘E’, after the cells get the higher biomass, the cells transform into the sporont flattening laterally (stage ‘F’). The differences in the morphology of the mature specimens allow the identification of the specimens to species. In the case of mature Spatulodinium, a tentacle is projected from the ventral region.
The distribution of the kofoidiniaceans is mainly restricted to warm to tropical waters, whereas Spatulodinium (= Gymnodinium) pseudonoctiluca is only known from boreal waters and from the northern Mediterranean [7–9] and the Black Sea [10]. Recently, Spatulodinium spp. has been recorded from the tropical and temperate waters of the Pacific Ocean [11].
In the eutrophic and turbulent waters of the English Channel, the dinoflagellate assemblage has a low diversity. However, after the spring, Phaeocystis Lagerheim and diatom bloom, S. pseudonoctiluca and its life stage ‘D’, misidentified as G. lebouriae and G. viridis is a highly distinctive species [1,2]. The present study investigated the seasonal and interannual distribution of the life stages of Spatulodinium, often considered as separate species, during eight years, in the proximity of the type locality. Illustrations of the transformation from stage ‘D’ into the mature stage are provided. This study is the first to describe an unusual reproductive stage in Spatulodinium that consisted of a cluster of pairs of small cells that were similar in form to the previously described species G. lebouriae.
2 Material and methods
Within the context of the SOMLIT monitoring program (‘Service d’observation en milieu littoral') 127 cruises were carried on board R/V Sepia II from November 1997 to December 2005 off Boulogne-sur-Mer (northeastern English Channel). Two permanent stations were sampled during the high tide. One station was located at 2 km offshore (50°40′75N; 1°31′17E; 21-m depth) and other station was located at 8 km offshore (50°40′75N; 1°24′60E, 50-m depth). The sampling frequency was planned to be biweekly, but cruises were often cancelled or were restricted to the most coastal station, due to meteorological constraints. During the spring and summer of 2003, 2004, and 2005, additional cruises were carried out with a sampling frequency planned to be once a week. Seawater samples were collected with a Niskin bottle at the surface and the bottom. Lugol-fixed samples of 25 or 50 mL were settled in composite settling chambers. The entire chamber was scanned at 200× with an IX71 inverted Olympus microscope equipped with an Olympus digital camera and each specimen was photographed at 400× with the DP70-BSW software.
3 Results
3.1 Temporal distribution in the northeastern English Channel
Spatulodinium was observed from May to October each year from 1998 to 2005 (Fig. 1). The highest abundance, 320 cells L−1 (8 specimens in 25 mL) was encountered in June 2004 after the bloom of Phaeocystis and diatoms, mainly Guinardia delicatula (Cleve) Hasle. Anomalously in spring 2005, the bloom of Phaeocystis spp. was not observed and only one specimen of Spatulodinium was encountered. The abundance of microplankton as well as Spatulodinium was higher in the coastal stations. No clear differences in the vertical distribution of Spatulodinium were observed in this highly turbulent environment. During and after the spring Phaeocystis-diatom bloom, the dinoflagellate assemblage showed a low diversity. Heterotrophic species such as Gyrodinium spirale (Bergh) Kofoid & Swezy and Protoperidinium spp. were dominant. The only noctilucacean observed was the distinctive sporont of Spatulodinium and its immature stages, with the exception of a very few records of Noctiluca scintillans.
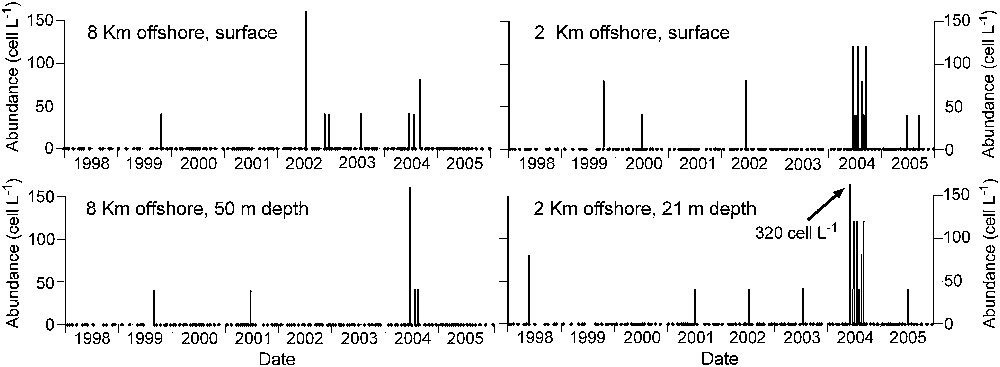
Spatio-temporal distribution of the abundance (cells L−1) of Spatulodinium pseudonoctiluca in the northeastern English Channel off Boulogne-sur-Mer from 1998 to 2005.
3.2 Morphology and life stages
The mature specimens of S. pseudonoctiluca (80–180 μm long) tend to be more oval than rounder in shape. The elongate tentacle was present in all the specimens (Figs. 11–14). The undulate flagellate often appeared unattached (Figs. 12–13). Stage ‘D’, precursor of the mature stage, was the most distinctive of the immature stages and co-occurred with the sporont in nearly all the samples. This form, described as Gymnodinium lebouriae, showed a cylindrical shape with an anterior cingulum that resembles a large highly pigmented Amphidinium-like cell (Figs. 6–10). One of the specimens showed two small ‘wings’ during the transformation from stage ‘D’ into the sporont Spatulodinium (Fig. 9). Coinciding with the period of highest abundance of Spatulodinium in June 2004, a cluster of four pairs of smaller cells of the form G. lebouriae joined at the elongate episome was observed (Figs. 4–5). This may be the origin of the elongate episome in stage ‘D’ (Fig. 6). In addition, co-occurring with the previous forms, cells with a globular hyposome and a visible net of fibrils were observed. These cells, with variable degrees of pigmentation, may correspond to stages ‘B’ or ‘C’ of Spatulodinium (Figs. 2–3). In the case of other smaller Gymnodinium-like cells, it is difficult to discern if they are gymnodiniaceans or immature stages (‘A’, ‘B’) of Spatulodinium.

Photomicrographs of Spatulodinium pseudonoctiluca. 2–3. Stage ‘C’, usually reported as Gymnodinium pseudonoctiluca. 4–5. Cluster of pairs of small cells of stage ‘D’. 6–10. Stage ‘D’, usually reported as Gymnodinium lebouriae (= G. fulgens) or G. viridis (= G. conicum). The arrows in Fig. 9 indicate two ‘wings’ in the specimen that correspond to the flattening in the transformation from stage ‘D’, G. lebouriae, into the mature stage. 11–14. Mature specimens. The arrows indicate the undulate flagellum. Scale bar=50 μm. Masquer
Photomicrographs of Spatulodinium pseudonoctiluca. 2–3. Stage ‘C’, usually reported as Gymnodinium pseudonoctiluca. 4–5. Cluster of pairs of small cells of stage ‘D’. 6–10. Stage ‘D’, usually reported as Gymnodinium lebouriae (= G. fulgens) or G. ... Lire la suite
4 Discussion
4.1 Distribution in Boreal–Arctic waters
The complex morphological transformation along the life cycle is responsible for the confusion in the identification of the species of kofoidiniaceans. The morphology of the immature stages of Spatulodinium and Kofoidinium are so similar that it is difficult to identify the genera to which the immature specimens belong.
Spatulodinium pseudonoctiluca has been reported along the boreal Atlantic European waters, Russian and Canadian Arctic waters and near the Japan Sea [12]. However, in several checklists in the Atlantic European waters, Spatulodinium (= Gymnodinium) pseudonoctiluca and G. lebouriae are both included as separate species [13–17]. In British waters, Dodge considered G. lebouriae as an immature life stage of Kofoidinium [14]. If this consideration is valid, Kofoidinium should appear associated with G. lebouriae in the English Channel. However, the only species co-occurring with G. lebouriae is Spatulodinium. All the species, G. pseudonoctiluca, G. lebouriae, and G. viridis were described in the English Channel in June or July, which is the period of higher abundance of Spatulodinium, as reported in the present study. No species of Kofoidinium have been reported in the boreal Atlantic Ocean [16,17], the Russian Arctic waters [18] or the Japan Sea [19], with a very few exceptions in western Ireland [20] and in the Norwegian Sea [14,21]. Bursa [22] described K. arcticum in the Canadian Arctic waters, where G. pseudonoctiluca has been reported [23]. As reported by Cachon and Cachon [7 (p. 437)] and Taylor [24], K. arcticum is a doubtful taxon, described from a single fixed specimen evidently deformed due to the preservation. Records of Kofoidinium in very cold waters of high latitudes are very rare compared to the more common Spatulodinium.
4.2 Records beyond Boreal–Arctic waters
The records of Kofoidinium in the Boreal–Arctic waters are rare. However, Kofoidinium is common in the Mediterranean Sea [25], whereas the records of Spatulodinium are scarce. Cachon and Cachon illustrated the sporont in the Ligurian Sea [7]. In the Adriatic Sea, both Spatulodinium and Kofoidinium have been listed [8,9] as well as G. pseudonoctiluca and G. lebouriae [26]. The distribution of Kofoidinium and Spatulodinium overlaps in the colder basins of the Mediterranean Sea. In the Black Sea, the only record of S. pseudonoctiluca corresponds to Stoyanova [10]. No records of Kofoidinium exist in the Black Sea [27].
The records of Kofoidinium in temperate to tropical waters are numerous [24] and its immature stages may be referred to as G. pseudonoctiluca [28,29]. Following Cachon and Cachon [7], Balech illustrated stage ‘C’ of Kofoidinium pavillardii as G. pseudonoctiluca [28]. He reported K. pavillardii and K. velelloides, and he did not find S. pseudonoctiluca. A recent study reported Spatulodinium spp. in the tropical and temperate Pacific Ocean [11].
4.3 Ecological aspects
The northeastern English Channel, near the Dover Strait, is a shallower environment subjected to intense winds and a tidal range of ∼8 m [30]. A bloom of Phaeocystis dominates this highly turbulent environment in spring and diatoms throughout the year. The early immature stages of Spatulodinium feed on small particles such as bacteria, whereas the large stages, especially stage ‘D’, feed on larger particles such as diatoms. The Phaeocystis post-bloom conditions, usually around June, provide abundant detritical material from the decomposition of Phaeocystis that may favour the development of immature stages. The present study for the first time illustrates a unique reproduction of the immature stage ‘D’ (Figs. 4–5). Cachon and Cachon observed an 8-cell chain of an immature stage of Kofoidinium that in only 2 h divides into a 16-cell chain [7]. Stage ‘D’ seems to develop a strategy of fast division in the short period of favourable conditions (Fig. 15). The high abundance of diatoms in late spring and early summer is the source of preys for the larger stages of Spatulodinium.
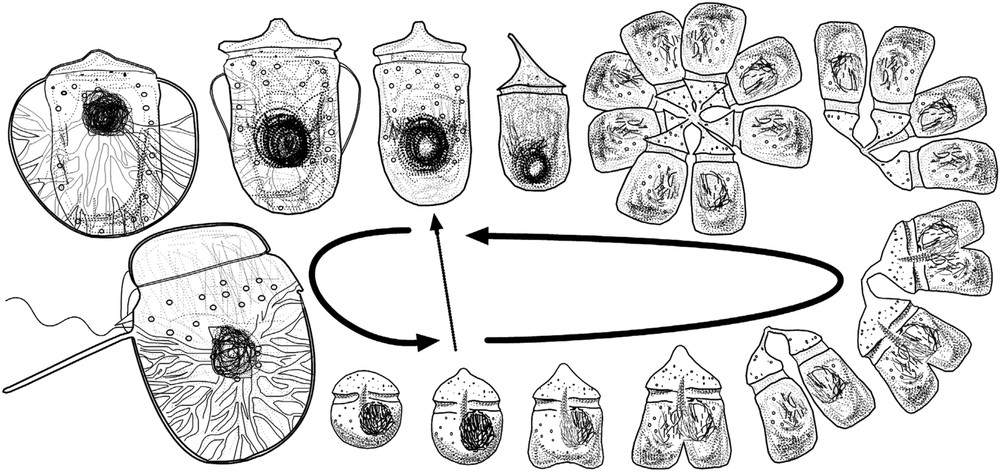
Scheme of the life stages of Spatulodinium pseudonoctiluca in the English Channel.
The present study investigates for the first time the ecological distribution of members of the family Kofoidiniaceae. Spatulodinium is especially adapted to the strong phytoplankton fluctuations in the turbulent northern English Channel. The unique mechanism of reproduction, which is for the first time illustrated here, facilitates the fast division and the occurrence of the mature stage in the short period of favourable conditions.
Acknowledgements
Samples were collected within the context of the SOMLIT programme on board R/V Sepia II (INSU–CNRS). We thank N. Degros, E. Lecuyer, D. Devreker, and G. Flamme for their help on sample collection. This is a contribution to the French IFB ‘Biodiversité et changement global’ and PNEC ‘Programme d'environnement côtier’ programmes.


