1 Introduction
Monogamy is generally uncommon and unevenly distributed among mammals (3–5% according to Kleiman [1]): up to 15% in Primates, common in Canids, occasional in Rodents. However, in small nocturnal mammals, monogamy seems more frequent than previously thought. As early as 1981, Foltz [2] believed that their elusive behaviour prevented detection of monogamy in various rodent taxa. In effect, since this statement, evidence for pair bonding or other monogamic traits has been evidenced in several rodent families, and it could even be widespread in Microtines (review in Smorkatcheva [3]). Nevertheless, the mating system of murids remains largely to discover, with apparently controversial reports. For example, populations of the wood mouse Apodemus sylvaticus seem to be organized around a dominant male that patrols a large range including that of several females and other subordinate males [4–6], suggesting a polygynous mating system [7]. Other observations have led to descriptions of male–female pair bonds typical of monogamous systems [8–10], whereas a genetic analysis in an enclosed population revealed multiple paternities [11]. Studies of the mound-building mice Mus spicilegus show also various issues leading to postulate a flexible monogamous mating system, with facultative polygyny or promiscuity [12–17]. Even the so-called monogamous prairie vole (Microtus ochrogaster) was found to engage during a laboratory experiment [18] in multi-male mating acts similar to those observed in related promiscuous voles. In fact, the mating system may vary in a species-specific manner depending on socioecological factors at the populational level [19,20], or even at the individual level, each individual being a priori presumed to act adaptively, even though it behaves differently from other individuals [21 (p. 169)]. In the same way, Kay [22] predicts that flexible social monogamy should occur in elusive mammals with dispersed social system.
This is the case for the Algerian mouse, Mus spretus, an exclusively outdoor species, morphologically close to M. musculus domesticus, occurring in the western Mediterranean region. Under laboratory conditions, wild males belonging to a population from southern France (Languedoc) showed a highly aggressive behaviour particularly oriented toward other males housed in similar conditions, i.e. heterosexual pairs [23] (see below). Hurst et al. [24] proposed that intermale aggression in Mus spretus is used for resource defence. Another hypothesis is that the fierce intermale aggressions occurring in this species could be related to mate guarding, involving high intolerance towards potential rivals. Indeed, several features observed in a natural context or in outdoor enclosures suggest the occurrence of a monogamic mating system in M. spretus, such as exclusive mating, paternal behaviour, and female dispersion (Table 1). Although insufficiently conclusive by themselves, some of these observations favoured a monogamous mating system, others fail to agree, but the whole set suggests monogamy at least facultative as a parsimonious hypothesis.
Some ecological and behavioural features of the Algerian mouse Mus spretus resulting from previous works and inferences for a possible monogamous mating system in this species
| Data from | Design | Previous works | Results | Inference on the mating system | |
| Laboratory experiment | Wild individuals were reared by pairs before a 10-min encounter in a 0.65-m2 terrarium. | – male vs. male – female vs. female | [23] [23] | – 70% aggression 5% investigatory items – only 1 encounter out of 10 was aggressive | – Possible mate guarding. – Contradictory to literature data about monogamous rodents. |
| Laboratory-born individuals from a Spanish strain | – male vs. female | Present paper | See below. | See below. | |
| Outdoor enclosures | Individuals were caught in Spain and genetically characterized. The observations were carried in two 50-m2 enclosures provided with feeding and cover places: – in the first one, three together-reared males were released with three females reared also together; – in the second one, three male–female pairs were released. | Unpublished thesis [25] | – In both cases, one male became dominant and killed the two other males; – based on the genetic constitution of the pups, the male reproduced with only one female in each enclosure; – in several instances, the male was caught with (its?) youngs, including subadults. | – Possible link with mate guarding. – Suggests a genetic monogamy. – Suggests paternal investment. | |
| Field | CMR in a garrigue near Montpellier. The trapping sessions were made during two-week periods over two years, on a 300-ha grid with traps 30 m apart. | Recalculated from [26] | The mean nearest-neighbour distance between adult females was greater than intermale or female–male distance, without overlapping between ranges. | “Females living in separate ranges appear to be a more common starting point for the evolution of social monogamy in mammals.” [27 (p. 18)] | |
| The between-sex distances were always the weakest. | |||||
| After each session, a multiple-catch trapping was achieved during one week | [28] | The only adult multiple catch achieved were male–female pairs, and this during the higher density periods. | We interpreted multiple catch as a as a social phenomenon, and male–female pairs seemed exclusive [28]. |
According to Møller [29], “the close spatial co-occurrence of a single male and a single female is the pre-eminent criterion for defining monogamy”; this seems to be the case for M. spretus (Table 1). Numerous authors [29–31] first characterize a monogamous mating system by the mutual partnership between a male and a female. The neurological basis of such a long-term association is now well known, and it affects affiliation, pair-bond formation, and paternal care [31,32].
Since mice are discrete, small-sized nocturnal animal, this feature has to be assessed by an experimental assay. In this paper, we use dyadic heterosexual encounters to investigate experimentally the degree of mutual pair bonding between sexual partners in Mus spretus in comparison with the related sympatric polygynous species, M. m. domesticus. We predict that (i) the social behaviour directed by males to their mate differs from that toward unknown paired individuals; (ii) in M. spretus, these differences should involve more affiliative (i.e. ‘amicable’) behaviours expressed by body contacts in the first case, and more agonistic or avoidance behaviours in the second one. On the contrary, in the polygynous M. m. domesticus, the male should exhibit a high interest for unknown potential matings expressed by investigatory acts. If there is acceptance by the female, we should observe more affiliative or sexual bouts from the male of this species than in the case of encounters with its usual mate; (iii) the social behaviour of the females of both species should be relatively similar toward their mate, but the polygynous house mouse female is expected to react in a similar way toward the unknown males.
2 Material and methods
Mice were provided by the Genetic Conservatoire of the Mouse (UMR 5171; http://www.genetix.univ-montp2.fr/souris.htm). They were reared in standard laboratory cages () under a reverse 12L:12D photoperiod. Water and standard laboratory food were available ad libitum. We used a Tunisian strain (STF) for Mus spretus and a Moroccan strain (DMZ) for M. m. domesticus. Laboratory strains were used for their easy breeding. Preliminary observations showed that these mice behaved similarly to wild individuals in their exploratory, social, and reproductive behaviour during laboratory experiments.
Mice were weaned when 30 days old and were separated by gender until 60 days old. At this time (day 0), 22 heterosexual pairs of M. spretus and 28 pairs of M. m. domesticus were formed at random excluding brother/sister pairs. Some pairs were separated at day 7, to constitute the ‘isolated males’ class (14 M. m. domesticus and 8 M. spretus). So 14 pairs of each species were available for the experiments (among which one M. spretus male and one M. m. domesticus female died before all experiments were done). Eight M. spretus males and 8 females, and 14 M. m. domesticus males as females were used as isolated males and virgin females. In both species, the virgin female were not those resulting from the separated pairs, but they were females that were bred with their sisters since weaning. As there were fewer mice available in the M. spretus strain, so five isolated male and five virgin female were used twice as target. There was no difference between the first encounter involving all eight individuals and the second one involving five individuals (test U Mann–Whitney: exact p from 0.284 to 1.000 for the eight behaviours expressed by females towards isolated males, and from 0.171 to 0.943 for the seven behaviours expressed by males toward virgin females). So all virgin females, on the one hand, all isolated males, on the other hand, were pooled in single category, whether they were used once or twice.
At day , a set of 10-min dyadic encounters was carried out. This procedure is more informative on the social relationships and the strength of the social bond than experiments based on olfactory preference, because it provides a qualitative expression of behaviours. Preference is evidenced by amicable behaviour, preceded or not by preliminary investigation; intolerance is revealed by passive avoidance or by overt aggression. All paired females used in the analysis were pregnant at the time of the experiment (a posteriori comparison between the three kinds of encounters showed that this had no decisive influence on the recorded differences: see Section 4). Mice were placed in a large PVC box () with a frontal glass 10 min before the encounters began. Observations showed that this duration was usually sufficient for mice to exhibit an exploratory behaviour. As mice, notably M. spretus, sometimes required a longer time to move, the record of behavioural elements only started when the first interactions between mice were apparent. All behaviours were recorded using a Psion Organiser event recorder with the Observer 3 software (Noldus Information Technology). All observations were made during the diurnal period.
Each individual was involved in three encounters. In the first one, which took place after a one-day separation period, the protagonists were always the usual mate. We operate in such a way because of the emotionality of Mus spretus mice, for which handling was more stressful than for the other species, leading to a longer time of familiarization in the experimental box. Thus, by following this procedure, we avoided an experimental skew due to stress generated by both experimental device and meeting an unknown opponent. At the same time, because little or no aggression occurred toward the usual mate, we thus avoided the possible effect on subsequent encounters described par Chase et al. [33]. In the case of males, the two other encounters involved an unknown paired female and an unknown virgin one. The virgin female had been housed with her sisters since weaning. They were in an oestrous or anoestrous state determined by cervical vaginal smears before the encounter. Statistical analyses were made by a three-factored ANOVA ‘species × parturition × oestrous state’ for each item with Bonferroni corrections. Neither the oestrous state, nor the interaction with each other factor or both factors together had a significant influence on the nine behaviours expressed by the male. Consequently, all virgin females were pooled for the analysis. In the case of females, the encounter with the familiar partner was the same that was previously described for the male, the behaviours of each individuals being separately recorded. In the following encounters, order of which was random, the protagonists were a paired male and an isolated male. The former was a male from another pair reared in similar conditions. The isolated male had been paired seven days with a female and then isolated for at least seven days prior to the encounter. Both of these males were unknown to the female. Such a scheme allowed us to compare results between the familiar male and two types of unknown males, both of which were socio-sexually experimented. The only difference between the latter males was that the paired male was housed with a female at the time of the encounter (and thus could maybe bear female odours), which was not the case for the isolated one. We did not use virgin males, since we wished to compare males that were sexually experienced.
The behavioural interactions were classified into five main categories and consisted in 22 elementary items (some of them were never recorded): (1) investigation (approach, naso-nasal, naso-body, and naso-anal sniffing, social attention); (2) amicable behaviour (body contact, following, allogrooming); (3) aggression (defensive and offensive posture, attack, chasing, fighting, avoidance); (4) sexual behaviour (mount attempt, mount, inaccurate mount – that is a mount exerted toward any part of the body, except the sexual zone –, lordosis, copulation); (5) self-oriented behaviour (locomotion, grooming, resting). This latter category was not used in the statistical analysis.
3 Statistical analysis
Most of the items were brief and every occurrence was counted as one unity. Some of them can be prolonged (body contact, chase...); in that case, every sequence of ten seconds was equal to one unity. These values were transformed to to obtain a normal curve and homoscedasticity of variances. The statistical analyses were performed using the Statistica-7 software. The results of dyadic encounters were analyzed with a two-factor ANOVA (species × opponent). When the ANOVAs on the classes of items or the only items were significant, the post-hoc test of Bonferroni was used. For interactions between factors, LSD (Least Significant Difference) tests were used for each item, corrected by the sequential test of Bonferroni.
4 Ethical considerations
This experiment was conducted under J.C.'s authorisation (No. 006555) to perform behavioural investigations by the veterinary service of the French ‘Ministère de l'Agriculture et de la Pêche’. It was performed in accordance with the ethical prescripts of this institution, which share the spirit and rule of the ‘guidelines for the treatment of animals in behavioural research’ concerning animal observations, maintenance, and handling. The mice were kept in good health, and kindly manipulated only before and following encounters in large cages.
5 Results
In general, our first prediction was verified, as males from the two species behaved differently toward the three kinds of opponents, that is the usual mate, the unknown paired female, and the virgin female. This was particularly true as regards amicable behaviour, which decreases markedly from the first encounter to the one involving the foreign-paired female, and finally the virgin one. When the two factors ‘species’ and ‘opponent’ are taken into account, the multivariate test yields a probability of 0.003 () for males, indicating that the two species react differently according to the nature of the opponent (Fig. 1). For females, there was also a significant difference (Fig. 2) for the interaction species × opponent (). In the following detailed analysis, only significant results will be provided concerning the behavioural items and the categories of items with the corresponding test value.
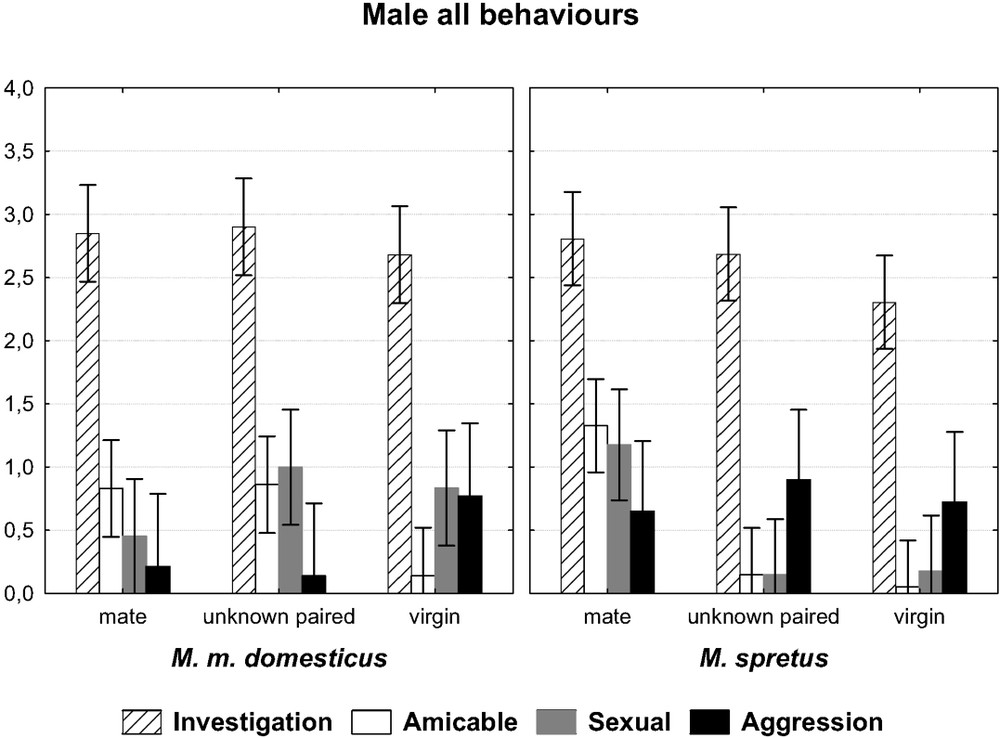
Comparison of 10-min encounters between a male of each species towards three kinds of females: its familiar mate, an unknown paired female and an isolated virgin female. Graph of the two-factor ANOVA species × opponent. (F(8,144)=3.0587, p=0.0033). The symbols show the main categories of behaviours (log-transformed mean values with 0.95 confidence intervals).
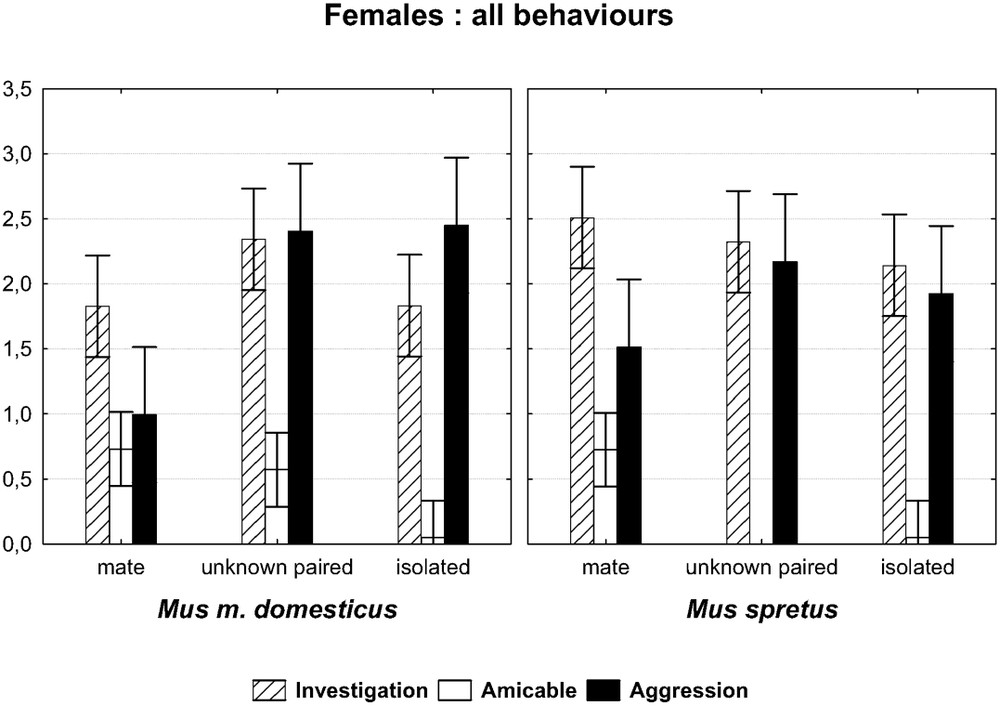
Comparison of 10-min encounters between a female of each species towards three kinds of males: its familiar mate, an unknown paired male, and an isolated male. Graph of the two-factor ANOVA species × opponent. (F(6,152)=6.6013, p=0.0000). The symbols show the main categories of behaviours (log-transformed mean values with 0.95 confidence intervals).
The male from the two species differed for the category ‘amicable behaviour’ (two-factored ANOVA: ). The spretus male showed more body contact with his usual mate, and significantly less with the unknown paired (Bonferroni post-hoc test, ) and virgin () females (Fig. 3). It also showed more sexual behaviours towards his mate than towards the unknown females (, ) (Fig. 4). This was true whether the paired (post-hoc test ) or the virgin (post-hoc test ) female was involved. For these two categories of behaviour, this was never the case for the M. m. domesticus male, which showed as many amicable behaviours towards his mate as he did toward the paired unknown female (Fig. 3) and more sexual behaviours towards the paired and the virgin unknown females (Fig. 4), but this was not significant. Otherwise, the M. spretus male exhibited less nasal sniffing towards the virgin (post-hoc test ) female than towards his partner, but the difference for the whole investigation category did not differ significantly from that of M. m. domesticus. In contrast, the domesticus male showed as many body contacts towards the unknown paired female as he did towards his familiar partner, and less towards the virgin female, but the difference was not significant.
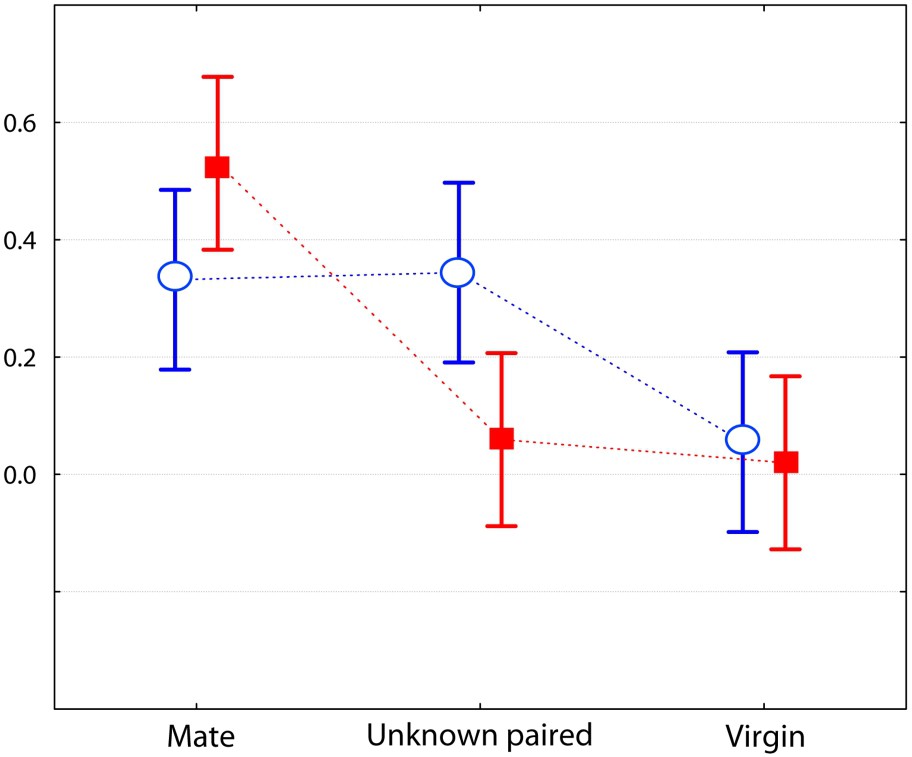
Comparison of 10-min encounters between a male of each species towards three kinds of females (the same as in Fig. 1). Graph of the two-factor ANOVA for the category ‘amicable behaviour’ (F(2,75)=5.1310, p=0.0082; log transformed mean values with 0.95 confidence intervals), with Mus spretus (red) and M. m. domesticus (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
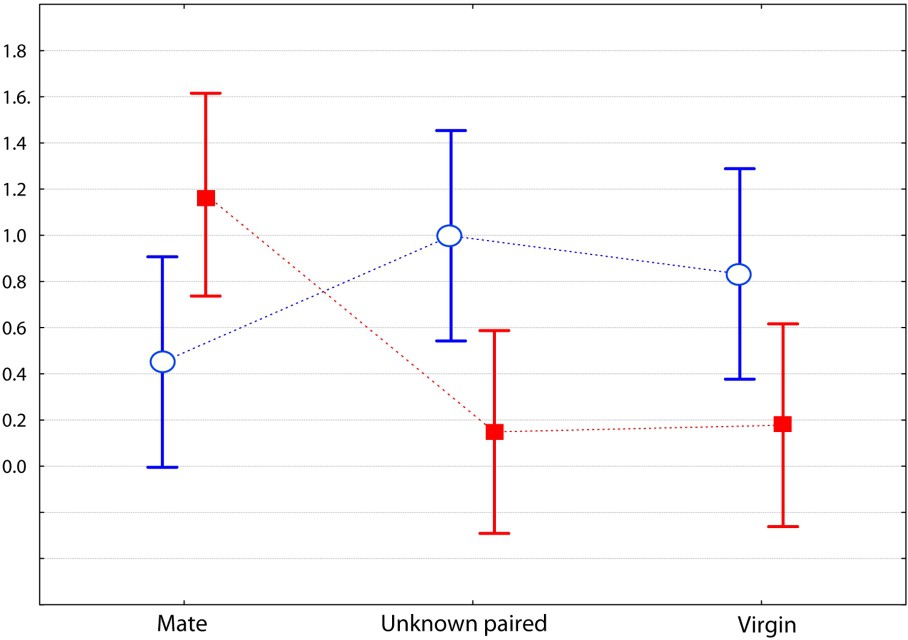
Comparison of 10-min encounters between a male of each species towards three kinds of females (the same as in Fig. 1). Graph of the two-factor ANOVA for the category ‘sexual behaviour’ (F(2,75)=7.307, p=0.0013; log transformed mean values with 0.95 confidence intervals), with Mus spretus and M. m. domesticus.
There was a significant difference between females of the two species as measured by the interaction species × opponents (see above). In detail, both species showed so much frequent investigatory acts whatever the opponent, as did their conspecific counterparts. However, the M. spretus females showed non-significant decreasing investigatory trends from her mate to the isolated male. They showed body contacts only towards their mate (difference mate vs. unknown paired male: post-hoc test , mate vs. isolated male: ), since they exhibited almost no such behaviour towards the unknown male (Fig. 2). The M. m. domesticus females behaved differently, being less amicable towards the isolated males, but so many towards their mate or the unknown paired males. Furthermore, they were more aggressive towards the two kinds of unknown males (isolated: , paired: ; see Fig. 2), which was more expected for the M. spretus females, which showed, but not significantly, the same trends. The emotionality mentioned in the individuals of this species, sometimes observed at their insertion in the device, seemed to have no influence on their behaviour, and we observed no pregnancy failure after the encounter, maybe too brief and too late to have such an effect.
6 Discussion
In this study, we evaluated the strength of the social bond that occurs between heterosexual pairs of Mus spretus mice and compared it to that in M. m. domesticus mice. The interactive behaviours were recorded during dyadic encounters; each individual was involved in three encounters, one with its usual mate from whom it was separated for one day, and two with unknown individuals (single or paired) from the opposite sex. Results are unequivocal and agree with our first prediction: males of the two species behaved differently toward the three kinds of opponents. The most significant behaviour of social attachment (affiliative behaviours such as prolonged body contact) was very strong between usual mates in M. spretus, whereas M. m. domesticus males were friendlier towards the unknown paired female than their own mate (Fig. 3). This confirms our second prediction. Moreover, the M. spretus male often showed sexual behaviours, but solely towards its own mate, whereas the opposite situation was observed in the house mouse (Fig. 4). An interesting point concerns the ‘inappropriate mount’ item, i.e. non-sexually oriented mounts, for which the motivation is likely not a sexual one. It could be an example of a ‘striking’ behaviour called pair-display by Wachmeister [34], that is a display exhibited after pair formation, “necessary for maintaining efficient reproductive cooperation and thus for keeping the relationship”. In the present work, the pair-display observations of affiliative and pseudo-sexual behaviours exhibited by M. spretus confirm our second prediction. However, the males did not show more aggressive items toward the unknown opponents than to its mate, contrary to what was expected from previous results [23]; this maybe due to the fact we used a laboratory strain originating from Spain wild mice in the present experiment.
The paired and virgin females were in different reproductive states. Had this a decisive effect on our results? We do not think so since (i) some behaviours, belonging to investigation or aggression category, showed no significant differences between species and kind of opponent, pregnant or not, and (ii) when there is a difference, it acted in a dissimilar way in the two species. Therefore, if the virgin females were less amicable than the paired females in M. m. domesticus species, both were treated in the same way by M. spretus male. The same two kind of opponents elicit more sexual behaviours than the mate by M. m. domesticus (but the difference was not significant), and less by M. spretus (difference mate vs. unknown paired male: Bonferroni post-hoc test , mate vs. isolated male: , and see Fig. 2). Thus, the reproductive state seemed to have no effect on the issue of the encounters.
The M. spretus females showed few differences with regard to M. m. domesticus in their interactions with their mate. However, compared to the other opponents, both behaved as expected according to our third prediction, with amicable behaviours expressed at first only towards their mate, and a little more aggression towards unknown males, whereas the house-mouse females showed more frequent investigatory, amicable as well aggressive drives towards the unknown coupled male than its own mate. There is a reciprocal social interest, but aggressive behaviour expressed by the female may be a response to the sexual willingness on behalf of males, as it is widespread in animal societies [35]). The lack of female–female aggression in M. spretus females, mentioned besides [20,36] seems to run against our third prediction (see below).
Even though scarce, field data collected in previous works [26,28] on a scrubland population of Mus spretus around Montpelier (Hérault, France) indicated that both members of heterosexual pairs inhabited the same area or very close ones. They were caught together in the same trap, whereas such spatial associations involving two adult males or two adult females were never recorded. On the contrary, associations of two or more adult females were observed in M. m. domesticus [28,37]. Thus, these observations, when added to the laboratory results on the behavioural interactions between the sexes, suggest the existence of a socio-spatial link between a single male and a single female in M. spretus. This is a prerequisite for the occurrence of a social monogamous mating system.
The pair display as evoked by Wachmeister [34] would have evolved because of the reproductive conflict between males and females. For this author, its exaggerated patterns serve as a manipulating behavioural mechanism. In the case of the M. spretus male, which is the only one of the pair to show such a behaviour (as the pseudo-sexual acts), what could be the females' decision-making that is being manipulated? The individuals of this species occupy large and complex ranges in habitat such as scrublands on limestone with rocky emergences. They most likely need a few days to patrol the whole range, as do mice of the New Zealand populations described by Fitzgerald et al. [38]. Consequently, there is a critical risk of separation during the oestrus period, which would make strengthening the pair bond necessary, and the manipulation by the male in that sense. The lack of aggressive behaviour on behalf of the female towards the unknown paired male (present experiment), as well as between females (previous results in [23], Table 1), remains unclear, as the means by which they establish territories, where intrasexual aggression generally plays currently a prominent role [39,40]. Indeed, there is a marked intolerance exhibited by females of other species of rodents in which a strong social link, with monogamous tendency, was shown [41–43]. This could be due to the lack of the currently invoked causation, that is resource distribution [44–46], which would not be limiting [26] in the case of M. spretus, at least during winter and early spring (since oak acorns are at this moment an abundant and rich food they eat), or to the fact that interference by other females is prevented by a sufficiently large distance between them (since their ranges are not contiguous). This could mean that in the mating system of this species, the female undergoes a forced monogamy, which would not necessarily resist a breeding opportunity.
Concerning the males, the relation between the mutual intolerance between mated males and a sexual aspect was confirmed by observations of enclosed populations (J. Cassaing, unpublished data). In this case, a male on a given territory had a single preferred female with whom it reproduced exclusively despite other breeding opportunities, and he eliminated all potential competitors. These observations, and the occurrence of a strong social bond between members of a pair, as revealed by the present study, suggest that social and sexual monogamy could occur in this species. To what extent remains questionable, and other features, as parental behaviour, or dispersing pattern, or the lack of aggression between females, must be investigated before concluding on a modal mating system.
Acknowledgements
The authors are thankful to Prof. Cezilly for preliminary discussions about the possibility of monogamy in a mouse that had never been described at that time. We particularly wish to thank Thierry Jacquart, who is no longer in the scientific field, for his work on genetic assignation of mice in the experiment in outdoor enclosures described in the introduction. We also thank Guilla Ganem and Janice Britton-Davidian for their helpful comments and English corrections, as well as two anonymous reviewers. Some parts of this research were supported by a grant from the Languedoc-Roussillon Region (France). This is publication ISE-M No. 2007-083.


