1 Introduction
On a daily basis, humans ubiquitously interact with their environment and, as a consequence, are exposed to a broad spectrum of synthesized chemicals present in the food they eat, the air they breathe, and the water they drink. Contaminants such as polychlorinated biphenyls (PCBs) are present at relatively high concentrations in food and show estrogenic, anti-estrogenic or anti-androgenic activity in biological test systems [1–3]. Although the concentration of the persistent organochlorines (POCs) in food has slowly decreased in some areas of the world, concentration is still high enough to be of concern for human health [4,5]. In addition, it was pointed out, in a number of studies, that these chemicals can adversely affect endocrine, reproductive, and immune systems [1,3]. Some sex-hormone-mimicking POCs might therefore affect bone mineral density and bone quality. This hypothesis is supported by a few experimental animal studies on PCB, DDT, hexachlorobenzene (HCB) and @-hexchlorocyclohexane (@-HCH) [6–8]. Tetradifon (Fig. 1), as an organochlorine pesticide, may therefore have important impacts on the environmentally exposed people, especially the farmers [9,10]. It has been reported to cause mutagenic effects [11] and impaired antioxidative system [12]. It has been extensively studied specially in D. magna [13]. In spite of the extensive use of tetradifon for the prevention of insect effects, there are, however, no reports about its effects on bone remodelling and mineral content.

The structure of tetradifon (C12H6Cl4O2S).
In the current study, we have analyzed whether a high dietary intake of tetradifon may produce skeletal damage and bone remodelling disturbance, using SEM, histomorphometric, and biochemical parameters. In addition, we have studied its effects on mineral composition in female Wistar rat serum and bone.
2 Materials and methods
2.1 Animals
Twenty-four sexually mature female rats, 10–12 weeks old and weighing about 190 g were purchased from the Pasteur Institute of Tunis (IPT, Tunisia). Animals were bred and kept in our animal facility under strict hygienic conditions and were free of major pathogens. Local vivarium conditions were controlled: temperature (24 °C), humidity (30–60%), and light (12 h:12 h light:dark cycle). Animals were provided standard laboratory diet (SICO Sfax, Tunisia). They were housed per group, six per cage and acclimated to holding facilities for 2 weeks prior to commencement of experimentation. A single cumulative dose of 2430 mg/kg body weight (BW) of tetradifon was administered orally for 90 days. Tetradifon [1,2,4-trichloro-5-((4-chlorophenyl)-sulphonyl)-benzene] was delivered by SEPCM (Megrine, Tunisia). The dose was chosen according to the works of Innes et al. [14] and Niepolomski et al. [15], and represented about one quarter of LD50. In a study on the fate of insecticides in an irrigated field, Yaron et al. [16] found a tetradifon concentration of 250 mg/kg (wet weight) in the peel of potato. This study involved two different exposure periods: a 6-week study and a 12-week study.
2.2 Euthanasia
The animals were sacrificed under anaesthesia by an intraperitoneal injection of 8% chloral hydrate (400 mg/100 g BW). The blood was obtained from heart puncture. The right distal femurs were shared; their lengths were measured. Subsequently, an incision was made parallel to the long axis of the femur after dividing the fascia and the M. biceps femoris. All the experiments were approved by the local Ethical Committee for animal experiments.
2.3 Histomorphometry
At the end of the experimental period, the right femur of each animal was carefully dissected out, defleshed and fixed in 10% formaldehyde for 24 h at 4 °C. The next day, the bone samples were embedded in methylmetacrylate without previous decalcification, as previously reported [17]. Seven micrometer-thick coronal sections were cut dry in parallel to the long axis of the femur, of the bone samples, using a heavy-duty microtome (Polycut S Reichert Jung, Germany) equipped with tungsten carbide knives. The sections were stained with a modified Goldner trichrome. Other sections were stained for tartrate-resistant acid phosphatase (TRAP) detection, and Giemsa fast (10%). The trabecular bone volume (BV/TV, expressed in %), osteoclast surface (Oc.S/BS, expressed in %) and osteoid volume (OV/BV, expressed in %) and surface (OS/BS, expressed in %) were measured by a point count method [18] using a 25-point integrating filter. The thicknesses of the bone cortices (Ct.Th, expressed in μm), trabeculae (Tb.Th, expressed in micrometres), growth plate and the trabecular separation (Tb.Sp, expressed in μm) were determined. The osteoclast surfaces (i.e., percentage of endosteal bone surface presenting features of bone resorption with osteoclasts) were also measured. Bone samples from different groups were examined blindly. According to the area of the femoral section, the grid was projected over ∼40 fields in each sample. For each individual animal, the combined fields examined covered completely the secondary spongiosa section area of the right femoral bone cut.
2.4 Scanning electron microscopy
SEM was performed on the femur, nearly 1 cm proximal to the knee joint space, of control and treated animals. The femurs were cut longitudinally with a scalpel, immersed in 50% sodium hypochloride for 3 h to remove organic material. They were rinsed for 30 min in distilled water and fixed overnight in 1% osmium tetroxyde dissolved in 0.1 M cacodylate buffer (pH 7.2). Subsequently, the samples were then rinsed for 30 min in distilled water, dehydrated in an ascending series of 70, 95, 100% ethanol. Further treatment was conducted in hexamethyldisilazane (HMDS) for 1 h, followed by air drying using a filter paper. They were SEM analyzed at 20 kV (SEM Philips series XL 30) as described by Libouban et al. [19].
2.5 Biochemical analysis
Serum alkaline phosphatase activity (AlkP), as a bone formation biochemical marker, was measured by a colorimetric method, using the alkaline phosphatase kit test optimized (Fluitest® AlkP). The kit was used according to the manufacturer's instructions. Calcium and phosphorus levels on femurs were determined after nitric acid mineralization, and in serum by calorimetric method using kits from Biomerieux France (Ref: 61041 and 61571, respectively).
2.6 Statistical analysis
Data are expressed as mean and standard error of the mean (mean ± SEM). The one-way analysis of variance (ANOVA) and the Student–Newman–Keuls post hoc test were performed on the data for intergroup comparisons. Database management and statistical analysis were performed using SPSS (SPSS Inc, Chicago, IL, USA) statistical software package. The nominal statistical significance level was set at 0.05.
3 Results
3.1 Biological findings
The animal treated with tetradifon remained healthy and showed no signs of toxicity from either direct observation or autopsy examination. However, those treated with tetradifon had a reduced rate weight gain in the first period of treatment, which was attributed to a decrease in the consumption of drinking water () and food intake ().
Taking into account the possibility of bone parameter differences between left and right limbs, as a result of functional bilateral asymmetry [20], the lengths of femur and tibiae were expressed as the mean for the left and right limbs for each animal. The femoral length varied between 34.25 and 36.5 mm; for the tibia, this range was 35.25 and 39.1 mm. There was no significant difference between treated animals and their correspondent control in the femoral and the tibial length. Elsewhere, the final growth plate thickness (μm) of the rats treated with tetradifon was slightly higher than in the control animal, though the differences were not statistically significant.
3.2 SEM and histomorphometric findings
Fig. 2 displays four images obtained by SEM examination of the endosteal femur, which differentiates two types of features. In fact, the endosteums (endocorticals) of both treated groups showed a heterogeneous aspect, while control ones appear homogeneous. For the cancellous bone, compared to control, treated rats displayed fewer and more separated trabeculae.
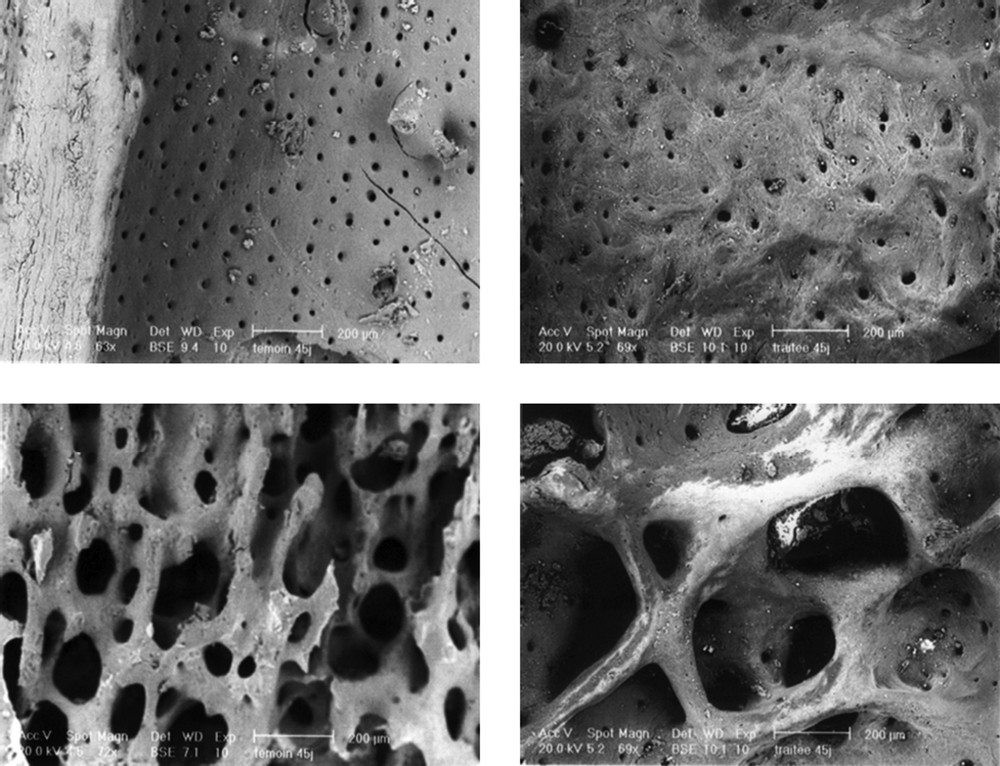
SEM micrographs of the endosteum (top), and the trabecular bone (bottom), compact bone, in control (left) and treated (right) rats. The work differentiates two types of features in the endosteum diaphysis and spongy bone. (A) The bone at the endosteum seems to be completely remodelled. A completely remodelled bone structure was observed. (B) The remodelling process in the endosteum zone was not yet complete. Bone neoformation seems to be in full activity. (C) and (D) Notice the apparent increase of the trabecular separation.
Histomorphometric data did not show any significant changes as regards the femora in 6-week treated animals compared to controls (Table 2), whereas those of 12-week treated rats exhibited significantly higher osteoid surfaces (OS/BS) and trabecular thickness (Tb.Th)
Bone histomorphometric data, comprising trabecular bone volume (BV/TV), osteoid volume (OV/BV), osteoid surfaces (OS/BS), osteoclast surfaces (Oc.S/BS), trabecular separation (Tb.Sp), growth plate thickness, and cortical and trabecular thicknesses (respectively, Ct.Th and Tb.Th)
| 6 weeks | 12 weeks | |||
| Control () | Treated () | Control () | Treated () | |
| BV/TV (%) | 32.17±2.21 | 30.17±2.02 | 32.83±2.72 | 34.17±2.07 |
| OV/BV (%) | 0.23±0.02d | 0.31±0.03 | 0.25±0.03 | 0.41±0.04a,c |
| OS/BS (%) | 3.06±0.25d | 4.53±0.52 | 3.4±0.34d | 5.4±0.65a,c |
| Growth plate thickness (μm) | 61.9±3.7 | 80.8±9.2 | 63.7±6.2 | 69.5±5.0 |
| Ct.Th (μm) | 143±9 | 151±10 | 137±11 | 144±8 |
| Tb.Sp (μm) | 161±12 | 181±14 | 180±10 | 206±6 |
| Oc.S/BS (%) | 3.8±0.3 | 4.7±0.3 | 4.9±0.6 | 4.9±0.7 |
| Tb.Th (μm) | 40.5±2.6d | 50.3±5.8d | 55.1±3.2d | 67.7±4.2a,b,c |
a versus control of 6 weeks;
b versus treated rats of 6 weeks;
c versus control of 12 weeks;
d versus treated rats of 12 weeks.
3.3 Biochemical findings
The biochemical data (Fig. 3) showed that serum AlkP level, which reflected bone formation, underwent a highly significant increase (), in the 12-week-treated rats compared to the corresponding controls. Table 1 shows the results of the calcium and phosphorus measurements on femoral bone after nitric acid mineralization and in serum by calorimetric method. Analysis pointed out that the bone levels of calcium and phosphorus were dramatically increased after 6 weeks of treatment.
Bone and serum levels of calcium and phosphorus
| 6 weeks | 12 weeks | |||
| Control () | Treated () | Control () | Treated () | |
| Bone levels (mg/g) | ||||
| Calcium | 95.83±5.87 | 128.16±8.38⁎ | 95.5±4.11 | 117.08±7.09 |
| Phosphorus | 38.08±2.09 | 49.26±3.76⁎ | 45.83±3.02 | 48.66±3.70 |
| Serum levels (mg/l) | ||||
| Calcium | 2.42±0.05 | 2.26±0.05 | 2.32±0.04 | 2.44±0.04 |
| Phosphorus | 3.10±0.32 | 2.42±0.08 | 2.63±0.01 | 3.21±0.20 |
⁎ compared to the correspondent control group.
4 Discussion
The aim of the present study was to evaluate the potential of tetradifon, as an oestrogen-like pesticide, on bone, and to examine its effect on bone biochemical markers (AlkP) and contents (calcium and phosphorus).
Reports correlating bioavailable oestrogen with skeletal mass in both sexes likewise corroborate an important role for oestrogen in skeletal conservation in both sexes [21]. Up to now, the hypothetic control of the bone remodelling by neurotransmitters seems minor compared to its endocrine regulation (steroid hormones, estrogens and corticosteroids) [22]. And the detection of the effects due to the oestrogen-like pesticides arouses a big concern, as oestrogen, the key modulator of osteoclast formation, plays an important role in maintaining bone mass in adult women by suppressing bone remodelling and maintaining a balance between osteoblastic and osteoclastic activity [23]. Its withdrawal results in an increased osteoclast formation and postmenopausal bone loss [24,25]. The decline of its level is accompanied with a disturbance of bone remodelling, in favour of resorption activity involving an osseous fragility [21] and postmenopausal bone loss [24].
The present study's treatment with an oestrogen-like substance led to an increase of bone formation. The femur proved to be a susceptible bone, especially the femoral diaphysis, which is composed of cortical bone, but also the distal femur, rich in trabecular bone. The scanning electron microscopic results of our experiments revealed that treated animals bone tissue did not show a structural alteration in comparison to the control rats (Fig. 2). However, we noted in the animals treated during 6 weeks as well as during 12 weeks a relative increase of the intertrabecular spaces of the spongy bone. This could result in a relative reduction of the trabecular density. Elsewhere we noted a heterogeneous aspect on the endosteum level of the treated rats during 6 and 12 weeks contrary to the control ones for which the endosteum appears homogeneous (Fig. 2C and D). This could be explained by remainders of bone reworking, in connection with a relative delay of ossification. Thus, the remodelling process appears still incomplete and the bone neoformation seems to be in full activity.
This heterogeneous aspect of the endosteum could be also explained by an exaggeration of the calcium deposition, corroborated with the cortical thickness, which appeared more important for the animals 12 weeks after the treatment. In addition, the bone mineral composition study revealed that tetradifon disturbed the mineral composition of the endosteum femoral bone, especially calcium and phosphorus (Table 1). In fact, after 12 weeks of treatment, the contents of calcium, a major element in the bone and many cellular metabolisms [26–28] in the treated rats' bones were much more important than those of the control rats. So tetradifon altered bone mineral composition especially calcium and phosphorus contents. These data concurred with recent studies by Lind et al. [29] who had shown that pesticides impaired bone strength and provoked changes in the bone tissue composition in rats and in female juvenile Americans alligators [30]. Disturbances in calcium metabolism have also been reported by Andrew et al. [7] and Samet et al. [31] in rats exposed to hexachlorobenzene and dimethoate, respectively.
Alkaline phosphatase (AlkP), which is an enzyme produced in liver and bone tissue, indicates, at least in part, the bone neoformation [32] and its mineralization [33]. Our serum samples of the treated animal for 12 weeks show a significant increase in the concentration of AlkP in comparison to the control rats (Fig. 3). This increase could occur during hepatic affections when there is an obstacle on the bile ducts and/or in the osseous affections that are accompanied by an exaggeration of osteoblastic activity so reflecting the bone formation. This last possibility is most probable since we did not note a hepatic damage (data not shown). Thus, the osteoblastic hyperactivity could be explained by the oestrogen-like activity of tetradifon.
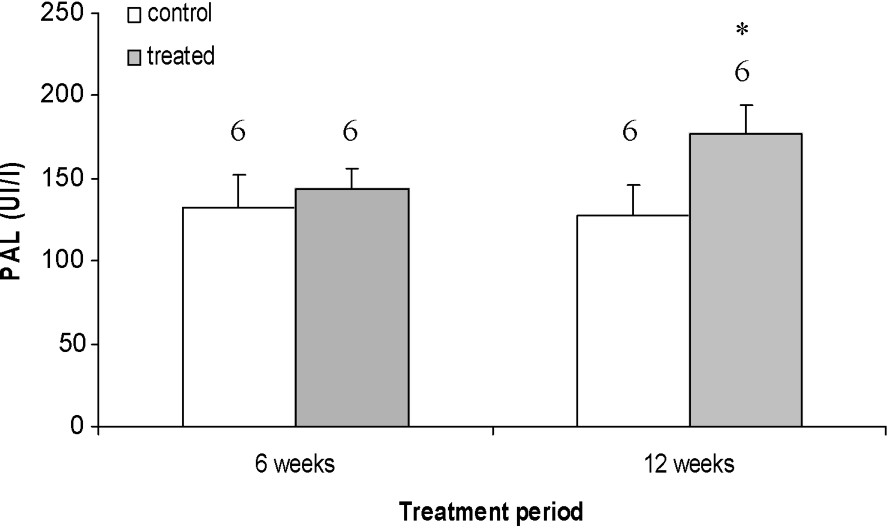
Effects of 6- and 12-week treatments with tetradifon on alkaline phosphatase (AlkP). Data are expressed as means ± SEM. The number of rats is represented above the columns of each diagram. * Statistically significant with p<0.05 from the corresponding control.
Organochlorine pollutants tend to concentrate in tissues with high lipid content because of their lipophilic properties [34]. Organochlorines are transferred to dental tissues and bone either [35], their distribution is especially due to the lipophilicity of particular organochlorines and the lipid content of individual tissues [36]. Hence, the increase in bone mass could be explained by the kinetic and the metabolism of tetradifon. In fact, the highest percentages of tetradifon, after its distribution, in intact rats were found in the faeces and urine, followed by fat [37]. The residual quantity in fatty tissues was found to exceed the plasma level by a factor of 50 at 96 h [38]. The activation of oestrogen receptors caused simultaneously an increase of the bone mineral density and the cortical thickness and activated the formation of the trabecular bone [39]. These findings are in accordance with ours. In fact, after up to six weeks of treatment, non-significant differences for all investigated quantitatively-measured histomorphometric characteristics were found between treated and control animals (Table 2). Moreover, after 12 weeks of treatment, the trabecular bone volume was increased without a statistical significance.
Taking into account the physiological change of the femoral histomorphometric parameter, BV/TV (%) decreased with or without tetradifon treatment. The histomorphometric data resulted in a relative increase of the BV/TV (%) range of the animals treated for 12 weeks to a level not similar to that of the corresponding control. The slightly higher average BV/TV in the present study may be, at least partly, due to a slightly higher number and/or activity of osteoblasts. On the other hand, the increase of calcium rate could be the result of osteoblasts stimulation. In the present study, increased levels of the investigated bone formation markers were correlated to each other, suggesting that osteoblasts' activity was stimulated by subchronic exposure to tetradifon.
Recent studies indicate that androgens may have positive effects on BMD by decreasing the bone resorption [40]. It is hypothetically possible, therefore, that the xenoendocrine disrupters, as tetradifon, cause negative effects on bone. This hypothesis is supported by a small experimental study on pigeons, where a PCB reduced the medullar bone formation during oestrogen treatment [6]. It was currently advanced that the organochlorine pesticides caused damage of the skeleton and skeletal malformations as reduction and/or delaying ossification process [41].
In conclusion, the effect of tetradifon on bone seemed to be predominantly anabolic, leading to a relative increase of bone formation, due to the significant increase of osteoid surfaces and trabecular thickness in treated animal compared to controls. Our biochemical findings confirmed the microscopic examinations. In fact, tetradifon seems to disturb the mineral composition.
Acknowledgements
The authors express their gratitude to Mr. D. Chappard (INSERM EMI 0335, LHEA, France) for critically reviewing the manuscript, and to Mr. M. Feki and Mr. K. Jamoussi for their contribution to the dosage of AlkP, calcium and phosphorus. This work was supported by the 99/UR/0860, Tunisia.


