1 Introduction
To people living in temperate areas, a vascular plant must usually grow connected to the ground which allows its root system to access to water and nutrients (especially phosphate and nitrogen), implying the expected death of the plant if the relationship to the ground is interrupted. To people living near tropical forests, the growing of plants on other ones, especially trees (called phorophytes), without connection to soil and without parasitism, is not exceptional. This particular type of growing is called epiphytism. Epiphytic plants are, indeed, one of the major components of rainforest plant diversity and the first community to decline when such fragile ecosystems are threatened [1]. Most horticultural tropical orchids are naturally epiphytic and mostly cultivated in draining growing media made of pine barks, a media able to serve as a substitute for the natural substrate (i.e., the cork of the phorophyte). If cultivated in standard cultivation soils such as loam, these orchids will inevitably die. This suggests that epiphytism and thus lack of the relation to the ground are obligatory. Epiphytic plants are therefore naturally selectively adapted to their unusual and quite constraining habitat.
The most famous group with frequent epiphytism is probably orchids, because of their high specific diversity (more than 20,000 reported species, of which 73% are epiphytic and likely represent two thirds of the total vascular epiphytes [2]) and because of their attractiveness. They are followed by the widely cultivated epiphytic bromeliads and indeed most studies on epiphytic plants and adaptive traits related to epiphytism focus on flowering plants, and particularly on orchids and bromeliads (see Benzing's review [2]). This overrepresentation tends to obscure the fact that epiphytism occurs in many other groups of plants, involving ferns (extant Monilophytes). Indeed, the proportion of epiphytic ferns in tropical forests is not negligible. In many palaeotropical rainforests (e.g., in Australia, New Zealand, and Micronesian islands), ferns and other seedless vascular plants (especially clubmosses) represent from 36 to 72% of the epiphytic diversity, and are more diverse than orchids [2]. Furthermore, ferns and other lineages of seedless vascular plants root their origins in Palaeozoic (upper Silurian for clubmosses and Devonian for ferns), while epiphytic angiosperms belong to modern lineages (and not older than the Cretaceous period). The fossil record indicates that the oldest occurrences of epiphytism are at least in the Carboniferous; most of these fossils are herbaceous extinct ferns growing on Psaronius, an extinct arborescent fern [3]. Their non-negligible current diversity and their old history make ferns a quite interesting model for studying the origin and diversification of epiphytic forms and strategies in vascular plants. The aim of this study is to review: (i) the diversity of living epiphytic ferns and their supposed epiphytic adaptive strategies; (ii) the origin of epiphytism in ferns and their diversification in current tropical forests; and (iii) an example of a family (Hymenophyllaceae), illustrating an epiphytic hygrophilous strategy that is unique in vascular plants.
2 Diversity and adaptive strategy in epiphytic ferns
Ferns are the second group of vascular plants in terms of epiphyte diversity, with 29% of the species occupying this habitat [4]. The first group in importance is monocots with 31% of epiphytic species, but most belong to the orchid family. The majority of epiphytic vascular plant diversity is localized in tropical areas. Except for epiphytes belonging to eudicot families such as Rubiaceae and Ericaceae, most vascular epiphytes are perennial herbs and not able to survive frost, explaining the absence of vascular epiphytes in higher latitudes, in temperate and boreal areas. The epiphytic habitat is quite constraining; the lack of relation to the soil implies a high risk of desiccation and the obligatory capacity to quickly and directly use rainwater or moisture. A relative small number of vascular plants is poikilohydrous, able to survive and be reborn after a long period of dehydration. Pleopeltis polypodioides (Polypodiaceae), called the resurrection fern, is one rare example. This strategy is, however, more widespread in mosses. Because most vascular epiphytes are homoiohydrous (i.e., relatively desiccation intolerant), other adaptive strategies are needed in order to avoid dehydration, to limit water loss and/or to store water. According to their tolerance level to drought, vascular epiphytes, including ferns, could be divided into three categories: hygrophytes, mesophytes and xerophytes [2].
Hygrophytes are strictly adapted to hygrophilous habitats and are drought intolerant. Owing to this drought intolerance, hygrophytes are observed only in places constantly submitted to abundant rains, such as the tropical mountain nebulous forests, also characterized by the highest diversity in epiphytes. In such habitats, epiphytes directly absorb rainwater or water flowing on trunks and branches, and the dehydration is limited by the high moisture. Hygrophytes often display anatomy and morphology regressive traits facilitating direct water absorption, often by the blades. Roots, if present, are used for anchorage on the phorophyte rather than for nutrition. Hymenophyllaceae are a typical hygrophilous fern family and will be detailed in the last part of this review.
Mesophytic epiphytes do not exhibit particular adaptive features but grow in places where water is easily available, such as in hygrophilous forests (but hygrophytes are typically and strictly adapted to hygrophilous habitats, mesophytes are a priori not). They are thus often observed sympatrically with hygrophytes, or they are able to create a suspended soil and are thus called humus-collectors, displaying growth habits allowing them to accumulate humus and to entrap besides nutrients and water. Asplenium nidus (Aspleniaceae), the bird's-nest fern, uses the base of its spirally tightly clustered fronds forming sinkhole to humus. Similar growth forms can be found in other epiphytic ferns belonging to Polypodiaceae. In drynarioid ferns, there are several strategies to accumulate litter: some Drynaria species display distinct specialized humus-collecting blades while other ones and some Aglaomorpha species possess fronds that are all basally enlarged [5]. Platycerium species, the staghorn ferns, exhibit hanging fertile fronds and distinct sterile fronds adpressed on the phorophyte (Fig. 1). The successive agglomerated sterile fronds form a nest that entraps flowing water and protects rhizome and roots from dehydration. A different strategy to respond to limited access of nutrients is found in ferns living in a mutualistic interaction with ants. Two highly specialized cases are known from the Polypodiaceae. The Neotropical Microgramma (Solanopteris) bifrons and relatives bear modified caulinary urn-like structures with included roots that absorb entrapped water and nutrients. The modified swollen lateral shoots explain the English name for these ferns, potato ferns. Different strategies to house ants can be found in species of the Malaysian ant fern genus Lecanopteris. Most species form cavities either in lateral shoots or in all shoots that are colonized by ants. One species of this genus, L. mirabilis from New Guinea, shows a different strategy. Ants are colonizing the space between the flattened rhizome and the host tree bark. In an exhaustive study, it was shown that the ants provide the fern with important nutrients such as nitrogen and phosphate [6]. Less advanced interactions between ferns and ants are reported for taxa forming nests and other structures for litter collection. These nests are often also colonized by ants. Ant-plant mutualism is observed in epiphytic flowering plants too, displaying diverse adaptive features, and some “convergences” with fern strategies, such as swollen hypertrophied stems (e.g., Hydnophytum and Myrmecodia genera, Rubiaceae) [2]. Furthermore, the epiphytic ant-mutualistic angiosperm Dischidia rafflesiana (Apocynaceae) exhibits foliary urn-like structures that are functionally analogous to the caulinary urns observed in Microgramma (Solanopteris) bifrons, as described above [2].

A staghorn fern, Platycerium sp. (Polypodiaceae), an epiphytic humus-collector, showing pendant fertile fronds (F) and agglomerated sterile fronds (S) that form a nest protecting rhizome and roots and entraping humus (“Serres d'Auteuil” collection, Paris).
Xerophytes display adaptive traits allowing them to avoid and/or endure drought. Xerophilous strategies (i.e., adaptations for limiting water loss – sclerophylly – and/or for storing water and nutrients – succulence –, in order to survive the drought period) are more widespread in epiphytic flowering plants: succulent blades with thick cuticle in most Peperomia species (Piperaceae), epiphytic cacti (e.g., Rhipsalis or Epiphyllum spp.), succulent stems as pseudo-bulbs in sympodial orchids, etc. Xeromorphic features are also observed in numerous ferns: stout blades covered by a thick cuticle, analogous to succulent foliage of xerophilous flowering plants, are common in epiphytic polypods, such as in the genus Microgramma, often combined with stout suberified stems storing water and amylaceous reserves; water storage tissue is also observed in Platycerium and Pyrrosia leaves (succulence); thick cuticles with waxes are observed in Belvisia (sclerophylly). Such foliar adaptive traits are also observed by convergence in other families, such as in the genus Elaphoglossum (Dryopteridaceae) or in Vittariaceae. Many flowering plants limit the loss of water and avoid drought effects by losing their blades during the drought season (caducifoly), as observed in several epiphytic orchids. This strategy seems to have been selected mostly in seasonal terrestrial taxa, such as the bracken fern (Pteridium aquilinum, Dennstaedtiaceae), but it also concerns some epiphytes in the Davalliaceae–Polypodiaceae clade involving species that shed leaves when dry. Most xerophilous epiphytic ferns are therefore drought endurers. In conclusion, as for flowering plants, the epiphytic ferns are quite diversified as well specifically as in their epiphytic adaptive strategies, including numerous ones not detailed above (reduction of lamina, dense indumentum, both reported as sclerophyllous adaptations).
3 Diversification of epiphytic ferns in the shadow of angiosperms
Most studies on epiphytism in ferns are only descriptive and only recent works include evolutionary approaches (as reported below). These evolutionary studies have benefited from the impressive increase, during the last decade, in available phylogenetic data, providing a robust historical framework for major fern groups [7–9] (Ophioglossales, Psilotales, Marattiales, Osmundales, Hymenophyllales, Gleicheniales, Schizaeales, Salviniales, Cyatheales and Polypodiales). Current studies mostly focus on the infra-generic level, allowing studying morphological evolution and testing evolutionary scenarios at this level.
The Polypodiaceae family (sensu lato, including Grammitidaceae, and also called polygrammoids), comprise more than 95% of epiphytic taxa (out of more than 1200 species) and display the most diverse adaptive strategies (as highlighted above). As such, they have been quite studied to investigate epiphytism evolution. Evolution of specialized frond structures in the drynarioids has been investigated in a phylogenetic context, suggesting numerous evolutionary transitions, including reversal to non-specialized fronds [5]. Phylogenetic studies on Platycerium seem to indicate a fast diversification in staghorn ferns, accompanied by the early appearance of frond dimorphism and specialization [10]. A fast diversification, illustrated by the rapid appearance of cavities and domatia (chambers produced by plants that house insects), has also been evidenced in Lecanopteris, another Polypodiaceae [11]. This diversification may have been facilitated by the ant-plant mutualism in an epiphytic context, but results also suggest that selection of xerophilous traits (i.e., succulent rhizome) may have preceded the mutualism (the phylogenetic inference indicates, indeed, that succulence may have been selected in nodes older than those suggesting ant-plant mutualism acquisitions). A study on the whole polygrammoids indicated that numerous rapid radiations (including those cited above) may have occurred and could coincide with the establishment and diversification of tropical forests dominated by flowering plants, likely related to epiphytism acquisition [12]. Within polygrammoids, the most striking radiation concerns the grammitids, which are typical epiphytic displaying a range of adaptive strategies including sclerophyllous habits. Studies often focus on the dominant sporophyte generation but grammitids are also promising candidates for investigating the role of gametophyte installation in ecological preferences of the sporophyte. The success of grammitids may be caused by the establishment of long living gametophytes versus short living ones in their relatives. A similar transition can be found in filmy ferns (Hymenophyllaceae). A gametophyte long-living strategy may be favoured in an epiphytic context, by increasing the probability of cross-fertilization by gametes from distinct gametophytes [13].
The fossil record for epiphytic ferns is quite sparse: except for few examples in Carboniferous concerning extinct taxa [3] and in Early Cretaceous concerning living Lindsaeaceae [14], evidence of epiphytic ferns is more documented in the Tertiary and may concern polygrammoids [15] and more especially a Grammitis, supposedly epiphytic, found in amber from the Late Oligocene [16]. Epiphytism was likely not rare in the Palaeozoic or the early Mesozoic, but epiphytic plants are usually very poorly preserved with the exception of amber fossils (see [16]). We could suggest that Palaeozoic and Mesozoic trees, and especially arborescent fern trunks, were potential phorophytes for a diversified epiphytic flora (likely seedless vascular plants) but further investigations on fossil data are still expected. Evolution of epiphytism in ferns has thus been indirectly inferred from phylogenies of living lineages. Schuettpelz has recently reviewed this evolution by proposing, using molecular data and fossil calibration, dating estimations for the diversification of major living epiphytic lineages [17]. This author demonstrated that diversification of four major epiphytic clades (polygrammoids plus davalloids, vittarioids, elaphoglossoids, asplenioids) may have occurred in the Tertiary rather than in the Cretaceous. This is in accordance with a former study which showed that diversification of major fern groups occurred in the Cretaceous, “in the shadow of angiosperms” [18]. This Cretaceous diversification may thus have preceded the diversification of epiphytism in the Cainozoic. Indirectly, occurrences of epiphytic ferns, strongly related to tropical forests dominated by angiosperm trees, suggest that current tropical forests and their biodiversity may also have originated in the Cainozoic. Interestingly, we could note that the origin of epiphytic orchids is dated from the same period [19].
4 The bryophyte-like strategy of epiphytic Hymenophyllaceae
The Hymenophyllaceae (also called filmy ferns or filmies) are the most speciose (more than 600 species) family of the basal leptosporangiate ferns (Hymenophyllales are the sister group of a clade comprising Gleicheniales, Schizaeales, Salviniales, Cyatheales and Polypodiales, according to [8]). There is little fossil evidence and the oldest putative filmies are from the Triassic [20]. Divergence time estimates based on cpDNA rbcL sequences suggest a Palaeozoic origin [7,21], but the division into the two extant lineages, the hymenophylloids (corresponding to the genus Hymenophyllum) and the trichomanoids (regrouping the remaining genera according to the most recent classification [22]), may have occurred in the Early Jurassic (see Fig. 3) [21,23]. Hymenophyllaceae exhibit a remarkable diversity in terms of morphology (detailed in Table 1) and the habitats they occupy (terrestrial, epiphytic and/or saxicolous, hemi-epiphytic and lianescent). Hemi-epiphytes grow first as creeping terrestrial, then climb on a phorophyte, becoming epiphyte if the relationship to ground is secondarily lost, while a true liana keeps the relationship to ground via a perennial rooted terrestrial part. Hymenophyllaceae are characterized by a single-cell thick lamina (hence the English vernacular name), lack cuticle, differentiated epidermis and stomata. They depend upon environmental moisture, because no barrier exists to prevent unregulated loss of water. Filmies are thus strict hygrophytes. Some are poikilohydrous, but dehydration is allowed only for a short time (some hours per day). A recent study on a reduced Polynesian sampling demonstrated that epiphytic filmy ferns are more desiccation tolerant than the terrestrial ones [24]. More than 60% of species are epiphytic and localized in hygrophilous forests at the pantropical level, with few occurrences in wet temperate areas. Filmy ferns are therefore ideal candidates for studying the evolution of epiphytism and adaptive strategies in a hygrophilous context. Furthermore, this study benefits from the availability of a robust historical framework for the family [22,25].
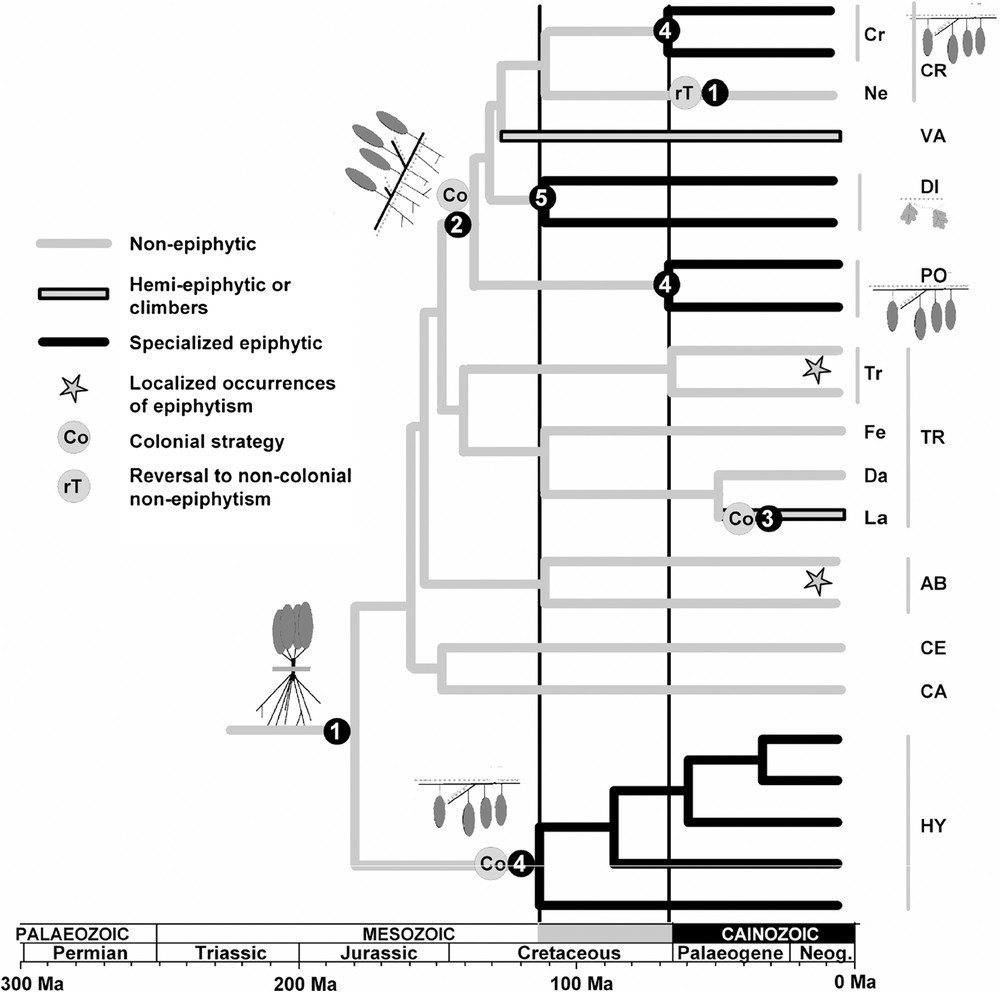
Evolutionary relationships and dating of major living lineages of Hymenophyllaceae [21–23,25] and evolution of ecology and growth forms. Divergence time estimates are from Schuettpelz and Pryer [21]. The growth forms (numbers in black circles) are detailed in Table 1. Left vertical line (in Cretaceous) and grey portion of time scale indicate the diversification of modern fern families providing more than 80% of living species, in parallel to the angiosperm diversification. On the right, abbreviations correspond to genera and subgenera according to Ebihara et al.'s classification [22]: CR = genus Crepidomanes, VA = genus Vandenboschia, DI = genus Didymoglossum, PO = genus Polyphlebium, TR = genus Trichomanes, AB = genus Abrodictyum, CE = genus Cephalomanes, CA = genus Callistopteris, HY = genus Hymenophyllum, Cr = subg. Crepidomanes, Ne = subg. Nesopteris, Tr = subg. Trichomanes, Fe = subg. Feea, Da = subg. Davalliopsis, La = subg. Lacostea.
Description of the five different growth forms observed in the Hymenophyllaceae with detailed morphological features and the observed associated ecology, according to a former study [26].
| Type 1. See Fig. 2A | Type 2. See Fig. 2B | Type 3⁎ | Type 4. See Fig. 2C | Type 5. See Fig. 2D | |
| Stem branching | exceptional | usual | in climbing parts | usual | usual |
| Stem thickness | stout (≫1 mm) | stout (≫1 mm) | stout in terrestrial parts; fine (∼1 mm) in climbing parts | mostly filiform (<1 mm) | mostly filiform (<1 mm) |
| Stem habit | short-creeping to erect | long-creeping | long-creeping in climbing parts | long-creeping | long-creeping |
| Root system | developed | developed in parts connected to soil | developed in terrestrial parts; climbing stems rootless | reduced or rootless | rootless |
| Frond disposition on the stem | clustered | distanced | clustered in terrestrial parts; distanced in climbing parts | distanced | distanced |
| Frond size | medium-size (10–20 cm) to large (more than 60 cm) | medium-size (10–20 cm) to large (more than 60 cm) | medium-size (10–20 cm) | small (<10 cm) to exceptionally large (20–30 cm) | dwarf (<4 cm) |
| Frond habit | mostly erect | mostly erect | adpressed on support | mostly pending | pending or adpressed on support |
| Frond architecture | highly divided to simply-pinnate | highly divided to simply-pinnate | simply-pinnate to bipinnatifid | mostly highly divided | mostly pinnatifid to simple or lobed |
| Observed associated ecology | mostly terrestrial, some individual epiphytic | creeping terrestrial, or hemi-epiphytic climber | lianescent (true liana) | mostly colonial epiphytic or saxicolous | mostly colonial epiphytic or saxicolous |
⁎ This type combines two growth forms: a juvenile terrestrial part providing mature climbing stems.
Five growth forms are distinguishable in the Hymenophyllaceae [26]. They are described and detailed in Table 1 and four are illustrated in Fig. 2. Hymenophylloids all are of type 4 (see Fig. 2C, with a few type 1 exceptions and some type 5) and colonial epiphytes (i.e., able to form a large colony on the phorophyte) or saxicolous. The trichomanoid lineage is the most diversified. Callistopteris and Cephalomanes genera cluster type 1 terrestrial taxa. Abrodictyum and Trichomanes taxa are mostly of type 1 and terrestrial (see Fig. 2A), but include some individual epiphytes (i.e., not forming a colony and growing often on tree-fern trunks) as well as some type 2 species as creeping terrestrial or climber. In Trichomanes, the subgenus Lacostea is of type 3 and thus includes true lianas. Vandenboschia species are of type 2 and are creeping colonial terrestrials or hemi-epiphytes (see Fig. 2B). Polyphlebium taxa are exclusively type 4 colonial epiphytes. Crepidomanes taxa are mostly type 4 or type 5 colonial epiphytic or saxicolous but the subgenus Nesopteris clusters type 1 terrestrial species and one type 2 climber (Cr. aphlebioides). Didymoglossum is a typical clade grouping dwarfish type 5 colonial epiphytic or saxicolous taxa (see Fig. 2D).

Ecological and morphological diversity in the hygrophilous Hymenophyllaceae. A. Trichomanes elegans, a typical type 1 growth form terrestrial species, fronds can exceed 50 cm long (Basse Terre, La Guadeloupe); B. Vandenboschia speciosa, from Tenerife, a typical type 2 growth form hemi-epiphytic species (habitus, Paris Herbarium); C. Hymenophyllum tenellum, a type 4 growth form colonial epiphyte, fronds do not exceed 10 cm long (Mare Longue, La Réunion); D. Didymoglossum punctatum (Hymenophyllaceae), a colonial epiphyte “resembling” a bryophyte, more particularly a liverwort, fronds do not exceed 1 cm long, this species illustrates a typical type 5 growth form (Basse Terre, Guadeloupe). Growth form types are detailed in Table 1.
We inferred the evolution of ecology and growth forms on the lastly obtained phylogeny (Fig. 3, [23]). Type 1 growth form is inferred as the ancestral morphology and may be related to ancestral terrestrial habitat in the Hymenophyllaceae. With a parsimony approach, equivocal results are obtained for the node grouping genera Didymoglossum, Polyphlebium, Crepidomanes and Vandenboschia. An inferred epiphytic habitat is nevertheless suggested with a Bayesian approach [23], implying reversal from epiphytism to terrestrial (in subgenus Nesopteris) and transition from epiphytism to hemi-epiphytism in Vandenboschia. Because all strict epiphytes belonging to this clade are of type 4 and type 5 and thus clearly exhibit anatomy and morphology regressive traits (frond size reduction, root system simplification, and anatomy regression as recently evidenced [25]), likely related to their habitat (as discussed below), we could hypothesize an irreversible evolutionary scenario, suggesting that acquisition of epiphytic regressive features is fixed. There are 4 major colonial epiphytic clades, related to type 4 or 5 morphology, corresponding to genera Hymenophyllum, Polyphlebium, Didymoglossum and subgenus Crepidomanes and numerous localized occurrences of individual epiphytism in clades mostly grouping terrestrial taxa. By using parsimony and irreversibility constraint, this suggests at least 4 major diversification events related to strict epiphytism: in the Cretaceous for Hymenophyllum and Didymoglossum and in the Cainozoic for Polyphlebium and Crepidomanes. Numerous occurrences of individual epiphytism (not shown on the figure) are in the Cainozoic too. These diversifications are also synchronized with the diversification of major modern fern lineages and with the diversification of epiphytism in other fern families (as suggested above). Colonial strategy would have appeared two times in the Jurassic in hymenophylloids and in trichomanoids, in addition to localized occurrences in Cainozoic in subgenus Lacostea and in some Abrodictyum and Trichomanes species (not shown here). The ancestral habitat of the clade clustering Didymoglossum, Polyphlebium, Crepidomanes and Vandenboschia remains equivocal, even with an irreversibility constraint, but we could propose a hemi-epiphytic strategy, as observed in Vandenboschia. Hemi-epiphytism would have preceded and “prepared” colonial epiphytism, a widespread habitat in the clade and in the whole family. More than 80% of epiphytic filmies are indeed colonial epiphytes of type 4 or 5. By contrast, the few individual epiphytes (of type 1) do not display any particular epiphytic specialization. The colonial strategy, combined with reduction in anatomy and morphology, has thus been favoured in the epiphytic context. Reduction is particularly exacerbated in the genus Didymoglossum involving taxa resembling bryophytes (Fig. 2D), and including the smallest filmy fern species with fronds less than 5 mm long. Reduction in size may contribute to decrease water loss, a favourable strategy in a constraining epiphytic habitat, but this strategy requires a high hygrophilous environment. As for bryophytes, nutrients and water absorption and conduction are performed by diffusion directly by the blade lamina rather than by roots and vascular tissues. Dwarfism is not rare in epiphytic plants but it is generally associated with xerophilous features (as in polygrammoid and vittarioid ferns, in Peperomia and orchids) [2,26]. The “resemblance” to bryophytes, combining hygrophily and reduction/regression of main parts of the vascular plant body, is a filmy fern feature that is unique in vascular plants.
5 Conclusion
With 29% of epiphytic species, contribution of ferns in the epiphytic habitat is quite important. Ferns and other seedless vascular plants (such as clubmosses) even represent the dominant epiphytic flora in some palaeotropical places. Some adaptive strategies are remarkable, especially for humus-collectors that recreate, without connection to soil, adequate growing conditions by entrapping water and nutrients in a nest or in specialized organs. The origin of vascular epiphytism is quite old, at least in the Carboniferous. However, diversifications of major living epiphytic fern groups are quite more recent; most occurred in the Tertiary. Since the Cretaceous, the diversification of angiosperms and the first forests dominated by flowering plants created numerous opportunities for modern ferns that evolved in “the shadow of angiosperms”. Epiphytism in living ferns may have appeared in Cretaceous too, as evidenced by some Hymenophyllaceae genera. But the diversification of major epiphytic clades, especially in the polygrammoids, may have coincided with the origins of extant tropical forests in the Cainozoic. The impact of angiosperm diversification on ecosystems and diversification of many other organisms, especially animals, and more particularly insects and tetrapods, has been fully documented. Little has been discussed, however, on other groups of plants, such as ferns, often considered as primitive surviving groups. Studies on fern evolution and epiphytism evolution in ferns evidence strong relationships between flowering plant and seedless vascular plant diversity, leading to the current observed and endangered high biodiversity, especially in tropical areas.


