1 Introduction
In terms of conservation, marine biodiversity and, in particular, marine fish species have long been considered much less vulnerable than the terrestrial biodiversity [1]. This perception, based on high abundance of many marine fishes and their capacities to undergo important variations of densities, was seriously called into question by the collapse of several populations such as, for example, the Atlantic Cod, Gadus morhua [2]. In spite of an important debate on the criteria of evaluation of their state of conservation, it appears clear today that the risks of extinction of populations and species also apply to marine fish species [2,3]. A comparative study [1], indeed, showed that fish would not have a lower extinction vulnerability than the mammals, birds or butterflies. Among marine fishes, Serranidae, which includes groupers, is one of the families classified as priority by the Global Marine Species Assessment (GMSA). This program aims at evaluating, according to the criteria of the World Union for Nature (IUCN), the status of conservation of approximately 20 000 marine species from now to 2010 [4]. Because of their economic value, their wide distribution and their biological characteristics, groupers are the subject of numerous conservation plans [4]. Within the Commission on the Survival of Species (SC) of the IUCN, a workgroup (Groupers and Wrasses Specialist Group) was created in 1998 to focus on the grouper. In 2007, out of the 161 described species of groupers, only 45 were evaluated including 3 classified CE (Critically Endangered), 5 classified EN (Endangered), 13 VU (Vulnerable) and 9 NT (Near Threatened) [4].
The dusky grouper, Epinephelus marginatus (Lowe, 1834) is an emblematic species of the Mediterranean Sea, it is also present in the Atlantic (Africa and South America) [5–8]. It was evaluated in 2004 and was classified “in danger of extinction” on the basis of important rate of decline in populations mainly due to overexploitation and the high vulnerability of this species to spear fishing [4,9]. Its biological (piscivorous, large size, hermaphrodism protogynous and longevity) and behavioral characteristics explain this situation [9]. This species is the only Serranidae registered with appendix III of the Convention of Bern (1995) and of the Convention of Barcelona (1995) [10]. In France, since 1993, the dusky grouper is partially protected by a renewable moratorium re-assessed every 5 years [11]. The current one is valid until December 31, 2013, when the renewal is re-evaluated. This evaluation is based on updated scientific knowledge about French populations, in particular the dynamics of colonization initiated from the Marine Protected Areas (MPA) which appear to constitute true “refuges” for the species [12]. These very specific characteristics explain in particular why even the simplest demographic models used in conservation biology of terrestrial species have limited applicability to marine species [13]. In this context the main question of this study was to determine the migratory pathway of the dusky grouper inside the Marine Reserve of Cerbère-Banyuls. This will allow bringing knowledge on the spatial organization of the grouper populations in a MPA presenting different levels of protection, such as no-take zone and partial reserve. In addition, this study highlights the benefits of using GIS software technology coupled with acoustic telemetry to answer key questions on the conservation of the dusky grouper and other marine fish species.
2 Methods
2.1 Study area
Created in 1974, The Natural Marine Reserve of Cerbère-Banyuls (France) is located in the north-western Mediterranean Sea, near the Spanish boundary. Along 6.5 km coastline and up to 1.5 nm wide, it covers an area of 650 ha [11] (Fig. 1). The MPA includes two zones with different levels of protection: 65 hectares of integral reserve on the level of the Cape Rédéris (created in 1979) and the remainder in Reserve known as “partial” (Fig. 1). In the integral reserve or “reinforced protection zone”, only navigation (speed limited to 9 km h−1) and swimming activities are authorized. In the remainder of the reserve (“partial reserve”), diving is authorized, as well as commercial fishing (authorizations limited to 12 fishermen) and recreational fishing (regulated and subjected to prior approval) [11]. Underwater spear fishing is prohibited in the entire reserve. The site was selected for its population of dusky groupers which are only present in the MPA: from 7 individuals listed in 1985, the population passed to 202 individuals in 2006. The population would be distributed for two-third in the reinforced protection zone and one-third in the partial reserve (Astruch, pers. comm.).
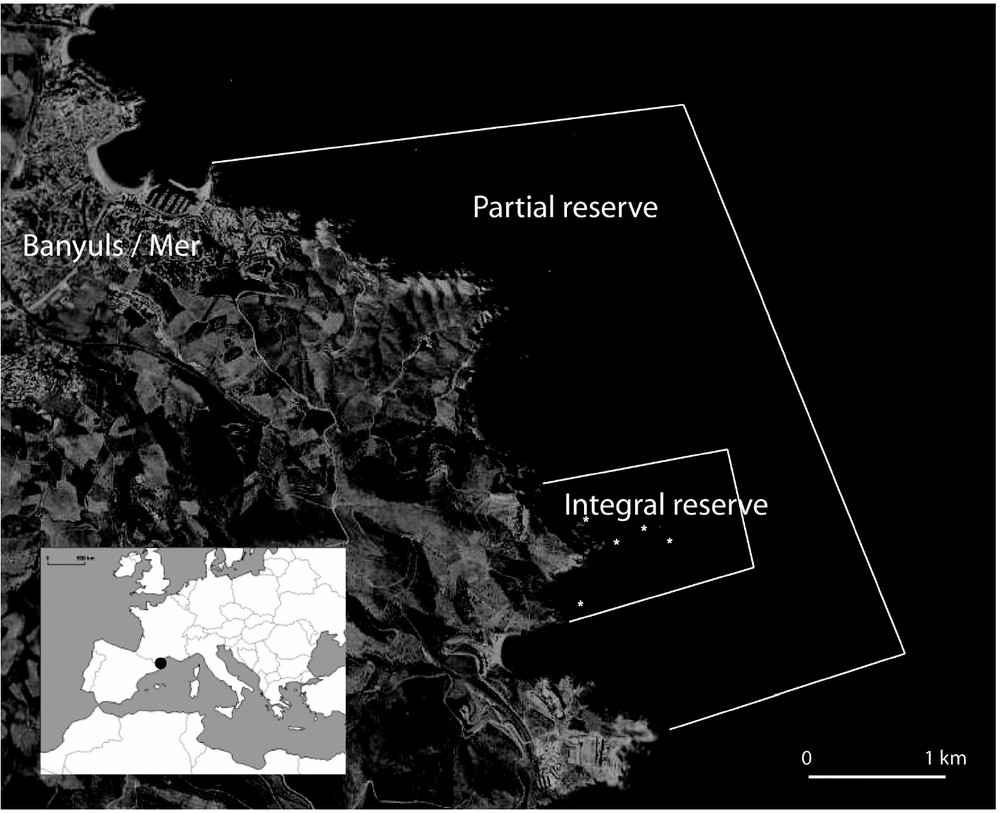
Map of the study area, the Natural Marine Reserve of Cerbère-Banyuls. The stars represent the positions of the VR2 fixed receivers.
2.2 Sampling
The survey by acoustic telemetry was carried out on 6 groupers (2 males, 100 and 105 cm of total length, 4 females between 52 and 79 cm of total length) between September 2005 and September 2006 in the no-take zone (Table 1). The capture of living animals is one of the principal constraints of tagging studies. Because risks of injury are important, the less traumatic method should be preferred.
Biological characteristic of tagged individuals (total length in cm), estimated age [34], estimation of sex and date of tagging.
| # Tag | Total length | Age | Sex | Tagging date |
| 156 | 65 | 8 | Female | 25/05/2005 |
| 157 | 100 | 17 | Male | 30/08/2005 |
| 158 | 52 | 6 | Female | 01/09/2005 |
| 159 | 75 | 10 | Female | 05/09/2005 |
| 160 | 105 | 18 | Male | 05/09/2005 |
| 161 | 79 | 11 | Female | 05/10/2005 |
Fish trapping was ineffective in catching dusky groupers. Hook and line was unsafe due to the possible fish barotraumas injuries. We are experienced in the use of anesthesia in Sparidae and Serranidae fishes in aquarium settings by using clove oil (eugenol), as a result this method was used to anaesthetize and collect the groupers in situ. Anaesthetizing fish with clove oil (eugenol) has been practiced for a long time in aquaculture [14], because it is a low cost and efficient method, and because fish recovery is fast. No solvent is required and the concentration level of application is not toxic for humans or the environment [15]. The method developed in closed environment was adapted to natural environment for the capture of groupers by scuba divers. The method was restricted to fish present in confined area (anfractuosity, cave). Cavities apertures had to be blocked, then the anesthetic could be injected in the environment with an approximate concentration of 0.2 ml eugenol per liter of sea water (using 30% eugenol diluted in ethanol). One to two minutes later, the fish was anaesthetized and could be gently handled and extracted from the cavity. Each fish was then placed in a bag with an important water renewal which is helpful to awaken it prior to a quick surfacing. The wake up lasted 2 to 10 min depending of the anesthetic exposure time. Because of logistical constraints, underwater tag implantation was avoided; therefore we tagged the fish on board. The groupers were anaesthetized and captured in depths not exceeding 15 m, which limited the effects of the increase on the gas bladder.
The fish was then deposited in a case filled with sea water on board. A second anesthetic was performed using eugenol (concentration 0.2 ml per liter of sea water). The mark could be implanted in the peritoneal cavity by a surgery. Two stitches closed back the wound. The fish was also marked by an anchor T-bar tag (Floytag) in order to follow it visually during its convalescence. The fish was then brought back in its capture place by a diver who will assist and supervise the fish waking which lasted from 2 to 20 min.
Each individual was equipped with a transmitter coded V13-1L (VEMCO Ltd, Nova Scotia) (emission of a signal each 30/40 seconds during at least one year). This allowed an active survey by boat using a hydrophone VH110 or VH165 connected to a receiver VR100 (VEMCO Ltd, Nova Scotia) and a continuous survey by 5 fixed receivers VR2 (VEMCO Ltd, Nova Scotia) deposited on bottom and covering the entire no-take zone. The active survey allowed recording the positions of each individual (GPS points of the boat in WGS 84 coordinates associated with a percentage of detection). The active survey was organized one day per week when the weather was favorable. The continuous survey generated datasets of (i) presence/absence in the field of detection of the VR2 (of a ray of approximately 200 meters per beacon) and (ii) the rhythm of daylight/nighttime activities.
2.3 Data analysis
Site fidelity, i.e. the tendency of the dusky grouper follow-ups to remain or not in the integral reserve, was studied using the two types of data. Detections by the fixed receivers initially allowed checking whether or not the marked individuals were continuously detected in the integral reserve. Tracking data provided by the active surveys are treated using the “site fidelity test” of AMAE (Animal Movement Analyst Extension) [16] under ArcView 3.2 (ESRI Inc., Redlands, CA). This allowed estimating fish site fidelity by estimating indexes of dispersion of the mean squared distances (MSD) and linearity of movements (LI), against the null assumption of random movements obtained from a Monte Carlo simulation (100 random trajectories) [16]. The MSD was calculated starting from the first point (“first fix”). LI (Linear Index) was defined as the ratio of the distance between the first and the last point of the trajectory divided by the distance covered. Only GPS points of detection higher than 60% were used in order to have a good precision for the positions of each individual.
Surfaces of habitats used correspond to vital domains of individuals which correspond to the real space used by each individual. These were estimated based on the data of active survey using the non-parametric statistical method of fixed Kernel where the coefficient of smoothing H was calculated automatically by the LSCV method (Least Squares Cross-Validation) [17,18]. This method is based on the density of the localizations and it attributes to each point space a probability of occurrence of the animal (“utilization distribution”). In this study, 95% (total vital domain) and 50% (zones of more supported activities or “core areas”) were calculated. In Kernel with 95%, the 5% of the most isolated points were not taken into account to define the perimeter of the vital domain [19,20]. Only GPS points of detection higher than 60% were used in order to have a high precision as for the positions of the individuals and to limit the spatial autocorrelation between the localizations. This method was implemented using AMAE (Animal Movement Analyst Extension) under ArcView 3.2 (ESRI Inc., Redlands, CA) [16].
3 Results
Between September 2005 and September 2006, the fixed beacons collected 600 000 signals. All individuals were present every day during all the study. The active survey recorded 1476 detections (between 136 and 455 per individual) corresponding to approximately 90 hours of acquisition. The mortality was null over all the period.
3.1 Site fidelity
For the entire year of the acoustic telemetry survey, the 6 dusky groupers remained in the integral reserve in a bathymetric zone ranging between 5 and 30 m depth. The fixed receivers detected all individuals for each month of the studied year. The indices MSD and LI observed for these 6 dusky groupers were significantly weaker than those obtained from the simulated random trajectories (Table 2). This indicates that the movements of the individuals are much more constrained than what would be expected if the movements were random. All dusky groupers show high site fidelity and use only a restricted zone of the habitat available.
Mean values of MSD and LI for trajectories simulated and observed.
| # Tag | Trajectories | MSD mean (m2) | LI mean |
| 156 | observed (n = 9) | 1663.3⁎ | 0.03⁎ |
| simulated (n = 100) | 13220.6 ± 7240 | 0.18 ± 0.08 | |
| 157 | observed (n = 19) | 1339.4⁎ | 0.02⁎ |
| simulated (n = 100) | 28741.5 ± 18271 | 0.13 ± 0.07 | |
| 158 | observed (n = 22) | 2512.9⁎ | 0.01⁎ |
| simulated (n = 100) | 45987.6 ± 30121 | 0.11 ± 0.06 | |
| 159 | observed (n = 22) | 2389.8⁎ | 0.01⁎ |
| simulated (n = 100) | 74205.6 ± 44019 | 0.08 ± 0.04 | |
| 160 | observed (n = 17) | 2445.2⁎ | 0.02⁎ |
| simulated (n = 100) | 37522.7 ± 23162 | 0.17 ± 0.08 | |
| 161 | observed (n = 23) | 2401.4⁎ | 0.04⁎ |
| simulated (n = 100) | 57618.2 ± 39289 | 0.12 ± 0.06 |
⁎
3.2 Surfaces of habitats used
The estimate size of the vital domains of the 6 dusky groupers followed is of 13 431 m2 (for 95% of UD (utilization distribution)) and of 2077 m2 (for 50% of UD), always related to rocky bottoms (Table 3). Dusky groupers were thus present predominantly only over 15%, on average, of their vital domain. Except for the grouper 156, females had larger vital domains (estimate size of 14 820 m2 for 95% of UD) that 2 followed males (estimate size of 10 655 m2 for 95% of UD) (Table 3). Females' vital domains overlapped (Fig. 2) contrary to the 2 males that had kernels with 95% of quite distinct UD (Figs. 3 and 4). Vital domains between females and males could also overlap. All individuals stayed inside the rocky zone (29 ha) representing only 45% of the integral reserve surface (65 ha). The potential carrying capacity of the integral reserve was estimated between 22 and 36 males regarding the male vital domain (Table 3). If we consider the fact that the visual census made in 2001 [11] showed 23% of male, the total carrying capacity (male and female) could be evaluated between 95 and 154 individuals.
Surface estimation of vital domain by fixed Kernel (LSCV), F: female, M: male.
| # Tag | Sex | Number of GPS points | Kernel 95% (m2) | Kernel 50% (m2) |
| 156 | F | 136 | 9459.4 | 1862.2 |
| 158 | F | 229 | 18533.1 | 1878.6 |
| 159 | F | 455 | 15326.9 | 2216.3 |
| 161 | F | 302 | 15961.2 | 2436.1 |
| 157 | M | 216 | 8127.2 | 1790.3 |
| 160 | M | 138 | 13182.9 | 2280.0 |
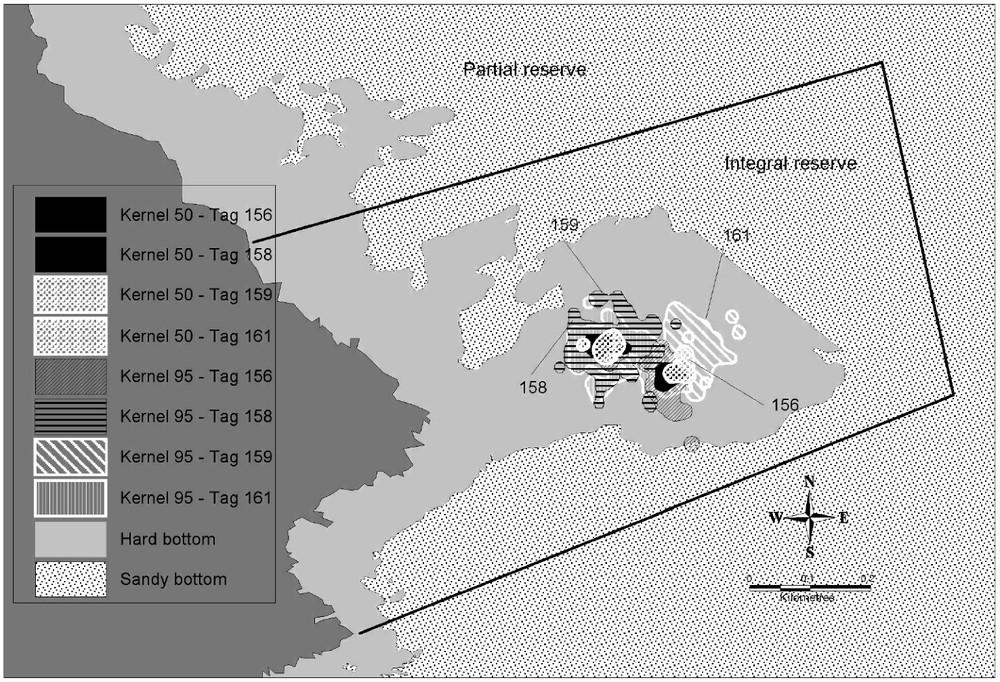
Cartography of vital domain (Kernel 50 and 95%) of females. Limit: Integral reserve – Available habitat (hard bottom).
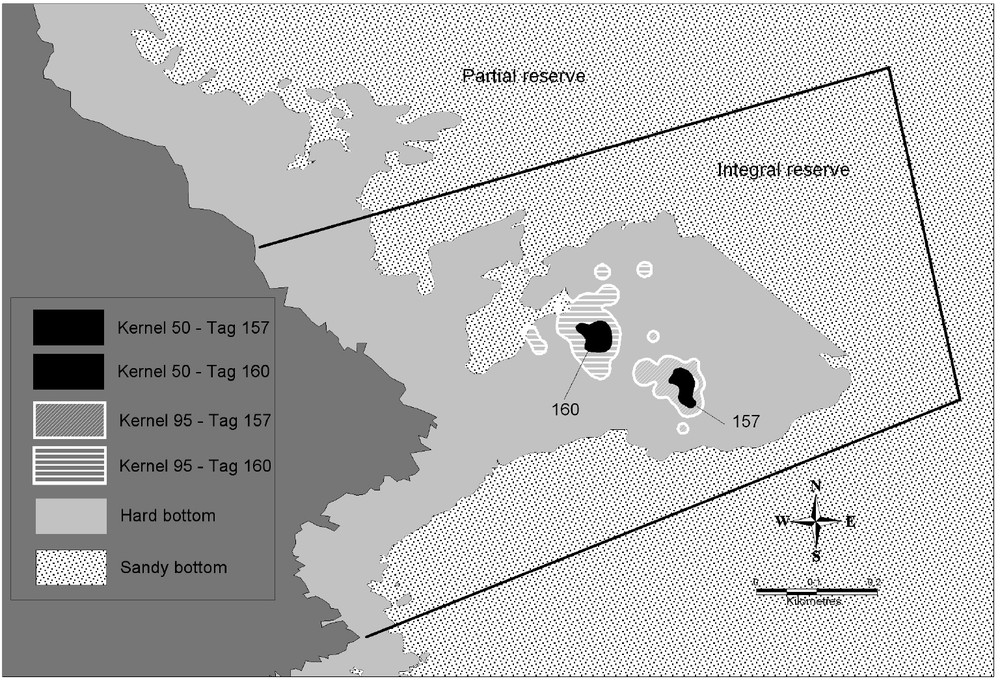
Cartography of vital domain (Kernel 50 and 95%) of males. Limit: Integral reserve – Available habitat (hard bottom).
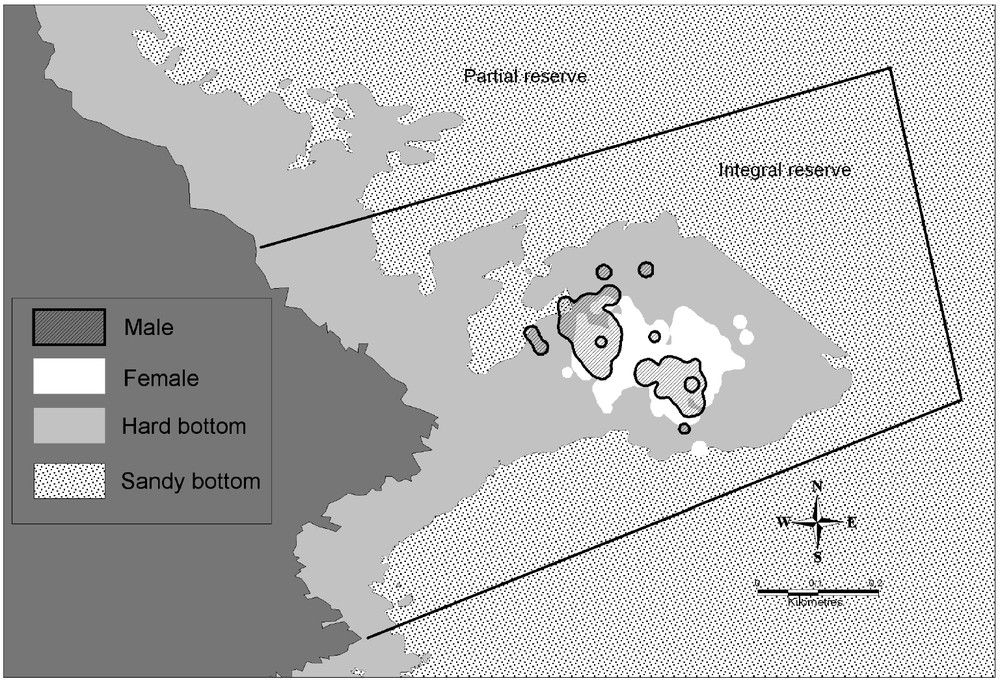
Cartography of vital domain (Kernel 95%) of males (black) and females (white). Limit: Integral reserve – Available habitat (hard bottom).
3.3 Daylight/nighttime rhythm
Individuals demonstrated a rather similar daily pattern over the various months of the survey (from October 2005 to January 2006). The number of detections was longer during the day than during the night (Fig. 5). The peak hours of activity were between 8–9 h and 19–20 h, decreasing at sunset. However, for some individuals, the pattern was not that clear.
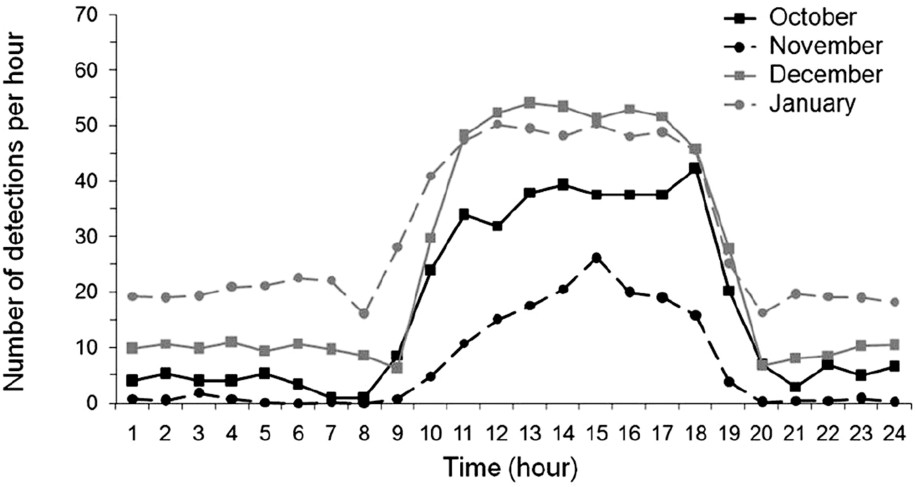
Number of detection per 24 h over 4 months of survey for one individual (#156).
4 Discussion
This study clearly shows that the tagged individuals remained at least for an entire year within the integral reserve; this zone offers the maximum level of protection in term of survival for immature individuals and females. The vital domains of the males and the females are smaller than the size of the integral reserve with high localized movements. During the second survey in September 2007 (still ongoing), the six individuals tagged in 2005 were detected again for the last time in their living area (Lenfant, pers. comm.). This tends to show that they certainly remained at their site well beyond the first year of the survey. These results are in agreement with the results found for dusky grouper population in the island of Ustica (Sicily) [21,22]. These authors [21,22] used acoustic telemetry and showed that dusky grouper has a very strong fidelity to the original site of tagging. This behavior was also observed on other grouper species such as Mycteroperca microlepis [23] and Epinephelus itajara [24,25]. These results confirm strongly the role of MPAs in the conservation of groupers. It has been indeed now demonstrated that the effectiveness of a marine reserve depends on the features of life history and the sizes of the vital domains of the species [26–30]. MPAs, which are often of small size, are much more effective conservation tools for those species with low population growth rates and restricted vital domains [29]. It is thus not surprising that the only populations that remain on the French coasts are mostly found in marine reserves [31]. It means that this kind of targeted species needs high level protection during long period to colonize some sites outside MPAs. Reserve ability to shelter males probably influences the level of colonization outside the reserve. To explore this hypothesis, we used the technique of estimating the vital domains using the method of the fixed kernel applied to the dusky grouper. Surfaces of territories between 1500 and 3800 m2 on 4 grouper males on the basis of regular observations by visual diving census in Lavezzi (Corsica) have been proposed in a study [32]. This is quite lower than the results obtained with kernels with 95% of UD but corresponds completely to the kernels with 50% of UD. This can be explained by the difficulties in following the movements of the individuals when diving; this technique reduces the possibility to delimit precisely the most frequently used zones. The interest of the fixed kernel method is to generate GIS polygons which can be compared to habitat maps. In our case, we confirm that groupers always stay related to rocky bottoms. Our precise knowledge of habitats (diversity and surface) permits to estimate the caring capacity of the integral reserve estimated between 95 and 154 individuals. The visual census made in 2001 [11] mentioned 134 individuals in the integral reserve which suggests that the carrying capacity is reached. Even if we did not observed migrations outside the integral reserve, the assumption of partial reserve colonization by individuals from the integral reserve can be proposed. This has been reinforced by diver observations for a few years. In the future, we will have to follow the demographic structure of the grouper population. The best candidate for the migration could be the old female, ready to change sex and to become a male. These results show the importance of the choice of MPA location regarding the habitat distribution. Without a precise characterization of habitats in quality and quantity of a large marine area, all MPA projects will be potentially limited in term of searched reserve effect.
Acoustic telemetry appears to be much more informative to study populations of dusky groupers [21,22]. This technique remains however very expensive and requires the tagging of individuals; this step is very problematic in the case of dusky grouper due to its difficulty to capture fish. These two points explain the reduced number of individuals followed in this study. We thus remain very careful when interpretating the results. As the fishes were tagged after the reproduction to limit disturbances, it is very likely that part of the population always stay in this zone. The next tagging campaign should rather be carried out including the beginning of the summer in order to correct this bias. This campaign began in May 2008.
These six individuals, due to their very different sizes are, however, at least well representative of the sections of population of the Natural Marine Reserve of Cerbère-Banyuls. It should be mentioned, however, that, like other studies, this project was confronted to the recurrent problem of small size of the studied sample; however, the convergence of the results between studies reinforces their relevance and accuracy [21,22]. Although the method of fixed Kernel is recognized as having the advantage of being not very dependent on the number of points, it has been recommended to have at least thirty independent localizations [18], which is largely respected in this study. Moreover, GPS points used follow the criterion of independence (i.e. the absence of spatial autocorrelation) of White and Garrott [33]. Authors consider that two localizations are independent, if the time between them, is higher than the time taken by the individual to cross its vital domain.
The number of continuously recorded detections allows analyzing the evolution of the daily behavior of the 6 dusky groupers on a cycle of 24 h. The fact that the number of detections is more important during the day than during the night can be interpreted like an increase of activity during the day. Some changes in daily pattern activities could be related to the distance separating individuals from the fixed receiver. Individuals living near the receiver have indeed a pattern much clearer than those living distant from it. The distance being a factor of loss of signals, this could partly explain the strongest variability of more detections received by fixed receivers.
Acknowledgements
The authors thank the “Fondation Total pour la biodiversité et la mer” for the financial support and the team of the Natural Marine Reserve of Cerbère-Banyuls for the technical support. We are also grateful to an anonymous reviewer for their most helpful comments on an earlier draft of this article. Thanks to P. Masanet (Aquarium de Canet en Roussillon) and P. Romans (Aquarium de Banyuls) for their help and knowledge in fishes anesthetics. We also thank Camille Mellin and Ronan Lucas for improving the English.


