1 Introduction
Due to their important Central Nervous System (CNS) depressant effect, benzodiazepines (BDZ) have been the subject of intense investigations in medicinal chemistry and are now among of the most widely prescribed psychotropic drugs [1,2]. BDZ act allosterically to influence central gamma-aminobutyric acid (GABA)-mediated neurotransmission [3]. GABA is a well known inhibitory neurotransmitter in the mammalian CNS [4,5]. BDZ derivatives produce their effect by interacting with GABAA receptors [6,7], chloride ion channels [8], via the BDZ receptors to increase chloride ion conductivity of the neuronal membrane. BDZ are clinically used in the management of anxiety [9], insomnia [10], seizures [11], skeletomuscular spasticity, panic and as premedication prior to surgery [12]. However, 1,4-BDZ also have some undesirable side effects which limit their clinical use such as memory impairment [13], cognition and motor disturbances, potentialization of alcohol effects and the ability to induce both physical and psychological dependence [14]. To our knowledge, only few experimental investigations comparing the effects of 1,5-BDZ and 1,4-BDZ [15] have been conducted although some studies have shown that a number of these adverse effects are less severe when using 1,5-BDZ [16]. The results pave the way for the screening of new active molecules structurally related to 1,5-BDZ [17]. Many studies have focused on the chemical synthesis of new heterocyclic compounds exhibiting specific activity, low toxicity and a better tolerance than those used nowadays in therapeutics [18,19]. For instance, the 4H-[1,2,4] triazolo [4,3,a] [1,5] BDZ derivative shows some anti-inflammatory and analgesic activities [20,21]. Moreover, several coumarine derivatives exhibit some beneficial health effect as antibacterial and antifungal agents [22]. In the perspective of studying new alternatives to treat neurological disorders, we report in the present study the pharmacological screening of two coumarine derivatives: 4-(2-hydroxyphenyl) 1,3-dihydro-1,5-benzodiazepin-2-one (RG0501) and Benzopyrano [4,3-c] 1,5 benzodiazepinone (RG0502). The current work aims to assess particularly, and for the first time, the hypnotic and the anticonvulsant activities of these two compounds in mice and to compare them with the reference drugs, Diazepam and Flunitrazepam.
2 Methods
2.1 Pharmacology
The tested molecules, RG0501 and RG0502 (Fig. 1), were prepared according to previously published experimental protocols [23,24].
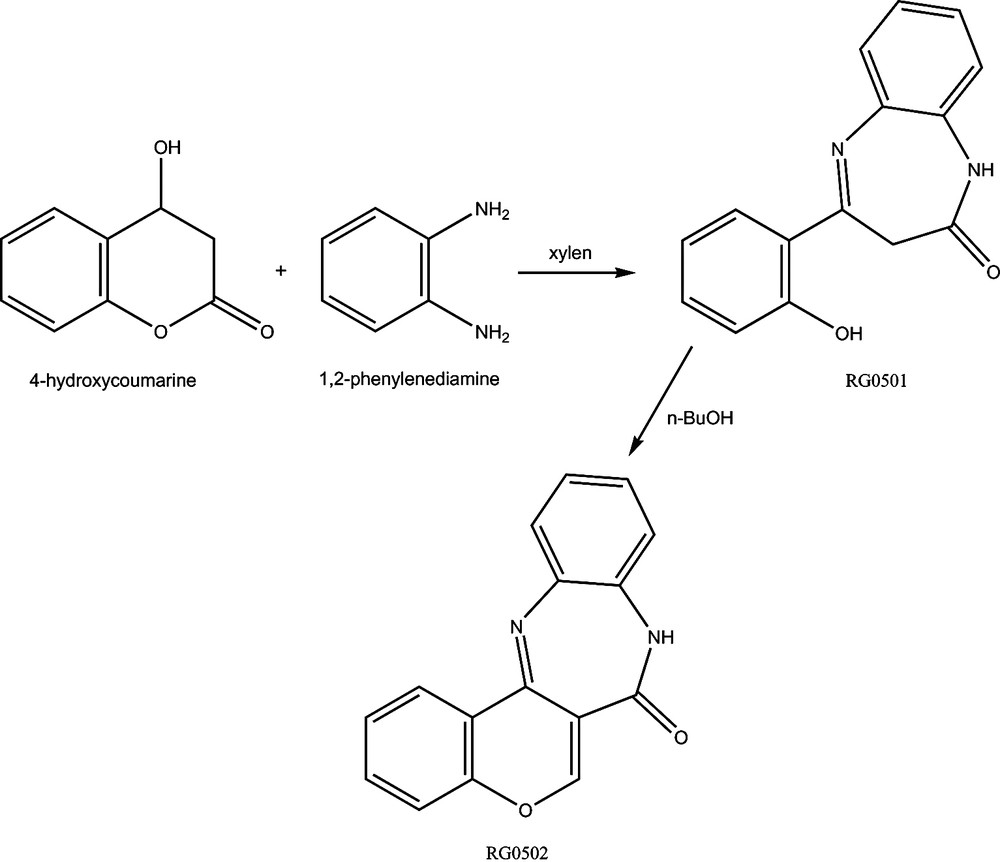
Chemical structures of 4-(2-hydroxyphenyl) 1,3-dihydro-1,5-benzodiazepin-2-ones (RG0501) and Benzopyrano [4,3-c] 1,5-benzodiazepinones (RG0502).
2.1.1 Animals
A total of 335 Swiss albino mice 10 to 12 weeks old (SIPHAT, Tunisia) were used for the study. Four to six animals were housed per cage in a controlled environment. Local vivarium conditions were controlled: temperature (22 °C), humidity (30–60%), and light (12 h:12 h light:dark cycle) during an acclimatization period of at least two weeks before starting the experiments. The mice were fed standard diets (SICO Sfax, Tunisia) and water ad libitum. For each test, the animals were used only once. To avoid any circadian variation, all the experiments have been performed at a fixed time: 11:00 a.m. All animal tests have been performed in accordance with the ethical recommendations of the Tunisian Association of Laboratory Animal Science (ATSAL) which adheres to the International committee on Laboratory Animal Science (ICLAS).
2.1.2 Drugs
Hexobarbital was dissolved in water containing a few drops of ethanol and Twin 80. Pentylenetetrazole (PTZ) was dissolved in sterile 0.9% NaCl solution. Because of their lipophily [25] and in order to avoid the use of organic solvents which can be toxic to the CNS [26], RG0501 and RG0502 compounds as well as the Diazepam and Flunitrazepam reference drugs were dissolved in inert corn oil [27]. All solutions were extemporanelly prepared and administered in an injection volume of 10 mL.kg-1 body weight b.w.
2.1.3 Hypnotic activity
The hypnotic effect of the two compounds was investigated by using the hexobarbital-induced sleep test [28]. One hundred and forty-four male mice were randomly divided into groups of three mice. Each group was intraperitoneally (i.p.) injected with each derivative 45 min prior to the subcutaneous (s.c.) hexobarbital injection (20 mg.kg-1 b.w.). The doses of the heterocyclic derivatives varied from 0.1 to 100 mg.kg−1. Sleep-time was considered as the difference between the loss- and recovery-time of the righting reflex. The control group was i.p. treated with corn oil alone before the hexobarbital injection to determine hypnosis latency and duration. The reference drugs: Flunitrazepam (0.015–4 mg.kg-1 b.w.) and Diazepam (0.01–40 mg.kg-1 b.w.) were i.p. injected 45 min before hexobarbital administration.
2.1.4 Anticonvulsant activity
The anticonvulsant activity of RG0501 and RG0502 compounds was evaluated by potency against PTZ-induced convulsions [29,30]. The standard drug used here is Diazepam.
One hundred and twenty-six mice were randomly divided into groups of six mice. Each group was i.p. injected by with each derivative (6.25 to 75 mg.kg-1 b.w.). Forty five minutes later, the convulsive dose 97 (CD97) of PTZ (85 mg.kg-1 b.w.) was subcutaneously injected in the back of the neck. Diazepam (0.4–3 mg.kg-1) was i.p. injected 45 min prior to the PTZ injection. Immediately after PTZ dosing, the mice were placed into individual cages and observed for 60 min. Animal response to PTZ administration was evaluated using the following criteria: latency to first clonic seizure, latency to the generalized tonic–clonic seizure, protection percentage against clonic and/or tonic seizure and protection percentage against lethality. A threshold convulsion was an episode of clonic spasms lasting for at least 5 s. The absence of convulsion over 60 min indicated a full protection. The number of animals protected in each group was recorded and the percentage of protection was calculated.
2.2 Statistical analysis
Data are expressed as mean ± S.E.M. The comparison of quantitative data between the experimental groups and their corresponding controls was carried out by unpaired Student t-test or a one-way analysis of variance (ANOVA), when applicable. The statistical significance of the differences between qualitative variables among the experimental groups was evaluated using Chi-square test. The results are given in the text as probability values (P). The differences were considered to be statistically significant at a value of P ≤ 0.05. All the statistical analyses were done using GraphPad InStat 3.0a for MacIntosh (Graph Pad Software, San Diego, California, USA).
3 Results
3.1 Hypnotic activity
The two new heterocyclic derivatives increased the hypnosis duration induced by hexobarbital in a dose-dependent manner (Fig. 2). RG0501 compound at 6.25 mg.kg-1 dose significantly increased hexobarbital-induced hypnosis from 41.7 ± 1.53 min to 84.7 ± 1.15 min (p < 0.01). This increase was significantly less important in pretreated mice with RG0502 at 50 mg.kg-1 dose (41.33 ± 2.08 min vs. 78 ± 2.64 min (p < 0.01)). Regarding Diazepam, the increase in sleep duration was well established. At the low dose of 0.15 mg.kg-1, sleep duration was almost two times (75.67 ± 2.08 min) higher than that of untreated mice (40.67 ± 0.57 min). The hypnotic effect was more pronounced with Flunitrazepam since sleep duration reached 71.66 ± 3.0 min at a lower dose (0.125 mg.kg−1) as compared to controls (40.66 ± 1.52 min).

Relationship between potentialization of sleeping time and structure of benzodiazepines. Top section (A): 1,5-benzodiazepines (RG0501 and RG0502) curve responses. Middle section (B): Diazepam curve response. Bottom section (C): Flunitrazepam curve response. Data of barbiturate narcosis potentialization are expressed as mean percent change ± S.D.
RG0502 compound showed a statistically significant reduction of sleep latency in a dose-dependent manner (p < 0.01). However, this reduction appeared less important when compared to reference drugs since it was obtained only with high doses. Indeed, at 100 mg.kg-1, RG0502 induced a sleep latency of 3.67 ± 0.57 min compared to 7.66 ± 0.57 min for untreated mice. The same sleep latency was obtained with 0.08 mg.kg-1 of Diazepam and with 0.06 mg.kg-1 of Flunitrazepam.
With the molecule RG0501 and the range dose 0.1–1.56 mg.kg-1, sleep latency significantly decreased from 7 ± 1 min to 2.67 ± 0.57 min (p ≤ 0.05). This decrease was higher than that observed with RG0502, since this dose range induced a sleep latency of only 6.33 ± 0.57 min in mice pretreated with RG0502. At a starting dose of 6.25 mg.kg-1, sleep latency remained almost similar to that observed with the 3.125 mg.kg-1 dose. Table 1 shows the effective dose that decreased to half the sleep latency of RG0501 and RG0502 as compared to controls (ED50). These results indicate that ED50 of RG0501 is almost equal to ED50 of Diazepam.
ED50 of tested compounds (RG0501 and RG0502) and the reference drugs (Diazepam and Flunitrazepam) in the hypnotic activity. ED50 of tested compounds (RG0501 and RG0502) and the reference drug (Diazepam) in the anticonvulsant activity. Flunitrazepam was not used as a reference drug in the anticonvulsant activity because this drug is not an antiepileptic drug. ED50 (mg.kg-1) is the effective dose that decreased to half sleep latency, clonic and tonic seizures as compared to controls. All experiments were performed at a fixed time: 11:00 a.m. The number of mice was (n = 6) per dose for the anticonvulsant activity and n = 3 per dose for the hypnotic activity.
| Drug | ED50 (mg.kg-1) | ||
| Sleep latency | Clonic seizures | Tonic seizures | |
| RG0501 | 0.17 | 37.5 | 37.5 |
| RG0502 | 50 | Inactive | 75 |
| Diazepam | 0.15 | 0.3 | 0.4 |
| Flunitrazepam | 0.06 | – | – |
3.2 Anticonvulsant activity
Control animals exhibited clonic and tonic seizures at the convulsive dose 97 (CD97) of PTZ (85 mg.kg-1 b.w., i.p.). In untreated mice, PTZ at 85 mg.kg-1 induced clonic seizures, tonic seizures, and mortality at 97%. Pretreatment with RG0501 induced a dose-dependent decrease in clonic seizure latency (p ≤ 0.05), but this decrease was significantly more important in mice pretreated with RG0502 (p < 0.01). Table 1 shows the tested compounds’ effective dose that delays to 50% the first seizure's latency as compared to controls (ED50). RG0501 compound showed a little increase in the latency of clonic and tonic seizures. Nevertheless, at 37.5 mg.kg-1 dose, only 83% of mice showed tonic seizures. This rate decreased dose-dependently to reach 67% at a 50 mg.kg-1, a dose which induced a protection against mortality in mice that not show any tonic seizures. The RG0502 compound increased the latency of the clonic and tonic seizures in a dose-dependent manner. At a 50 mg.kg-1 dose, RG0502 efficiently protected against clonic-tonic seizures and mortality (Table 2).
Protection against clonic and tonic seizures and against mortality (%) of compounds RG0501 and RG0502.
| Protection against clonic seizures (%) | Protection against tonic seizures (%) | Protection against mortality (%) | ||||
| Dose (mg.kg-1) | RG0501 | RG0502 | RG0501 | RG0502 | RG0501 | RG0502 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6.25 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 16.67 | 0 | 16.67 |
| 25 | 0 | 0 | 0 | 33.33 | 0 | 16.67 |
| 37.5 | 0 | 0 | 16.67 | 50 | 0 | 50 |
| 50 | 0 | 100* | 33.33 | 100* | 33.33 | 100* |
| 75 | 0 | 100* | 33.33 | 100* | 33.33 | 100* |
* (p < 0.05). All experiments were performed at a fixed time: 11:00 a.m. The number of mice was (n = 6) per dose.
However, this result remains less important when compared to that of the reference drug. Indeed, at 0.8 mg.kg-1, Diazepam lengthened threefold the latency time of the clonic seizures (224.5 ± 42.01 s in controls vs. 671.5 ± 50.9 s in pretreated mice). In this study, Diazepam decreased significantly clonic and tonic seizures incidence and also induced a significant increment in the latency time of both clonic and tonic seizures (Table 3).
Protection against clonic and tonic seizures and against mortality (%) of Diazepam.
| Dose (mg.kg-1) | Protection against clonic seizures (%) | Protection against tonic seizures (%) | Protection against mortality (%) |
| 0 | 0 | 0 | 0 |
| 0.4 | 0 | 25 | 100* |
| 0.8 | 0 | 100* | 100* |
| 1.5 | 100* | 100* | 100* |
| 3 | 100* | 100* | 100* |
* (p < 0.05). The number of mice was (n = 6) per dose.
4 Discussion
A great interest has been devoted to the pharmacology of BDZ [16] with a special emphasis on the use of 1,5-BDZ [31,32] in various diseases such as cancer [33], viral infection (HIV) [19] and cardiovascular disorders [2]. Some of these derivatives exhibited some interesting antipsychotic [18] or anticonvulsant properties [34–36]. One particular study, focusing on the evaluation of the psychotropic activity of 1,5-diakyl-1,5-BDZ-2,4-dithione, showed that this compound produces some sedative effects [37]. The present study is aimed at investigating the putative hypnotic and anticonvulsant activities of two synthesized poly-heterocyclic compounds belonging to the 1,5-BDZ family.
Accordingly, the two heterocyclic compounds used in pretreatment showed a significant hypnotic activity and an interesting in vivo activity in antagonizing chemically-induced seizures. The use of BDZ in the treatment of insomnia is usually associated with an increase in sleep duration [38]. In our study, we found that for both RG0501 and RG0502 molecules, sleep duration increased dose-dependently, although the RG0501 effect is statistically more prominent (p < 0.01). Regarding sleep latency, RG0501 compound in the 0.1–3.125 mg.kg-1 range exhibited a more important and significant decrease than RG0502 (p < 0.01). These features may be of great use in the design and the development of innovative hypnotic molecules among the 1,5-benzodiazepine derivatives.
Concerning the anticonvulsant activity, the effect of RG0502 compound was much more pronounced than that observed with RG0501. Indeed, there was a dose-dependent increase in the latency of clonic and tonic seizures. At 50 mg.kg-1, a total protection (100%) against seizures and mortality was obtained (p < 0.05).
Nowadays, it has become clear that the biological activity of chemical compounds must be related to their molecular structure [39,40]. The important role played by the position and nature of the substituent in position 7 in the BDZ pharmacological activity should be noted. Indeed, all physiologically active BDZ structures are generally 1,4-BDZ with a halogen or a nitro group at C7 position which is required for the pharmacological effect [41].
BDZ neuroleptic activity is generally enhanced by halogen substitution in position 7 [31] and we have to specify that all clinically-used BDZ have an electro-attracting group substituted at position 7. One study showed an important effect of a chloride derivative of 1,5-BDZ on sleep [16]. Another work showed that [1,2,4] triazolo [4,3-a] [1,5] BDZ exhibiting an anticonvulsant activity are substituted in position 7 with a chlorine atom [36]. Moreover, reference drugs used during our study were Diazepam (substituted in position 7 by Cl) and Flunitrazepam (substituted in position 7 by NO2). Interestingly, a recent study has confirmed the importance of NO2 substitution for the induction of a hypnotic activity [28].
Some speculation can be made on the relationships between the chemical structure and the pharmacological activity of the tested molecules. Substitution in position 7 seems particularly important, enough to induce the hypnotic and anticonvulsant properties of RG0501 and RG0502. These molecules were made less active than the reference drugs due to the absence of this substitution. Thus, the resulting less potent effect illustrates the importance of the electro-attracting atoms within the molecule to provide interesting pharmacological activities.
These pharmacological results indicate that the two tested heterocyclic compounds 4-(2-hydroxyphenyl) 1,3-dihydro-1,5-benzodiazepin-2-one and Benzopyrano [4,3-c] 1,5-benzodiazepinone showed some significant hypnotic and anticonvulsant activities. However, these activities were lower than those of the reference drugs tested in similar conditions. Furthermore, these data suggest a structure-activity relationship for RG0501 and RG0502 compounds and clearly confirm the importance of the 7-Chloro and the 7-Nitro substituent for their biological activity. The differential effect between the test compounds and the reference drugs suggests that even a small structural change could greatly modify the biological effects.
The two compounds show statistically significant effects. More particularly, the RG0501 molecule appears more efficient as a hypnotic agent, whereas RG0502 exhibits a higher anticonvulsant activity.
Some experiments are being conducted to clarify such an assumption and provide a better understanding of the relationship between the chemical structure and the biological activity in BDZ action mechanism, especially in the case of the 1,5-BDZ family.
Acknowledgements
The authors are grateful to Miss Marie-Thérèse Adeline (institut de chimie des substances naturelles, Gif-sur-Yvette, France) for her valuable help in chromatography and they also would like to thank Mr Adel Rdissi for proofreading this article. This work was supported by The Ministry of Higher Education, Scientific Research and Technology, Tunisia. The authors declare that there are no conflicts of interest.


