1 Introduction
The high conservation interest in temporary wetlands (TWs) in the Mediterranean biogeographical region has been long recognized [1–5], ever since the Isoetion communities were defined as “a floristical jewel” by Braun-Blanquet [6]. In Europe, some kinds of TWs are included in the “standing water group” of the Annex 1 of the Habitats Directive 92/43/EEC. They are mainly referred to as habitat types: (a) 3120-Oligotrophic waters containing very few minerals, generally on sandy soils of the West Mediterranean, with Isoetes spp.; (b) 3130-Oligotrophic to mesotrophic standing waters with vegetation of the Littorelletea uniflorae and/or of the Isoeto-Nanojuncetea; and (c) 3170*-Mediterranean temporary ponds [7]. The identification of the different habitat types inside this group is particularly complex because they are characterized by similar plant species and vegetation [7] and in some cases the differences are based on substrate and/or water quality [8]. Furthermore, these habitat types rarely, if ever, occur as isolated stands [8]: more frequently they are found in a dynamic mosaic of different habitats included or not in the Annex 1 of the Habitats Directive.
From a syntaxonomic point of view the benchmark vegetation classes are Isoeto-Nanojuncetea Br.-Bl. & Tüxen ex Westhoff, Dijk & Passchier 1946, which includes pioneer annual and dwarf perennial ephemeral isoetid communities on periodically flooded bare soils and Isoeto-Littorelletea Br.-Bl. & Vlieger in Vlieger 1937 (= Littorelletea uniflorae), which includes dwarf helophyte amphibious oligotrophic communities on shore dystrophic lakes, nutrient-poor standing or slow flooding water [9].
H3120, H3130 and H3170* should be considered emblematic of TWs, especially the latter which is a priority habitat type. It includes the communities which develop in “very shallow temporary ponds (a few centimetres deep) which exist only in winter or late spring, with a flora mainly composed of Mediterranean therophytic and geophytic species” as suggested in the Interpretation Manual of the European Union Habitats [10]. This description fits well with the Isoetion alliance [11].
Moreover, the term “temporary ponds” is used also in its wide sense of “habitat” as well as that of “habitat type” with the general meaning of TWs [12]. Therefore the emblematic type H3170* is sometimes referred to a mosaic of habitats of which it actually represents, if ever, a tessera.
Indeed, despite their small size, TWs present a large variability [11], which is mainly attributed to a typical trait of TWs: a fine-scale zonation of the vegetation, depending on the water depth and the flooding period [4]. To describe and classify the different types of plant communities repeatedly found in TWs, a sampling scale that is at higher resolution than that of the entire habitat should be used. It is then possible to capture the diversity of species assemblages within TW and to identify general patterns of plant diversity and distribution [13]. In California vernal pools, a fine-scale, non-concentric plant distribution has been recognized [13,14], while in some areas of the Mediterranean basin pool vegetation occurs in three concentric belts [5,15,16]: an inner or central belt (CB), an intermediate belt (IB), and a peripheral or outer belt (OB).
The objectives of this study were to: (i) define floristic, structural, and syntaxonomical features of plant assemblages within TWs and the corresponding habitat types; and (ii) identify their patterns at spatial scale adequate for conservation efforts.
2 Materials and methods
2.1 Study sites
The research was performed in Sardinia (Fig. 1), in the western Mediterranean area, which is considered to host the more relevant TW floristic assemblages of the Mediterranean biogeographical region [17].

Location of the nine studied TWs (see text for TW codes).
Although the territory of the island is mainly mountainous, the average elevation is just 334 m a.s.l. TWs are mainly located in the large tablelands that divide the mountain from the plains. They were more widespread in the past but many were converted to agriculture because they were considered to be unhealthy lands. Nowadays, they are poorly represented in the current protected area network [18].
Nine pristine TWs covering a wide range of shapes, elevations and substrates were selected (Fig. 1; Table 1): Buddusò (B), Montresta (M), Monte Minerva (MM), Mandra Puddata (MP); Punta Palai (PP), Suni (S), Scano Montiferro (SC), Santa Maria (SM), and Torralba (T). They ranged in elevation from 308 to 1120 m a.s.l. and 250 to 7743 m2 in surface area, when filled to capacity.
Characteristics of the nine studied TWs (see text for TW codes).
| TW (codes) | B | M | MM | MP | PP | S | SC | T | SM |
| Elevation m a.s.l. | 798 | 436 | 625 | 1033 | 1120 | 308 | 722 | 503 | 632 |
| Substratum | Granites | Calcalkaline volcanites | Calcalkaline volcanites | Calcalkaline volcanites | Calcalkaline volcanites | Alkaline volcanites | Alkaline volcanites | Limestones | Alkaline volcanites |
| n. HSUs | 3 | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 3 |
| Total size m2 | 928 | 594 | 620 | 716 | 1000 | 7743 | 820 | 250 | 300 |
Each TW showed a clear zonation based on maximum water depth and flooding duration (Table 2) and on the morphology of the TW. Therefore a rough concentric arrangement in a central belt (CB), an intermediate belt (IB), and an outer belt (OB) was individuated [5,15]. Each belt was indicated as a homogeneous spatial unit (HSU) because it presented uniform hydrological characteristics (e.g., water depth and flooding duration). Maximum average water depth in the CB varied from 17 to 39 cm with a flooding duration of 4–7 months, in the IB from 10 to 26 cm with a flooding duration of 3–6 months and in OB from 1 to 11 cm with a flooding duration of 1–4 months (Table 2). Water depth in some TWs was greater (e.g., PP and S). In two TWs, the OB was absent because of steep morphology at the boundaries. IB occupied the larger surface in total (Table 2) representing 56% of the monitored surface, while OB occupied just 13% of the total.
Characteristics of the belts in the nine studied TWs (see text for TW codes).
| TWs | B | M | MM | MP | PP | S | SC | T | SM | |
| Size (m2) | Tot | |||||||||
| CB | 168 | 270 | 555 | 625 | 190 | 1938 | 156 | 80 | 75 | 4057 |
| IB | 83 | 294 | 20 | 91 | 740 | 5815 | 56 | 60 | 95 | 7224 |
| OB | 677 | 30 | 45 | 0 | 70 | 0 | 608 | 110 | 130 | 1670 |
| Average max water depth (cm) | AVG ± sd | |||||||||
| CB | 21 | 29 | 29 | 17 | 49 | 42 | 20 | 32 | 19 | 29 ± 11 |
| IB | 12 | 12 | 10 | 11 | 26 | 27 | 10 | 10 | 10 | 14 ± 7 |
| OB | 1 | 6 | 5 | – | 11 | – | 6 | 1 | 9 | 6 ± 4 |
| Flooding duration (months) | AVG ± sd | |||||||||
| CB | 6 | 6 | 6 | 5 | 6 | 6 | 7 | 4 | 5 | 6 ± 1 |
| IB | 6 | 5 | 6 | 5 | 5 | 6 | 6 | 3 | 4 | 5 ± 1 |
| OB | 2 | 4 | 1 | – | 3 | – | 3 | 2 | 2 | 2 ± 1 |
Considering the single TWs, on average 40% of the total surface was occupied by CB with a range of 18–87%; 32% by IB with a range of 3–74%; and 28% by OB with a range of 0–74% (Fig. 2).
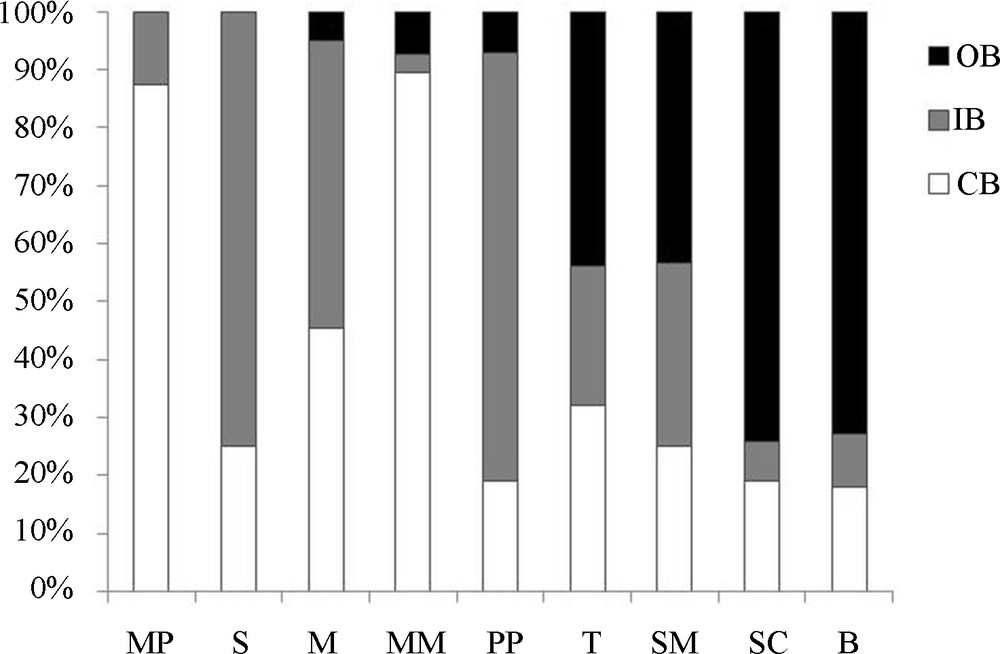
Percentage of the surface occupied by each belt (CB, IB and OB) in each TW (see text for TW codes).
The TWs presented different shapes: the arrangment in belts was rough and, in some cases, the belts were not complete because the morphology of the basin was not uniform (Fig. 3). This irregularity in shape affected mostly the OB which was absent in two sites (Table 1) and strongly reduced or phragmented in several cases.

Maps of the nine TWs: CB - white; IB - grey; OB - black (see text for TW codes).
2.2 Data collection and analysis
Vegetation surveys were carried out within each HSU three times throughout the year: March (1), April (2), and May (3). Plants were classified following Pignatti [19] and Tutin et al. [20] and categorized according to life forms [21] and phytosociological classes [9].
Plant cover was assessed within 30 × 30 cm quadrats located at 1 m intervals along transects (10 m in length). Cover was visually assessed: each quadrat was divided into nine 10 × 10 cm sub-quadrats, and a score was assigned to each plant inside each sub-quadrat: 0 (barren); 1 (cover = 1–25%); 2 (cover = 26–50%); 3 (cover = 51–75%); 4 (cover = 76–100%). The nine estimates were added up [22] and transformed to percent cover. Species cover values at each sampling date and HSU were used to realize a floristic matrix (F matrix) of samples × species describing the floristic space. Based on different aggregation criteria, two matrices were derived from the F matrix: a matrix of samples × life forms describing the structural space (S matrix) and a matrix of samples × phytosociological classes describing the phytosociological space (P matrix). From the F, S and P matrices by averaging the data across sampling dates a matrix of the mean values (e.g., Fm, Sm and Pm) was obtained.
For each matrix, the similarity matrix between each pair of samples was calculated using the Bray-Curtis similarity coefficient on untransformed data [23].
In each space (floristic, structural and phytosociological), a two-dimensional non-metric multidimensional scaling ordination (nMDS) was used to produce two-dimensional ordinations of the assemblages identified in each belt at each date, considering the mean coverage values of each species (in the floristic space), life forms (in the structural space), and phytosociological classes (in the phytosociological space). Formal significance tests for differences between the three groups, based on position within each pond (CB, IB, OB) and between sites and sample dates, were conducted with one-way analysis of similarities (ANOSIM) permutation/randomization tests [24]. The assessment of characterising variables which bring the main contribution to average similarity within each group was performed using the SIMPER routine [25]. PRIMER v6 package [26] was used for all multivariate analyses.
3 Results
A total of 113 plant species were recorded, referable to 18 phytosociological classes (S1 in the supplementary material). Therophytes were dominant representing more than the 50% of the total. Maximum richness was found in OB (α = 92) followed by IB (α = 52) and finally by CB (α = 38). The nMDS ordination and ANOSIM analysis of the F, the S and the P matrices did not point out any differences between sampling dates.
The nMDS ordination and ANOSIM analysis of the Fm matrix pointed out three groups, one for each belt (Fig. 4). Differences between groups were significant overall (Global R = 0.429; p = 0.001) as well as differences between each pair of groups (CB vs IB p = 0.002; CB vs OB and IB vs OB p = 0.001).

Two-dimensional nMDS of plant assemblages in the floristic space. Three date sample average for each belt and TW is reported (see text for TW codes).
Differences between sites were significant overall (Global R = 0.455; p = 0.001). Between pairs of sites differences were significant exept for S vs SC, MP vs B, PP vs T, MM vs SC.
Five top characterising taxa, accounted for a cumulative value of about 80% for the similarity of assemblages in CB: Apium crassipes, Glyceria spicata, Callitriche stagnalis, Lythrum borysthenicum, Ranunculus aquatilis.
In the IB the top characterising taxa were: Alopecurus bulbosus, Lotus uliginosous, Lythrum borysthenicum, Agrostis salmantica, Apium crassipes, Mentha pulegium, Isoetes tiguliana, Glyceria spicata.
In OB the top characterising taxa were: Trifolium subterraneum, Agrostis salmantica, Isoetes histrix, Alopecurus bulbosus, Carex divisa, Bellis annua, Lotus subbiflorus.
The coherence of the floristic groups was confirmed in the structural space analysed throughout the Sm matrix and in phytosociological space analysed throughout the Pm matrix.
In the structural space (Fig. 5), the ANOSIM was globally significant (Global R = 0.351; p = 0.001) as well as the differences between pairs of groups (p = 0.001 in all the comparisons: CB vs IB, CB vs OB and CB vs IB). The top characterizing life forms were hydrophytes (I) and geophytes (G) in the CB; hemicritophytes (H) and G in the IB; and therophytes (T) in the OB.

Two-dimensional nMDS of plant assemblages in the structural space. Three date sample average for each belt and TW is reported (see text for TW codes).
In the phytosociological space (Fig. 6), ANOSIM was globally significant (Global R = 0.455; p = 0.001) as were all the differences between pairs of groups (p = 0.001 for CB vs IB, CB vs OB and IB vs OB). The top characterizing classes were Potametea, Phragmito-Magnocaricetea and Isoeto-Nanojuncetea in CB; Juncetea maritimi, Isoeto-Nanojuncetea and Phragmito-Magnocaricetea in IB, and Poetea bulbosae, Isoeto-Nanojuncetea, Juncetea maritimi and Stellarietea mediae in OB.

Two-dimensional nMDS of plant assemblages in the phytosociological space. Three date sample average for each belt and TW is reported (see text for TW codes).
4 Discussion and conclusions
The TWs presented high heterogeneity in terms of environmental factors (e.g., elevation, size, substratum) and consequently in plant biodiversity. Elevation recognized in previous studies as one of the main environmental variables affecting biotic assemblages in ponds [27] and indicated as an important predictor of aquatic macrophyte richness [28] presented a range of 700 m which should be enough to influence plant assemblages in TWs. TW size is another environmental factor affecting plant richness and composition. For aquatic plants, a positive relationship between pond size and plant species richness has been considered to be generally valid [27] and the pond area is considered an important predictor of aquatic macrophyte richness [28]. Also, pond size and substrate are thought to be important environmental factors affecting plant assemblages [29]. In our case, the TWs ranged between 250 and 7743 m2 and substratum ranged from basic (limestone) to acid (granitic). As a consequence, the differences between TW plant assemblages were globally significant and each assemblage was generally different from all others.
In this study, differences between sample dates (1-March, 2-April, and 3-May) were not globally significant. Seasonal variability in plant assemblages has been previously considered a relevant characteristic in Mediterranean TWs [5,16]. Nevertheless, this result concerns homogeneous area where the differences between plant assemblages were not affected by elevation and substrata.
The application of the approach based on HSUs allowed us to identify distinct plant assemblages within TW characterized by different floristic, structural, and sintaxonomical features repeatedly present in each site in the same relative position.
The “within TWs” hydrological gradient was strong enough to drive the vegetation patterns and habitat types.
Plant assemblages presented a typical distribution within TWs following an arrangement according with the water-depth gradient and the flooding period confirming what already observed in different areas of the world [13,15,30–34]. The “within TWs” water gradient strongly affected the plant assemblages and their spatial arrangement in concentric belts. The belts should be considered homogeneous areas (HSUs) and the targets for conservation actions. Differences in the floristic space were more significant in the comparisons CB vs OB and IB vs OB than IB vs CB. Thus, OB is the more differentiated and well characterized of the three. In the phytosociological space, the Isoeto-Nanojuncetea class was listed among the characteristic classes for each of the three belts. This class is considered, together with the Isoeto-Littorelletea class, not represented in the study area, the benchmark for the identification of the habitat listed in the Annex 1 of the Habitats Directive [10] in TWs. Nevertheless, some relevant differences in quantitative and qualitative terms have been detected useful for the identification of the habitat types.
Typical aquatic species such as Glyceria spicata, Callitriche stagnalis, and Ranunculus aquatilis were relevant only in CB, where the structural space was mainly characterized by I and the phytosociological space by Potametea and Phragmito-Magnocaricetea classes. They generally fit the hydrology of a maximum water depth of 50 cm and an average flooding period of 6 months. The brevity of the inundation also allowed of amphibian species such as Apium crassipes, which is characteristic of Tyrrhenian suballiance Apienion crassipedis of the Preslion cervinae alliance [16] to be present. The Preslion cervinae has been ascribed to H3120 [16].
Among the characteristic species of IB was Alopecurus bulbosus which has been ascribed to the Juncetea maritimi class, including perennial grasslands growing along the coast and inland temporary wet areas or long-inundated salt marshes [9].
The dominant life form in this belt was H due to the dominance of A. bulbosus. Also, G were important, with a great contribution of the aquatic quillworts, Isoetes velata and I. tiguliana, which here reached their highest coverage values. The last was indicated as characteristic of Apienion crassipedis suballience together with Apium crassipes, wich was also abundant in this belt. Thus, the presence of H3120 in IB should also be recognized. The relatively long flooding period (5 months) and the maximum water level allowed species of the Phragmito-Magnocaricetea class (e.g., Glyceria spicata) to be present.
OB was clearly different from the other two zones. The water was very shallow (6 cm maximum average) and the flooding period very short (2 months). Such conditions are more suitable for the presence of the priority H3170* [10] which is considered a subtype of the H3120 in “temporary and very shallow water”. Its floristic space was notable by the presence of Isoetes histrix, among the characteristic species. This terrestrial quillwort, never found in CB or IB, is characteristic of the Isoetion alliance, which represents the most threatened alliance into the Isoeto-Nanojuncetea class [16]. The presence of the H3170* was confirmed in the structural species too, because the OB is mainly characterized by “therophytic and geophytic species” [10]. Therefore, OB should represent the main conservation target within TWs. On the other hand, it was the smallest in size (only 13% of the total surface) and in some cases absent (e.g., MP and S), inconspicuous (e.g. M and PP), or severely fragmented (e.g., MM). It should be considered more vulnerable in respect to the colonization by terrestrial species, especially those linked to human activities such as Poetea bulbosae and Stellarietea mediae classes. Otherwise traditional human activities have been recognized as having an important role in maintaining plant biodiversity in TWs [15,35,36], especially activities that prevent colonization by woody species [37]. The alteration of hydrological regimes should be another threat, which particularly affects OB plant assemblages. In fact, the response of “edge species” to inundation suggested that they suffer from moisture extremes, too dry or too wet, which cause them to become locally extinct [14].
The relevance of differences in plant assemblage diversity in the comparisons between sites and the pattern of habitat types within TWs suggest that the conservation programs are requested to pursue to aims in order to ensure the conservation of the highest variety of plant assemblages, species and habitat types. The first directs to TWs conservation of the maximum possible number of sites, and the second throughout special attention in conservation program for the priority habitat 3170* and then the OB. Finally, in order to avoid confusion, it is desirable that the terms “Mediterranean temporary ponds” should be used to designated only the emblematic priority habitat 3170*.
Acknowledgements
We sincerely thank Prof. Michael G. Barbour, University of California, Davis and Daniela Gigante, University of Perugia, Italy, for the review of the text.


