1 Introduction
The genus Teuthraustes was created by Simon [1] based on a new species, Teuthraustes atramentarius Simon, 1878 collected in Ecuador by M. Deville of the Brussels Museum. Both the genus and species descriptions are extremely reduced and poorly diagnostic. Between Simon's [1] description and the revision of the genus by Kraepelin [2], thirteen additional species have been described or transferred to Teuthraustes. With the exceptions of Teuthraustes amazonicus (Simon, 1880) and Teuthraustes glaber Kraepelin, 1912, both described from Peru, the other eleven species originated from Ecuador. Even if the taxonomic status of some Ecuadorian species may yet be the subject of question, this remarkable concentration of species in Ecuador is realistic and can be explained by biogeographic models [3].
From the revision by Kraepelin [2] and until the monograph work of Mello-Leitão [4], no new species of this genus were described. Subsequently, five new species have been described from Venezuela, Teuthraustes carmelinae Scorza, 1954, Teuthraustes adrianae González-Sponga, 1975, Teuthraustes akananensis González-Sponga, 1984, Teuthraustes maturaca González-Sponga, 1991 and Teuthraustes reticulatus González-Sponga, 1991, one from Brazil, Teuthraustes lisei Lourenço, 1994 and one from Colombia, Teuthraustes guerdouxi Lourenço, 1995 [5–7]. All the species of Teuthraustes, so far described, have been collected in the Andean mountains in Ecuador, Peru and Colombia, and in the Amazonian highlands of Venezuela and Brazil. These highlands are known as the ‘Tepuys’. One single exception remains. This is T. amazonicus, a species described from Pebas, a small town located on the banks of the Solimões River in Peruvian Amazonia.
In the present article, yet another new species of Teuthraustes is described from Brazilian Amazonia. This represents the second element of the genus to be found in the Amazonian lowlands. The pattern of distribution presented by the species of the genus Teuthraustes remains, however, a typical of highland formations of South America.
2 Geographical pattern of distribution of the genus Teuthraustes
The known geographical distribution of the genus Teuthraustes, clearly indicates its endemic and disrupted nature. Of the twenty species known at present, twelve are distributed in the Andean highlands of Ecuador and Peru. Another group of six species has been described even more recently from a different highland site located between Brazil and Venezuela. This area clearly corresponds with the Imeri endemic centre which has been defined both for plants and for animals [8–10]. It is located in the ‘Tepuys’ region which lies mainly in Guayana, a floristic province that has been delineated botanically [11]. Finally, one species is known from the highlands of Colombia and yet another has been reported from the lowlands of the Peruvian and Brazilian Amazonia.
This outstanding concentration of Teuthraustes species in the Andean highlands, and in the Imeri endemic centre, may be similar to the ‘explosive’ pattern of speciation proposed by Gentry [12] for plants of the genus Gasteranthus. This contains about 25% of the world total of species in the Andean region. According to Gentry [12], it is probable that an entirely different evolutionary mode may be operating in these areas. The exceedingly dynamic speciation is perhaps mediated more by genetic transilience associated with genetic drift in small founder populations in a kaleidoscopically changing milieu, than by fine-tuned selection of the type generally suggested to be typical of lowland rain forest.
Several botanists [12,13] and entomologists [14,15] agree that tropical rainforests are the most species-rich ecosystems on Earth. They also agree, on the basis of solid evidence, that the ‘epicentre’ of global biodiversity occurs in the tropical Andes, a region of the upper Amazonia which includes the North of Peru, Ecuador and the southern half of Colombia. This suggestion seems to be valid for plants, vertebrates and butterflies [10,12,13]. The region is also one of the world's greatest sites of alphadiversity on scorpions [16]. Consequently, the very high concentration of Teuthraustes species in the Andean region could perhaps be no more than the consequence of the great ecological diversity there.
It is obvious that scorpion speciation and differentiation is by no means recent. As stated by Haffer [17], the isolation of large populations due to Tertiary palaeogeographic changes undoubtedly played a major role in establishing the basic distributional and evolutionary patterns of the tropical floras and faunas at higher taxonomic levels of family and genera. At the same time, members of less ‘plastic’ groups with low evolutionary rates in the present flora and fauna have survived relatively unchanged since Tertiary times. Since the Andes arose in the form of strings of growing islands from a marine geosynclinal basin, there was no pre-Andean continuous and widespread lowland fauna occupying what was later to become the Andes and their forelands. Consequently, Andean elements must have come from abroad. The ‘Tepuys’ region which includes the Imeri endemic centre, is located in the Precambrian Guiana Craton (or shield). From a geological point of view, the ‘Tepuys’ are composed of sheer blocks of Precambrian sandstone and quartzite rocks. These ‘mesas’ are the remains of a huge sandstone plateau that once covered the granite basement complex between what is today the northern border of the Amazon Basin and the Orinoco, between the Atlantic coast and the Rio Negro [18].
Ecological, paleoclimatic and palynologic data [10] indicate that the apparent ‘stability’ of present day rainforests was interrupted by periods of climatic change through several dry/wet/dry episodes of the late Cenozoic period, and especially during more recent Pleistocene and Holocene epochs. During the earlier Quaternary period, temperate regions were glaciated. Cooler and drier conditions prevailed in the present tropical zones and reduced the rainforest to savannas or dry-forests except in localized regions where conditions of temperature and humidity allowed them to persist. During these glacial phases, more mesic species of scorpions, such as those of the genus Teuthraustes, and also of other mesic genera such as Chactas Gervais, 1844 and Vachoniochactas González-Sponga, 1978 probably experienced a more enlarged range of distribution than that observed today. With the return of the present interglacial phase, these genera are again restricted to the highlands where mesic conditions prevail. Some species of the genera Teuthraustes and Chactas, however, probably evolved in and became adapted to the tropical forest when this one expanded and coalescence throughout the entire Neotropical lowlands [6,19]. Consequently, one can expect new species of both genera still to be found in the lowlands of Amazonia (Fig. 1).

Map of Northern South America showing the distribution of Teuthraustes species in the Andean and Guayana highlands, and in the lowlands of Amazonia. 1. Teuthraustes adrianae. 2. Teuthraustes akananensis. 3. Teuthraustes amazonicus. 4. Teuthraustes atramentarius. 5. Teuthraustes carmelinae. 6. Teuthraustes dubius. 7. Teuthraustes festae. 8. Teuthraustes gervaisii. 9. Teuthraustes glaber. 10. Teuthraustes guerdouxi. 11. Teuthraustes lisei. 12. Teuthraustes lojanus. 13. Teuthraustes maturaca. 14. Teuthraustes oculatus. 15. Teuthraustes ohausi. 16. Teuthraustes reticulatus. 17. Teuthraustes rosenbergi. 18. Teuthraustes simonsi. 19. Teuthraustes whymperi. 20. Teuthraustes wittii. 21. Teuthraustes braziliensis sp. n.
3 Material and methods
Measurements and illustrations were made using a Wild M5 stereomicroscope with a drawing tube (camera lucida) and an ocular micrometer. Measurements follow those of Stahnke [20] and are given in mm. Trichobothrial notations are those developed by Vachon [21] and the morphological terminology mostly follows that of Hjelle [22].
Taxonomic treatment
Family Chactidae Pocock, 1893
Genus Teuthraustes Simon, 1878
Type material of T. amazonicus examined (Fig. 2). Originally three male syntypes. One male is herewith designated lectotype. The other two males are designated paralectotypes. The lectotype and one paralectotype are deposited in Muséum national d’Histoire naturelle, Paris (MNHN) RS-0767 (ES-3637). One lectotype is deposited in Zoologischen Museum Hamburg (ZMH).

Teuthraustes amazonicus, male lectotype. A. Carapace. B. Genital operculum and pectines. C. Metasomal segments IV–V and telson, lateral aspect. D. Chela, dorsal aspect, showing trichobothria.
Teuthraustes braziliensis sp. n (Figs. 3 and 4)
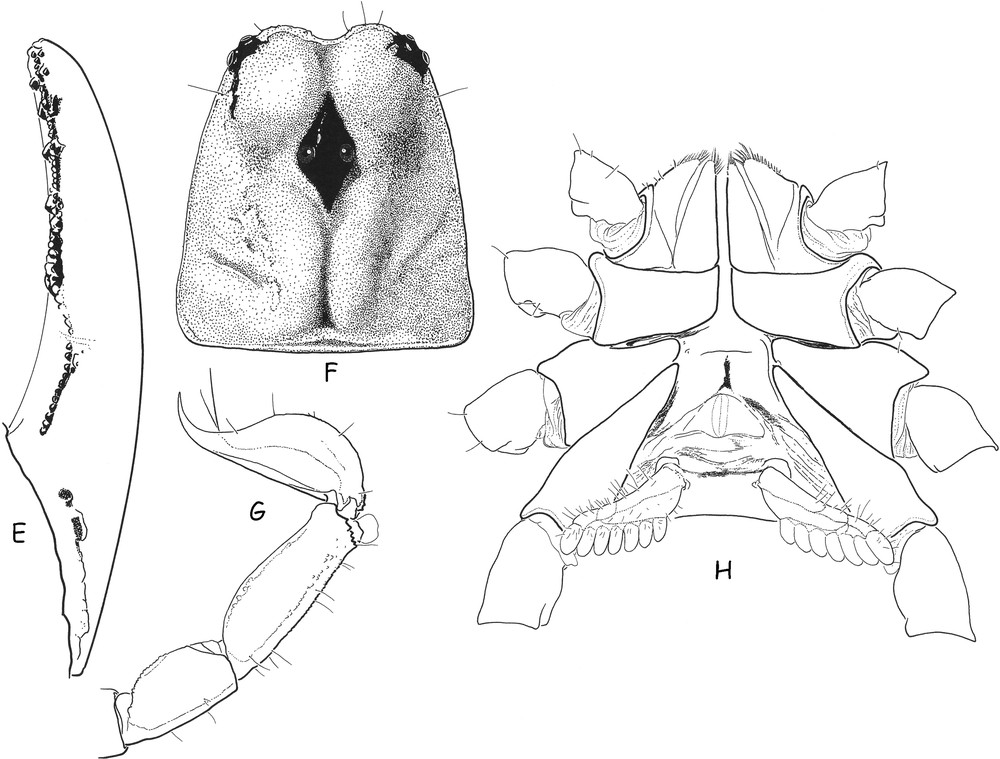
Teuthraustes braziliensis sp. n., male holotype. E. Cutting edge of movable finger with rows of granules; the most basal series is isolated from distal ones by a depressed zone in which the strong tooth of the fixed finger gets inserted. F. Carapace. G. Metasomal segments IV–V and telson, lateral aspect. H. Ventral aspect, showing coxapophysis, sternum, genital operculum and pectines.
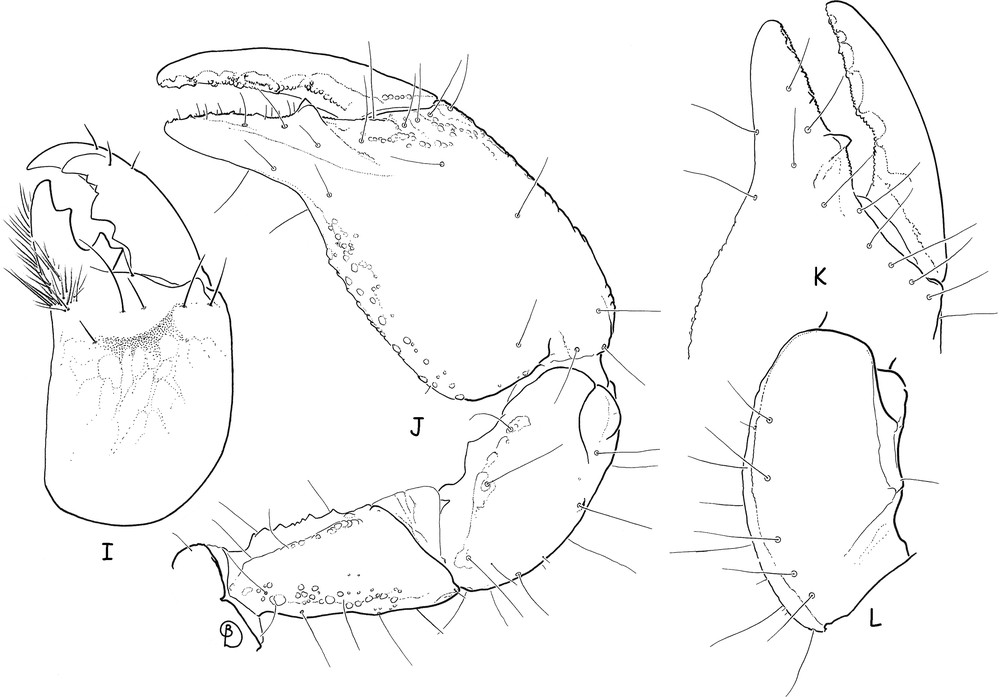
Teuthraustes braziliensis sp. n., male holotype. I. Chelicera, dorsal aspect. J. Right pedipalp, dorsal aspect, showing trichobothria. K. Chela, dorso-external aspect, showing detail of fingers and trichobothria. L. Patella, ventral aspect, showing trichobothria.
Material: Brazil, Amazonas, BR-319 km 350, trilha 1 ponto 1500 (5° 15′ 48.54′′ S – 61° 56′ 9.42′′ W), pitfall, 25/VII-1/VIII/2008 (H. Guariento & L. Pierrot), male holotype; Beruni, BR-319, km 350 (5° 16′ S–61° 54′ W), pitfall, 4/VIII/2007 (V. T. Carvalho), male paratype. Type material deposited in the ‘Instituto Nacional de Pesquisas da Amazônia’ (INPA-0560, 0639), Manaus, Brazil.
Etymology: The specific name is derived from Brazil, the country in which the new species was collected.
Diagnosis: Moderate scorpions, 44 mm in total length. Coloration reddish-yellow to reddish-brown. Body and appendages weakly granulated to smooth, with minute punctuation. Pectines with 6/7 teeth in males. Fixed and movable fingers of chela with 5–6 rows of granules. Ventral carinae absent on metasomal segments I to IV. Trichobothrial pattern of type C neobothriotaxic ‘majorante’.
Relationships: The new species can be distinguished from others in the genus Teuthraustes and in particular from T. amazonicus Simon, which is also distributed in the Amazonian lowlands, by the following features: (i) carapace, tergites and pedipalps weakly granulate to smooth, (ii) metasomal segments I to IV totally smooth and without ventral carinae, (iii) pectines smaller and narrower with less teeth.
Description (based on male holotype). Measurements after the description.
Coloration: Basically reddish-yellow to reddish-brown. Prosoma: carapace reddish-brown; eyes blackish. Tergites reddish-brown, slightly paler than carapace, with one incomplete yellowish longitudinal strip. Metasomal segments reddish, with darker zones over carinae; vesicle yellowish to reddish-yellow with the tip of the aculeus dark brown. Chelicerae yellowish, with some diffuse variegated reddish spots; fingers dark reddish. Pedipalps dark reddish; carinae blackish. Legs yellowish without spots. Venter and sternites reddish-yellow to yellowish; pectines and genital operculum pale yellow.
Morphology: Carapace lustrous and acarinate, with dense minute punctuation and some minute granulations on the lateral edges; furrows shallow. Sternum pentagonal, wider than long. Tergites acarinate, with punctuations. Pectinal tooth count 7-7, fulcra absent. Sternites, smooth and shiny; spiracles oval-shaped and conspicuous; VII acarinate. Metasomal segments I and II wider than long; metasomal tegument on segments I to IV lustrous without granulations; segment V with some spinoid granulations ventrally. Carinae on segments I–V weakly developed; ventral absent; only dorsal and laterodorsal are vestigial. Telson with a few ventral granulations, almost smooth; aculeur shorter than vesicle. Pedipalps: femur with dorsal internal, dorsal external and ventral internal carinae strongly marked; ventral external carina absent; dorsal, ventral and internal aspects smooth. Patella smooth with vestigial carinae. Chela smooth and acarinate; internal aspect with a few small granules. Dentate margins on movable and fixed fingers with 5–6 rows of granules. Chelicerae with dentition typical of the family Chactidae [23], and with dense setation ventrally and internally. Trichobothriotaxy of type C; neobothriotaxic ‘majorante’ [21]. Legs tarsi with short setae disposed in a single line.
Morphometric values of the male holotype of T. braziliensis sp. n. and the male lectotype of T. amazonicus. Total length excluding the vesicle, 44.5/41.2. Carapace: length, 7.5/6.8; anterior width, 4.5/4.2; posterior width, 7.2/6.7. Metasomal segments. I: length, 2.4/2.5; width, 3.6/3.2; II: length, 3.1/2.8; width, 3.2/3.1; III: length, 3.4/3.2; width, 3.0/3.0; IV: length, 4.1/3.8; width, 2.9/2.8; V: length, 6.7/6.3; width, 3.0/2.7; depth, 2.5/2.5. Telson length, 7.7/6.9; vesicle: width, 3.0/2.9; depth, 2.6/2.3. Pedipalp: femur length, 5.2/4.5, width, 2.3/2.3; patella length, 5.5/5.2, width, 2.9/2.6; chela length, 11.7/10.7, width, 3.7/3.4, depth, 5.5/5.2; movable finger length, 6.2/5.2.
Conflict of interest statement
There is no conflict of interest.
Acknowledgements
We are most grateful to Prof. John L. Cloudsley-Thompson, London, for useful comments to the manuscript, and to Elise-Anne Leguin, MNHN, Paris for the help with the preparation of the plates.


