1 Introduction
Resveratrol is a natural polyphenol found in grapes [1]. Several studies have shown its protective effects on the vascular endothelium [2–4], which could be beneficial in cardiovascular diseases [5]. Over the last 10 years, cellular effects of resveratrol have been described, such as antioxidative [6], antiinflammatory [7] and antitumoral properties [8]. These effects involve different metabolic pathways, which could suggest that resveratrol is able to act both on extracellular and intracellular targets. Interaction of resveratrol with membrane receptors, such as estrogen receptor [9], leads to a series of signaling pathways, targeting for example the activation of the adenosine monophosphate-activated protein kinase (AMPK) or the silent mating type information regulation 2 homolog 1 (SIRT1), resulting in biological effects [10,11]. Antioxidative effects of resveratrol could be explained by its capacity to activate enzymatic defenses through membrane receptors, but also to directly scavenge free radicals in the cell. Resveratrol could thus be able to penetrate into the cell and to interact with reactive oxygen species (ROS) and other molecular targets [12,13].
Bioavailability of resveratrol and stilbene derivatives is currently discussed. Indeed, the hydrophobic properties of this molecule are not in favor of a good bioavailability. However, some studies conducted in humans have shown that resveratrol was detected in plasma after a moderate intake of red wine [14,15]. In these studies, resveratrol was quickly metabolized into trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate that were secreted into the cell. However, only few studies have shown the presence of resveratrol in cellular models [16,17].
Thus, the protective effects of resveratrol have been described in several studies performed in vascular endothelium cells from various species in vitro, without showing if resveratrol was found inside the cell.
The aim of that study was to evaluate the ability of resveratrol to penetrate into bovine aortic endothelial cells (BAEC) in vitro after 24 h incubation, and to tentatively determine the active or passive way of transport.
2 Materials and methods
2.1 Cell culture
Bovine aortic endothelial cells (BAEC) were cultured in 75 cm2-flasks, in Dubelcco's Modified Eagle Medium (DMEM) without phenol red (Sigma Aldrich) supplemented with 10% FBS (Invitrogen), 1% L-glutamine (Sigma Aldrich), 1% antibiotics (penicillin/streptomycin, Sigma Aldrich) at 37 °C, in a humidified 5% CO2 incubator, until they reached 80% confluence.
2.2 Cytotoxicity assay
To evaluate the toxicity of resveratrol (purity ≥ 98%, Coger) on BAEC, a neutral red assay in 96-well plate was performed.
A 50 mM stock solution of resveratrol was prepared in ethanol (Sigma Aldrich). Cells were incubated with 200 μL of various concentrations of resveratrol (1, 5, 10 or 50 μM) diluted in DMEM (1% ethanol) for 24 h at 37 °C in a humidified 5% CO2 incubator. One hundred microlitres of a solution of neutral red (Sigma Aldrich) were added to each well. Cells were incubated during 3 h at 37 °C in a humidified 5% CO2 incubator. The plate was emptied by reversal. One hundred microlitres of a solution of formol-calcium (Sigma Aldrich) were distributed in each well and let in contact with the cells during 1 min. The plate was again emptied by reversal and 100 μL of a solution of ethanol with acetic acid (Sigma Aldrich) were added to each well. The plate was let under stirring during 5 min and absorbance was then measured at 540 nm on a microplate reader (MultiskanEx, Thermo Electron Corporation).
2.3 Treatments
All treatments were made in DMEM without phenol red, supplemented with 1% FBS, 1% l-glutamine, 1% antibiotics (penicillin/streptomycin), 1% ethanol. A 50 mM stock solution of resveratrol was prepared in ethanol (Sigma Aldrich). Cells were incubated with 15 mL of various concentrations of resveratrol (1, 5, 10 or 50 μM corresponding respectively at 15, 75, 150 and 750 nmoles) for 24 h. Then, they were trypsinized, washed with ice-cold phosphate-buffered saline (PAA Laboratories) by centrifugation during 10 min, at 1500 g and at 4 °C and harvested into 0.2 mL of lysis buffer (Sigma Aldrich). Supernatants were conserved and cell pellets were transferred into an Eppendorf tube before centrifugation at 3000 g during 15 min to separate the cytosolic and the membrane fractions.
2.4 Determination of total protein concentration
For each experimental condition, protein concentration was determined by Biorad DC Protein Assay kit.
2.5 Determination of resveratrol intracellular and membrane concentrations by HPLC
Measurements of resveratrol in intracellular and membrane fractions were determined by reverse-phase High Performance Liquid Chromatography (HPLC) with UV detection (304 nm) on a C18 5-μm Kromasil column (AIT) (25 cm × 4.6 mm internal diameter) as described by He et al. [18] with slight modifications. The mobile phase, consisting of a mixture of methanol, distilled water and acetic acid (52: 47.5: 0.5, vol/vol/vol), was pumped at a flow rate of 1 mL/min and the column temperature was maintained at 40 °C. To analyze the different fractions, the separation was performed in isocratic conditions. The concentration of resveratrol found in the sample was determined by external calibration. The resveratrol calibration curve was obtained by dilution of a stock solution of resveratrol (50 mM, 100% ethanol) in the mobile phase. Concentrations of resveratrol used were: 0.1 μM, 0.2 μM, 0.3 μM, 0.4 μM, 0.5 μM, 0.6 μM, 0.7 μM, 1 μM, 5 μM, 10 μM and 50 μM. The limit of resveratrol detection was 35 nM.
Fig. 1 shows the structure of resveratrol (A) and a chromatogram profile of a solution of resveratrol 50 μM in DMEM (1% ethanol) (B).
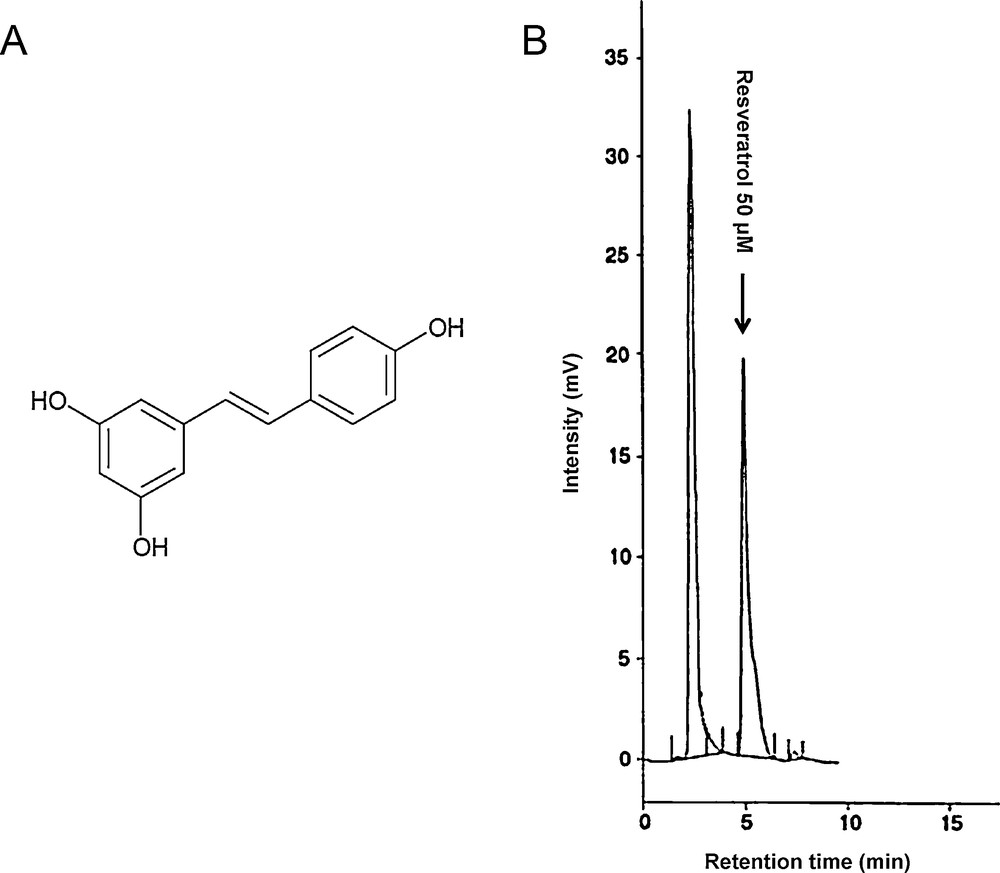
Structure of resveratrol (A) and chromatogram profile of a 50 μM solution of resveratrol (B). Retention time was at 5.2 minutes.
For membrane fractions, 200 μL of mobile phase were added and centrifuged for 10 min at 8000 g; 100 μL of supernatant were injected into the column.
For cytosolic fractions, 100 μL of methanol were added to 100 μL of cytosolic fraction and centrifuged for 10 min at 8000 g, then 100 μL of supernatant were injected into the column.
Concentrations of resveratrol in membrane and cytosolic fractions were expressed per milligram of protein.
2.6 Determination of resveratrol concentrations in culture media by UV spectrophotometry
The concentration of resveratrol in culture media was determined by UV spectrophotometry at 304 nm, as previously described by Camont et al. [19]. Measurements were not performed by HPLC at this step because culture media contained a great number of compounds making interferences with resveratrol determination by hiding its peak.
2.7 Study of the transport mechanism of resveratrol through the cell
Cells were incubated with 15 mL of DMEM without phenol red containing 5 μM of resveratrol, for 6 h at 37 °C, 5% CO2 or at 4 °C. After incubation, cell culture media were conserved and cells were trypsinized, washed and harvested into lysis buffer as previously described.
Controls without cells have been treated in the same conditions (incubation at 37 °C or 4 °C for 6 h) to consider the autoxidation of resveratrol.
2.8 Statistical analysis
The results are expressed as the mean ± SEM of at least five different cultures. For all experiments, each condition was measured in triplicate. Cells were used between the sixth and tenth passages. Statistical significance was determined by the non-parametric Mann-Whitney test. P values < 0.05 were considered as statistically significant.
3 Results
3.1 Study of resveratrol cytotoxicity on BAEC
Cytotoxicity of the four resveratrol concentrations used during all the series of experiments (i.e., 1, 5, 10 and 50 μM) has been studied after a 24-h incubation, by an incorporation assay with neutral red. For any concentration, no cytotoxicity of resveratrol was observed.
3.2 Determination of intracellular and membrane concentrations of resveratrol in BAEC
BAEC were incubated for 24 h with resveratrol solutions at different concentrations (ranging from 1 to 50 μM). Intracellular concentrations of resveratrol in cellular lysates were obtained by HPLC and were as follows for each concentration tested (i.e., 1, 5, 10 and 50 μM): 0.038, 0.066, 0.132 and 0.645 nmol/mg of protein, respectively (Fig. 2A). Similarly, membrane concentrations of resveratrol were as follows for each concentration tested (i.e., 1, 5, 10 and 50 μM): 0.017, 0.032, 0.040 and 0.142 nmol/mg of protein, respectively (Fig. 2B).
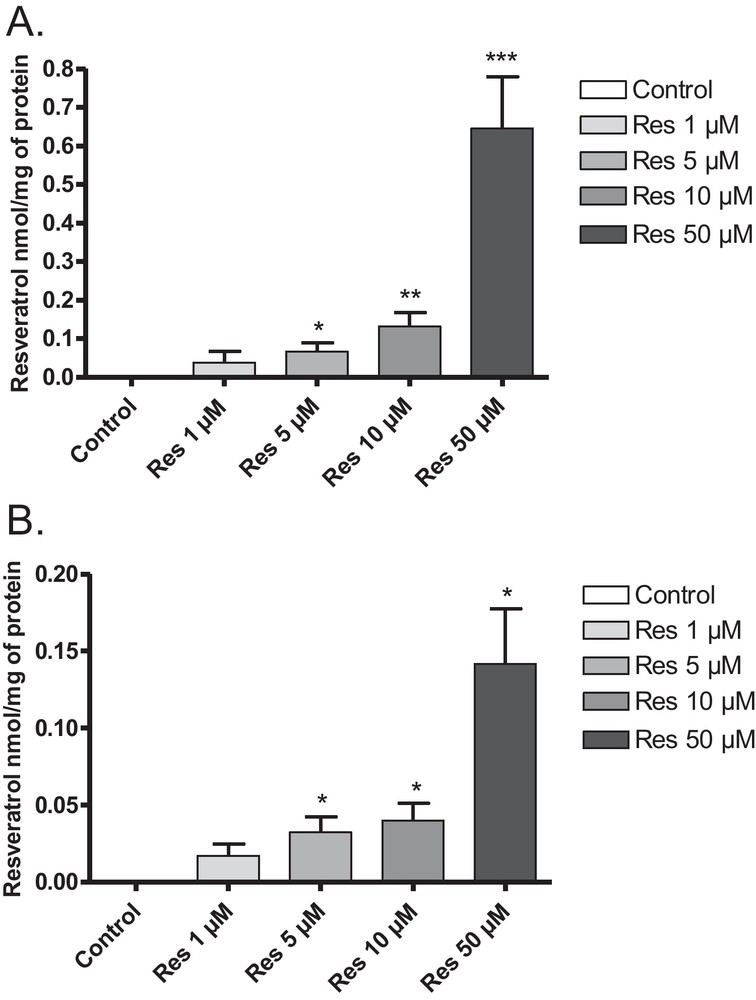
Intracellular (A) and membrane (B) concentrations of resveratrol in BAEC. Cells were incubated with various concentrations of resveratrol (1, 5, 10 and 50 μM) for 24 h. Resveratrol was detected by HPLC in intracellular fractions (A) *P < 0.05 vs 1 μM; **P < 0.05 vs 5 μM; ***P < 0.05 vs 10 μM and membrane fractions (B) *P < 0.05 vs 1 μM. Data are means ± SEM.
3.3 Determination of concentrations of resveratrol in culture media
Amounts of resveratrol in culture media were measured by UV spectrophotometry at 304 nm as described by Camont et al. [19], for the different concentrations of resveratrol used in this study after a 24-h incubation with BAEC. This value allowed us to determine the amount of resveratrol consumed by the cell after 24 h, for each concentration (Fig. 3). Amounts of resveratrol consumed in 24 h for the different concentrations tested (1, 5, 10 and 50 μM, corresponding to 15, 75, 150 and 750 nmol resveratrol, respectively) were 4.88, 32.50, 63.75 and 281.70 nmol/24 h, respectively.
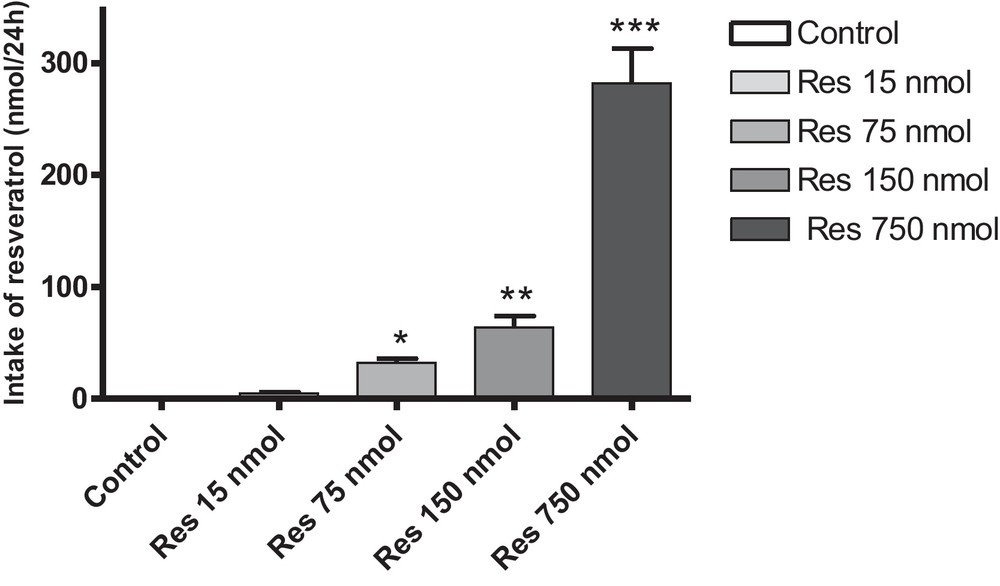
Intake of resveratrol by BAEC after 24 h of incubation. Cells were incubated with various amounts of resveratrol in culture media (15, 75, 150 and 750 nmol) for 24 h. *P < 0.05 vs. 15 nmol; **P < 0.05 vs. 75 nmol; ***P < 0.05 vs. 150 nmol. Data are means ± SEM.
3.4 Determination of the intracellular concentration of resveratrol in BAEC at 4 °C and 37 °C
To determine the transport mechanism of resveratrol through endothelial cells, BAEC were incubated with resveratrol at a concentration of 5 μM for 6 h at 37 °C or 4 °C to inhibit active cellular transport. Our results did not allow us to show any significant difference in the intracellular concentrations of resveratrol, either at 4 °C (0.046 nmol/mg of protein) or at 37 °C (0.043 nmol/mg of protein) (Fig. 4).
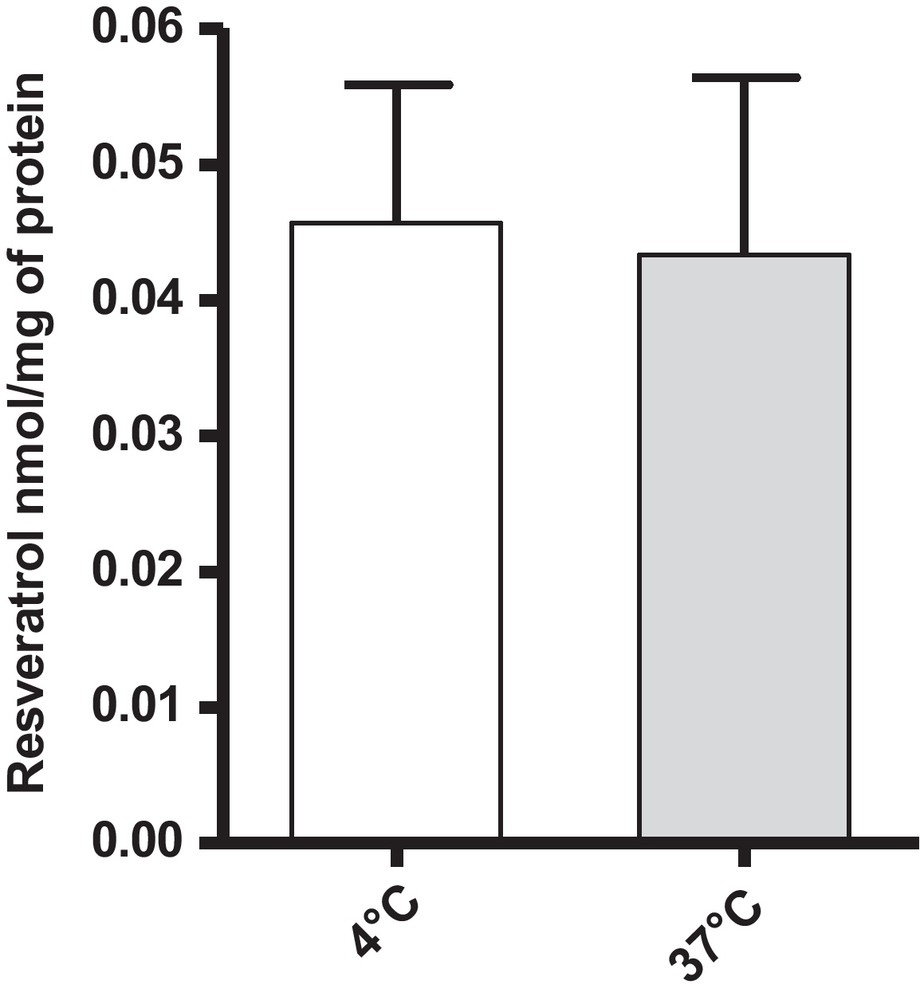
Cellular concentrations of resveratrol in BAEC at 37 °C and 4 °C. Cells were incubated with 15 mL of resveratrol 5 μM for 6 h. Data are means ± SEM.
4 Discussion
The aim of this study was to determine if resveratrol was able to penetrate inside bovine endothelial aortic cells (BAEC) and which kind of transport mechanism was involved.
For each concentration tested (ranging from 1 to 50 μM), resveratrol was found in a dose-dependent manner in cellular lysates, according to the amount of resveratrol added to the culture media. Resveratrol concentrations were lower in membranes than inside the cells. As previously shown for intracellular concentrations of resveratrol, membrane concentrations increased as a function of the amount of resveratrol initially added. However, the presence of resveratrol in cell membranes was in agreement with the fact that resveratrol is a membrane-bound receptor ligand, as shown for estrogen receptor [9].
Lançon et al. [16] have shown that resveratrol at a concentration of 10 μM was completely metabolized by cells from hepatocellular carcinoma (HepG2) into monosulfate and disulfate trans-resveratrol after 8 h incubation. Patel et al. [20] have demonstrated that in colorectal tissues from cancerous patients, sulfated metabolites were found after ingestion of 0.5 or 1 g of resveratrol. However, they have shown that levels of resveratrol and its metabolites were different depending on the localization of the tissue in the colon.
In our experimental model, no sulfated metabolite of resveratrol has been detected in the cytosolic fractions but we could not exclude their presence. The reasons could be that these compounds are present at very low concentrations below the limit of detection or that our HPLC conditions are not optimal to separate them. The second hypothesis is that endothelial cells may not be able to produce this kind of metabolites, due to a lack of enzymes responsible for their production. Currently, no study allowed to define the presence or the absence of these enzymes in endothelial cells.
Resveratrol could thus be able to penetrate inside endothelial cells and to scavenge ROS in the cytoplasm under conditions of oxidative stress, even when cellular enzymatic defenses were not sufficient enough.
Amounts of resveratrol consumed in 24 h for the different concentrations tested appeared to be directly dependent on the concentrations of resveratrol added to the culture media. These results were in favor of a passive diffusion of resveratrol through cellular membranes since no saturation phenomenon was observed for any concentration tested.
Inhibition of the cellular active transport by dropping incubation temperature to 4 °C did not lead to a decrease in cellular concentrations of resveratrol. These results highly suggested that resveratrol was able to penetrate into endothelial cells by a passive diffusion mechanism.
5 Conclusion
As a conclusion, under our experimental conditions, resveratrol was detected both inside endothelial cells and in the membrane fraction. Moreover, resveratrol was able to penetrate inside the cells even when active transport was blocked. As a whole, our results thus suggest that resveratrol penetration occurred by a passive diffusion mechanism.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


