1 Introduction
In non-mammalian vertebrates, vitellogenesis is a key event in reproduction since it represents a main step in oocyte maturation and it is involved in early embryonic development. It consists in the synthesis of vitellogenin, its specific incorporation in primary oocytes in growth, and accumulation of yolk [1–3]. In the liver of all oviparous species, the synthesis of vitellogenin is under control of estrogen [4–14]. Growth hormone plays a direct role in potentiating the induction of hepatic 17β-estradiol [15–17], and its action could be associated with hepatic synthesis of estrogen receptors. More recent data argued the authenticity of this regulation; the growth hormone treatment of hypophysectomized rats increased the rate of estrogen receptor mRNA in hepatocytes [17]. The in vitro study of hepatocytes of immature eel revealed that both growth hormone and prolactin were required for a better 17β-estradiol dependent synthesis of vitellogenin [16,18].
Progesterone exerts multiple effects on different target organs. Indeed, in the lizard Iguana Dipsosaurus dorsalis, the progesterone inhibits the seasonal ovarian growth and vitellogenin synthesis induced by 17β-estradiol [19]. The cellular signaling of estrogen is mediated via two receptor subtypes ERα and ERβ, which may have different biological roles. Ligand binding promotes homo- or hetero-dimerization of receptors that then migrate into the nucleus where they interact specifically with estrogen response elements (ERE) whose DNA sequence is 5′-GGTCA … TGACC-3′, stimulating the transcription of specific genes. The role of these two subtypes of estrogen receptors in the hepatic synthesis of vitellogenin in oviparous vertebrates is still open to question: in the rainbow trout, ERα [20] as well ERβ [21] were reported separately as mediators in this process. In the juvenile goldfish, the treatment with 17β-estradiol increased the mRNA of ERβ [22] while in female wild newts [23] and in males of tilapia [24] ERα could be the first involved in the induction of vitellogenin synthesis.
The investigations on the distribution of estrogen and progesterone receptors in the liver seem to be very rare in reptiles, the only result available on the interrelationship between the presence of the estrogen receptors subtypes and the biosynthesis of the vitellogenin in this class of vertebrates was published by Verderame and Limatola [25]. In this context, we would detect in the lizard Uromastyx acanthinura the localization of these two subtypes and their significance in the synthesis of vitellogenin. U. acanthinura was observed over the whole of the saharan territories. There has been a number of studies that have examined the diet [26,27], its special features of nasal excretion [27,28] and on the reproductive cycle. Adulthood (adult age) was reached from a mouth cloaca length of 18 cm [27]. Courrier [29] and Kehl [30,31] have determined the sexual cycle; it is of the monoestrien vernal type, the mating occurring in May, single ovulation in June and hatch in September. The retention of eggs in the oviducts lasts 15 days and their number varies from 6 to 20 [30,31]. However, Grenot [27] described one to two ovulatory clutches in the cycle of the specie.
In U. acanthinura female, our previous research confirmed the seasonal ovarian activity which running from Spring until Summer and the existence of one ovulatory clutch [1]. Several aspects of endocrine control of vitellogenesis have been observed in female [32–36], and male treated with 17β-estradiol obtained the ability to produce vitellogenin [37]. In order to determine the estrogen receptor subtypes and the progesterone receptor isoforms involved in the regulation of the synthesis of vitellogenin in U. acanthinura, we analyzed the presence of estrogen and progesterone receptors in the liver of wild females and females treated with 17β-estradiol using an immunohistochemical method.
2 Material and methods
2.1 Animals
The animals studied were adult females captured in the region of Beni Abbes, situated at 30°7′ north latitude and 2°10′ west longitude, southwest of the Algerian Sahara in sexual rest period (Winter) and during reproduction (April, May and June) over a year. Two females in early vitellogenesis, five females in full vitellogenesis, one non-vitellogenic female and one female with egg retention were obtained during the breeding season. During the sexual rest period, two groups were formed: one involving two females who underwent hormone treatment and the second group consisting of three untreated females.
2.2 Immunohistochemical analysis
The hormonal treatment was performed by intraperitoneal injection of 17β-estradiol at 10 mg/kg body weight, diluted in 1 ml of olive oil at a rate of six times a week with an interval of two successive injections every 3 days during a period of 29 days and sacrificed on 30th day.
The lizards were sacrificed by decapitation after anaesthesia by intraperitoneal injection of urethane solution at 25% (2 ml/350 g body weight), the liver was rapidly removed and fixed by immersion in Bouin-Holland's fluid for immunohistochemical analysis.
The labeling was applied by the indirect immunoenzymatic technique using an Avidine Biotine Peroxidase detection system by amplification method with the Dako LSAB2 Kit. This technique allowed to investigate the localization of subtypes α (ERα) and β (ERβ) of estrogen receptor and the isoforms B (PRB) of the progesterone receptor and that recognizing both A and B (PRA/B) respectively, using a monoclonal antibody mouse anti-human ERα (DAKO, clones 1D5 and D75), a polyclonal rabbit anti-human ERβ (Santa Cruz Technology, H-150 sc 8974), a monoclonal antibody rabbit anti-human PRB (Cell Signaling Technology, C1A2) and a polyclonal rabbit anti-human PRA/B (Cell Signaling Technology).
Labeling of sections required heat-induced antigen retrieval including treatment of sections with heating at 95 °C for 1 hour in the retrieval solution (1 volume of 10X target retrieval added at 9 volumes of phosphate buffered saline: PBS) or using citrate buffer pH 5.5. It was determined using commercial peptide antibodies supplied by Dako. Hepatic sections were processed through standard protocols of immunohistochemistry. Sections were deparaffinized, rehydrated in graded ethanol and endogenous peroxidase was quenched with 0.3% H2O2 in H2O. Sections were preincubated in 10% normal goat serum in PBS for 1 hour to reduce background staining. The tissue sections were then incubated for 30 mins at room temperature with the primary specific antibody at a dilution of 1:100 in the PBS. Following a rinse two times in a bath of PBS for 5 mins each slide was incubated with pre-diluted secondary antibody (anti-mouse IgG for ERα labeling and anti-rabbit IgG for ERβ, PRA/B and PRB) for 30 mins at room temperature and then rinsed in the same buffer. The slides were incubated for streptavidine for 30 mins at room temperature and rinsed in PBS. The labeling was revealed in brown with 3,3’diaminobenzidine. The nucleus was sometimes counterstained with Mayer's hematoxylin.
A control of specificity consisted in omitting the primary antiserum step to test non-specific binding of secondary antibody to the tissue was performed. The primary antibodies were also tested in the U. acanthinura [35,38] and the rat Psammomys obesus ovaries [39].
3 Results
3.1 At the breeding period
At the beginning of vitellogenesis, ovaries contained small vitellogenic follicles of 7 mm of diameter. No positive staining of ERα was detected in the hepatocytes, neither in the cytoplasm nor at the nuclear level (Fig. 1A). The labeling of the ERβ was positive and the signal predominantly nuclear (Fig. 1B). No labeling was obtained for the PRA/B (Fig. 1C) neither for PRB isoform alone. In full vitellogenesis, the ovaries had large follicles, their diameter reaching 1.5 cm. Since the beginning of vitellogenesis, no labeling of ERα subtype was observed (Fig. 2A). The immunoreactivity of ERβ was still revealed, the signal being localized in both nucleus and cytoplasm around the lipid inclusions (Fig. 2B). No progesterone receptor staining was detected during vitellogenesis (Fig. 2C). In non-vitellogenic female, the labeling of ERα (Fig. 3A), of ERβ (Fig. 3B), of PRA/B (Fig. 3C) and of PRB alone (Fig. 3D) was completely repressed.
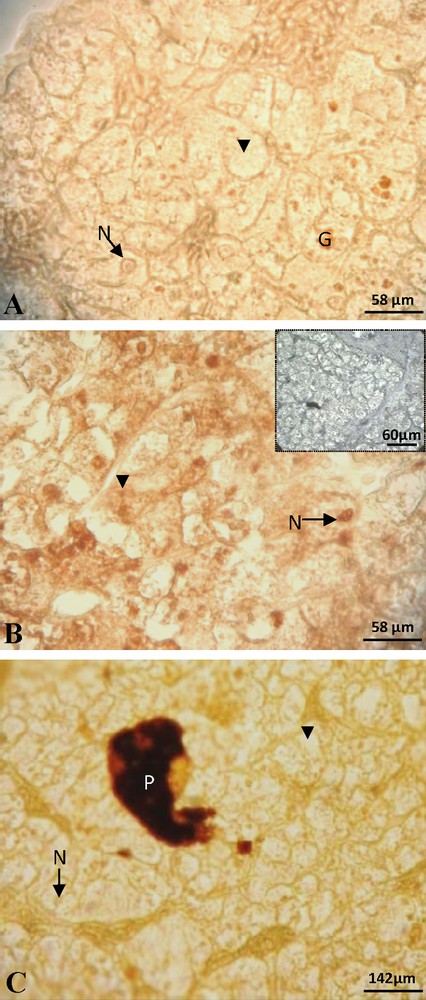
Female at the beginning of vitellogenesis: the staining of ERα was not visible, neither in the nucleus (N) nor in the cytoplasm (arrow head) (A). Positive labeling of ERβ was observed in numerous nuclei and some cytoplasms (arrow head) and the control was negative (insert) (B). No staining of PRA/B in the nucleus and the cytoplasm (C). G: granulation; P: pigment.
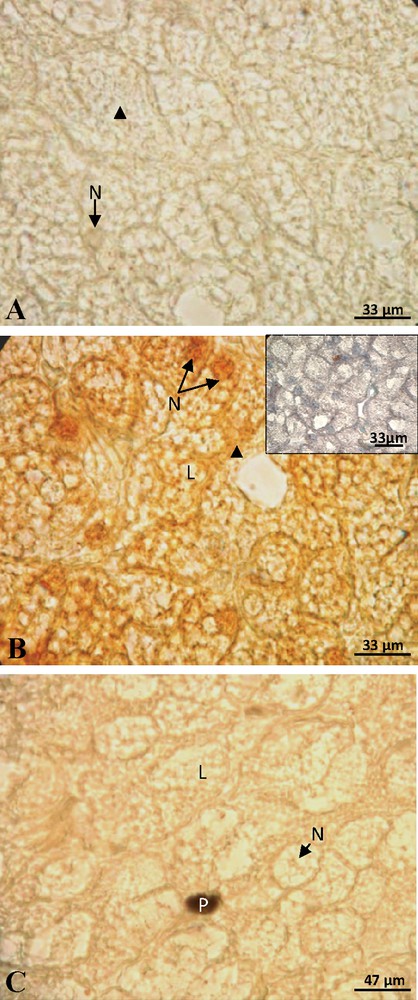
Female during vitellogenesis: no labeling of ERα was observed in the nucleus (N) and cytoplasm (arrow head) (A). The labeling of ERβ was visible in the nucleus and cytoplasm around the lipid inclusions (L) and the control was negative (insert) (B). The staining of PRA/B was absent (C).
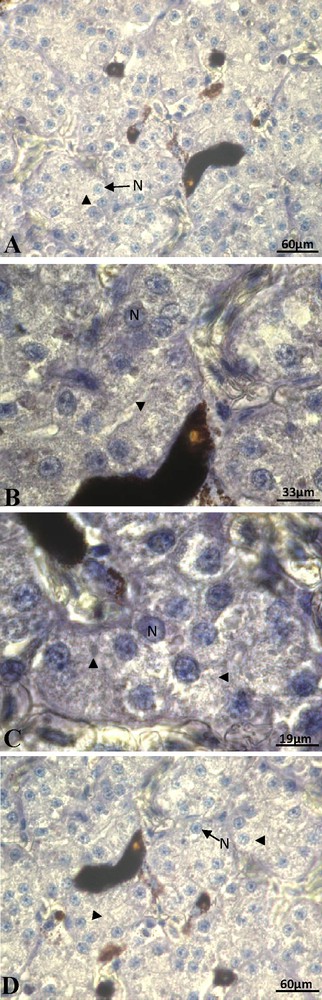
Non-vitellogenic female captured in active period: the immunoreactivity of ERα (A), of ERβ (B), of PRA/B (C) and of PRB alone (D) was absent. N: nucleus; arrow head: cytoplasm.
In the female with eggs retention, presenting numerous corpora lutea in the ovaries and eggs of 2.5 cm in the oviducts, the labeling of ERα subtype was still absent (Fig. 4A). A positive staining of the ERβ subtype was detected in cytoplasm and nucleus with a predominantly cytosolic signal and the control was negative (Fig. 4B). This signal was most intense and more widespread in most hepatocytes of this female compared to vitellogenic ones. In this female, a positive staining of PRA/B appeared in some hepatocytes. The extremely weak signal was exclusively localized in the cytoplasm and the control was totally negative (Fig. 4C). The labeling of PRB alone was absent (Fig. 4D), which means that the discrete cytoplasmic signal obtained with anti-PRA/B corresponded to the PRA.
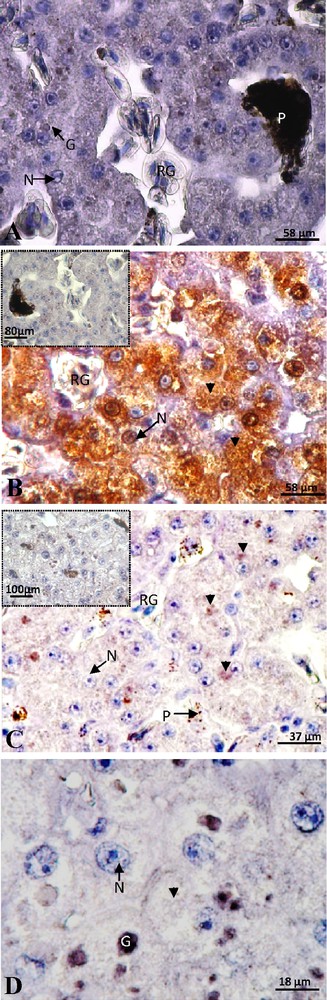
Female with egg retention: negative labeling of ERα (A). Intense labeling of ERβ was detected in the nucleus (N) and mainly in the cytoplasm (arrow head) and the control was totally negative (insert) (B). A low labeling of PRA/B was observed in the cytoplasm and the signal disappears in the control (insert) (C). The labeling of PRB alone was absent (D). G: granulation; P: pigment; RG: red globule.
3.2 At sexual resting period
In these females, the hepatocytes did not reveal ERα. An expression of ERβ was observed in hepatocytes with a signal that became strictly cytosolic (Fig. 5A). In hepatocytes, the presence of progesterone receptor became very intense at this phase of sexual quiescence. This positive labeling with anti-PRA/B was both cytosolic and nuclear (Fig. 5B). The staining of the PRB isoform alone was also positive; it was localized on nuclear level and in the cytoplasm of several hepatocytes (Fig. 5C). However, the intensity of the cytoplasmic signal was clearly reduced compared with the immunostaining obtained with the antibody recognizing both A and B, suggesting the simultaneous presence of PRA and PRB and the cytoplasmic signal correspond more at PRA.
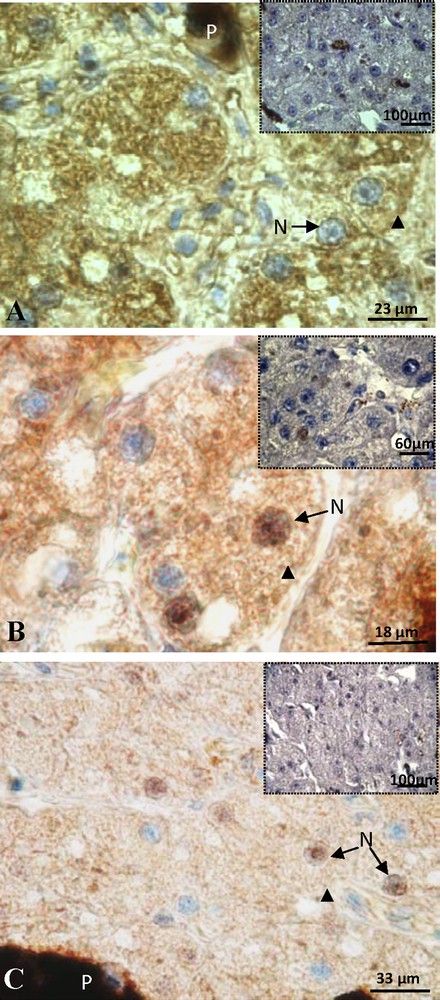
Sexual rest female: the staining of ERβ was detected exclusively in the cytoplasm (arrow head) and a negative control was visible in insert (A). Positive labeling of PRA/B was detected in the nucleus (N) and cytoplasm (arrow head) of hepatocytes and the signal disappears in the control (insert) (B). Labeling of PRB showing a reduced cytoplasmic signal (arrow head) and the control was negative (insert) (C). P: pigment.
In the rest females treated with 17β-estradiol, ERα subtype was still not present. A positive staining of the ERβ subtype was visible in both cytosol and nucleus of hepatocytes (Fig. 6A and B). The immunolocalization of progesterone receptors observed in the rest females disappeared completely after 17β-estradiol treatment at either nuclear or cytoplasmic level (Fig. 6C).
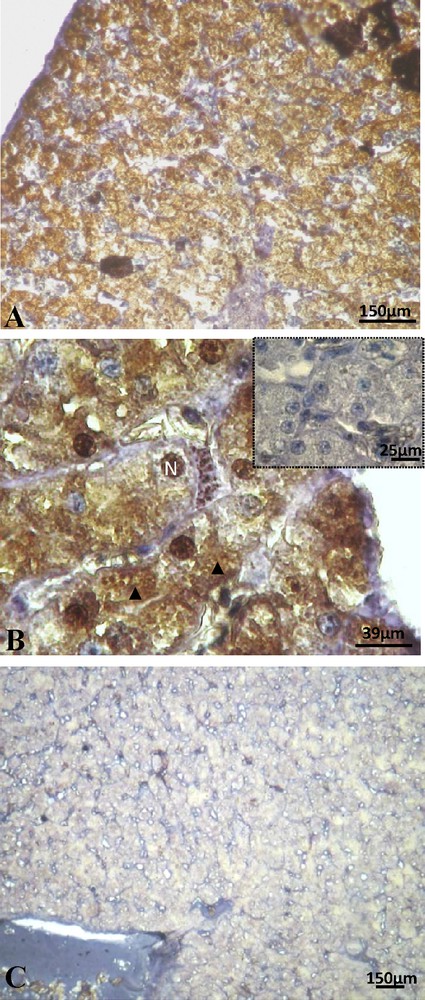
Sexual rest female after 17β-estradiol treatment: positive staining of ERβ in the hepatocytes (A), the signal was detected in the nucleus (N) and cytoplasm (arrow head) and the control was negative (insert) (B). Here we illustrate the absence of labeling of PRA/B (C).
In summarizing the results obtained (Table 1), it appeared that in U. acanthinura, only ERβ has been highlighted among the two estrogen receptor subtypes analyzed. Its variable cellular distribution according to the phases of the reproductive cycle (cytosolic and nuclear in vitellogenesis and after hormonal treatment, predominantly cytosolic in luteal phase and exclusively cytosolic in sexual rest) would indicate a positive correlation between the nuclear signal and the presence of 17β-estradiol. The progesterone receptors visualized by the respective presence of PRA and PRA/B, observed only during luteal phase and sexual rest would demonstrate the negative effect of progesterone on the hepatic synthesis of vitellogenin. Indeed, estrogen treatment induced their repression.
Summary hepatic labeling obtained for estrogen and progesterone receptors in Uromastyx acanthinura.
| Spring: breeding period | Winter: sexual rest period | |||
| Non-vitellogenic female | Vitellogenic females | Female at eggs retention | Non-reproductive females | Treated females |
| ERα absent | ||||
| ERβ absent | ERβ nucleus + cytosol | ERβ predominantly cytosolic | ERβ cytosol | ERβ nucleus + cytosol |
| PRA/B cytosol PRB absent |
PRA/B cytosol + nucleus PRB nucleus + some cytosols with a signal more reduced then with PRA/B |
|||
| PR absent | PR absent | Conclusion Presence of PRA cytosolic |
Conclusion Presence of PRA and PRB Nuclear fraction correspond more at PRB Cytosolic staining correspond more at PRA |
PR absent |
4 Discussion
The study of the hormonal regulation of vitellogenesis in U. acanthinura indicates the involvement of both steroid hormones in the hepatic metabolism. Indeed, the vitellogenic females and the rest females treated with 17β-estradiol showed a differential labeling of receptors ERα, ERβ, PRA/B, PRB compared to those studied in the sexual rest period. The physiological trend of 17β-estradiol during the reproductive cycle of this lizard was previously analyzed by immunohistochemistry and indicates that aromatase and 17β-estradiol were strongly present in the ovary during vitellogenesis, their signal considerably decreased after oviposition in summer and became totally absent in the rest period [35,36,38]. The action of 17β-estradiol on the induction of vitellogenesis in U. acanthinura has been demonstrated, the ovarian follicles acquire a yellowish and liver synthesizes two polypeptides of 79 kDa and 104 kDa corresponding of vitellogenin [37]. The vitellogenin synthesis appeared to be mediated via ERβ since only this subtype of the estrogen receptor has been demonstrated. It is present throughout the reproductive cycle with a nuclear and cytoplasmic distribution in the vitellogenic and treated females.
The contribution of each subtype of estrogen receptor in regulating the production of vitellogenin is up for debate. The cloning and the characterization at the molecular scale of ERα and ERβ has been made in the lizard Podarcis sicula and their differential presence in the liver during the reproductive cycle rather revealed the involvement of ERα in hepatic synthesis [25]. According to the latter, ERβ present throughout the cycle might modulate the vitellogenic response permitted by ERα detected only in vitellogenesis. Also, in the adult Iberian ribbed newt, the ERα transcripts seemed to be involved in vitellogenesis [23]. Our results are consistent with the study of Leonos-Castaneda and Van Der Kraak [21] which suggested the involvement of the ERβ subtype in the synthesis of vitellogenin in rainbow trout and with that of Soverchia et al. [22] which indicated that the exposure to 17β-estradiol modulated the amount of vitellogenin by increasing the transcription rate of hepatic ERβ-1 in the juvenile goldfish. In U. acanthinura, ERβ, would it be only involved as it is detected in the nuclear and cytosolic form during vitellogenesis and after treatment with 17β-estradiol or would it have a role in modulating the vitellogenic response initiated by ERα? The lack of ERα in U. acanthinura, should it be the case, remains unexplained.
The experimental studies revealed that 17β-estradiol was one of the most important inducers of the vitellogenin synthesis. Indeed, the synthesis of this precursor of yolk after treatment with 17β-estradiol has been demonstrated in vivo and in vitro in different species of vertebrates [15,40–42]. The 17β-estradiol induced mRNA translation of vitellogenin under the control of the ERE in the liver in both males and females of Xenopus laevis [43].
Concerning the expression of the estrogen receptors, if we refer to data of Guiochon-Mantel and Milgrom [44] which suggested a predominantly nuclear localization in the absence of the hormone and those of Kawashima et al. [45] which showed an increase in cytoplasmic receptors to the detriment of nuclear ones after a treatment with 17β-estradiol, thus the cytoplasmic receptor labeling in U. acanthinura rather justifies the absence of 17β-estradiol and would reveal a nonfunctional action. Indeed, during vitellogenesis, the distribution was both nuclear and cytoplasmic, the nuclear signal decreased during the luteal phase to disappear completely during the sexual rest. This nuclear signal was restored after 17β-estradiol treatment.
It is important to recall the absence of labeling against all the antibodies tested in the non-vitellogenic female, which remains incomprehensible. This female presents a mouth cloaca length of 19 cm, therefore, at a sexual maturity age, especially as other females during vitellogenesis analyzed in our study were shorter, measuring 18.5 cms. In the ovaries, only previtellogenic follicles exist. Would it have been a female between two ovulatory clutches, ovarian would have been follicles in the transition stage.
Numerous studies also indicate the involvement of the progesterone in regulating the synthesis of vitellogenin in the non-mammalian vertebrates. In lizards, the progesterone level during the follicular phase increases during vitellogenesis [46,47] and peaks at preovulatory stage [46]. Corpora lutea formed during the luteal phase, morphologically identical to those of mammals [48], induce the elevation of progesterone [49]. Progesterone allows, in oviparous species, the formation and retention of eggs and the inhibition of hepatic synthesis of vitellogenin [50]. These two functions require a physiological regulation of progesterone receptor in target tissues, namely, the reproductive tract and liver. Progesterone exerts an inhibitor effect on follicular growth in lizards P. sicula, [51] and Sceloporus cyanogenys [52,53] by preventing the release of gonadotropins by negative feedback.
In U. acanthinura, the physiological trend of progesterone during the reproductive cycle has been estimated by immunohistochemestry. The progesterone was partially present in the granulosa of vitellogenic follicles during vitellogenesis, more detected in the piriform cell of previtellogenic follicles and corpora lutea after oviposition [35,38] and absent in the sexual rest. In this specie, we obtained an early presence of PRA-like receptor in luteal phase, which became more intense during the sexual rest with the appearance of the PRB. This labeling was undetectable in vitellogenic females and in rest females treated with 17β-estradiol as it was demonstrated in the lizard P. sicula in which the immunoreactivity of progesterone receptor was visualized in liver only during the sexual rest phase [54]. These results obtained in Uromastyx indicated that the detection of these receptors was submitted to a hormonal control during the reproductive cycle and suggested a negative regulation of the gene expression of PRA and PRB by 17β-estradiol like it has been demonstrated in other species of reptiles [55] and amphibian [56]. Mosconi et al. [47] also confirmed the inhibitory action of progesterone on gene expression of vitellogenin estrogen-dependent. It acted via the two PRA and PRB isoforms by regulating different target genes. The presence of these two isoforms in the liver was differential during the seasonal cycle in the Turtle Chrysemys picta, the mRNA transcripts encoding both PRA and PRB was only found to vary significantly during the annual cycle and their rate was inversely proportional to the rate of vitellogenin mRNA [57]. Other studies found that the PRB isoform detected only in the luteal phase and in hibernation would be responsible for the inhibition of vitellogenesis [58,59]. The PRA isoform present throughout the seasonal cycle became dominant during the follicular phase and acted synergistically with 17β-estradiol in the vitellogenic process [58]. In the most recent study, the results showed that 17β-estradiol treatment enhanced the transcription of PRB mRNA whereas it made PRA proteins levels decrease [60]. These results were not similar to those obtained in U. acanthinura where we noted a complete alteration of the labeling of both progesterone receptor isoforms during vitellogenesis and suggested the involvement of both PRA and PRB in inhibiting this process at sexual rest and that of PRA during the luteal phase.
5 Conclusion
Our results suggest that the localization of ERβ in the liver of the lizard U. acanthinura was both nuclear and cytosolic in physiological and experimental conditions allowing the synthesis of vitellogenin and was repressed in the nucleus of all hepatocytes when this vitellogenic synthesis is inhibited. PRA and PRB were both involved in this inhibition. Further studies could clarify the role of ERβ and its interrelationship with ERα, if any, in regulating the vitellogenin synthesis.
Disclosure of interest
The authors declare they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to the Doctor F. Yesli of the C.P.M.C. of Mustapha Hospital and to A. Boubekri, member of team 3 L.R.Z.A. for providing some products, essentially the antibodies.


