1 Introduction
Multipotent stem cells capable of restoring damaged functions of altered organs is the Holy Grail of tissue and organ engineering in mammals. Early investigations had shown that differentiation gene products are present in tissues other than their main site of expression. Indeed, we have reported that bone marrow stem cells, as well as differentiated lineages to which they give rise, express the myelin basic protein gene, which encodes for a brain structural protein [1–6]. In parallel, it has been claimed that cells derived from bone marrow can give rise to various cell types including neural cells, pancreatic cells or cardiac muscle cells [7–11]. Taken together, these data suggested that bone marrow cells, or a subset of them, might be multipotent.
Another line of research has been to engineer pluripotent cell lines: as a first step, embryonic stem cells (ESCs) were derived from the inner cell mass of early embryo blastocysts, cells that are able to form each of the three embryonic germ layers: ectoderm, mesoderm and endoderm [12,13]. However, because of practical issues ESCs are problematic for human cell replacement therapy. Then an important step was the creation of a mouse clone from olfactory neurons showing that a differentiated cell could be reprogrammed back to an embryonic stem cell-like stage [14]. The more recent breakthrough has been the induction of pluripotent stem cells (iPSCs) by the genetic manipulation of normal adult somatic cells such as fibroblasts [15,16]. However, induction of pluripotency may evoke genetic changes leading eventually to tumorigenicity. This led us to investigate the existence of natural adult stem cells whose multipotency could lead to or take part in the generation of a new organ(s).
Indeed, we had previously reported that a sub-population of mouse bone marrow hematopoietic progenitors, with the CD34+ antigen, naturally express an array of ESC genes including Oct-4, Rex-1, Sox-2, Klf-4 and LIN-28, which that have been formerly used to induce iPSCs. We had also found that this same subset of adult stem cells expresses ectodermal neural lineage genes, mesodermal cardiac lineage genes as well as endodermal pancreatic and intestinal genes (Fig. 1 and [17]). Taken together, the fact that these bone marrow cells express both embryonic stem cell genes and lineage genes for each of the three embryonic germ layers suggests that they may be multipotent. Here we illustrate that when these adult bone marrow CD34+ cells are transplanted into mouse blastocysts, they give rise to organs of adult mice such as ectodermal derived brains, mesodermal cardiac cells, bone and bone marrow cells and endodermal pancreas and intestinal cells, indicating the multipotency of these CD34+ bone marrow cells.
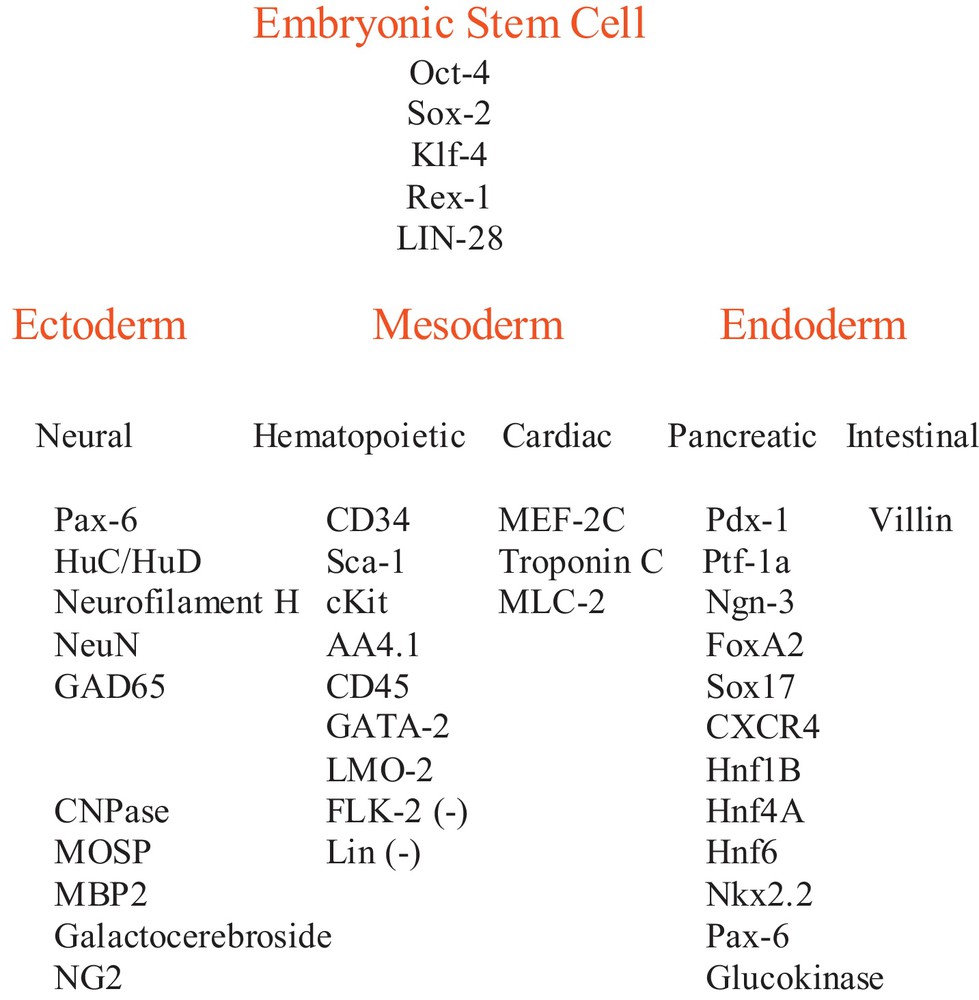
Adult mouse CD34+ bone marrow stem cell gene expression. Embryonic stem cell gene and embryonic germ layer lineage gene expression in adult CD34+ cells were reported previously [17].
2 Materials and methods
2.1 Bone marrow CD34+ stem cell cultures
Non-adherent CD34+ hematopoietic stem cells were cultured from adult mouse bone marrow with medium containing mouse IL-3, IL-6, SCF and β-mercaptoethanol as reported [6]. CD34+ cultures from two strains of mice were used: the wildtype C57Bl/6 J mouse and the C57Bl/6J transgenic mouse that expresses β-galactosidase, B6.129S7-Gt(ROSA)26Sor/J. The University of Maryland School of Medicine OAWA approved these animal studies.
2.2 Generation of chimeric mice
Adult CD34+ cell chimeric mice were generated by the University of Maryland School of Medicine Transgenic Animal Core Facility. Briefly, 8 to 15 adult CD34+ stem cells from a male C57Bl/6J transgenic mouse that expresses β-galactosidase, B6.129S7-Gt(ROSA)26Sor/J, were injected into each wildtype C57Bl/6 J blastocyst at E 3.5, the 64-cell stage. Eighteen to twenty CD34+ injected blastocysts were delivered to the uterus of each hormonally induced pseudo-pregnant wildtype C57Bl/6J surrogate mother mouse. Offspring were grown to weaning.
2.3 X-gal staining
Chimeric animals were killed and perfused with PBS followed by 2% paraformaldehyde plus 0.2% glutaraldehyde. Perfused tissues were immersed in the same fixative at 4 °C for 72 hours. Fixed tissues and organs were exchanged with PBS then 30% sucrose, frozen, and sectioned. X-gal staining was performed with an in situ β-galactosidase Staining Kit (Stratagene Cat. # 200384) following the manufacturer's protocol at pH 7.6, 37 °C for 10 to 12 hours. The same X-gal staining protocol was used for ROSA CD34+ chimera, ROSA transgenic and wildtype CD34+ chimera organs.
2.4 Immuno-cytochemistry
Cells were treated by standard immunochemistry methods and observed by fluorescence microscopy [6].
2.5 X,Y chromosome Fluorescence In Situ Hybridization (FISH)
XY-FISH was performed by Empire Genomics, Albany, NY using green 5-Fluroescein dUTP mouse X chromosome probe (Cat. # CLN-10048) and red 5-ROX dUTP mouse Y chromosome probe (Cat. # CLN-100920).
3 Results
3.1 Generation of chimeric mice
Expression of both embryonic stem cell genes, and lineage genes for each of the three embryonic stem cell layers, by a subset of adult CD34+ bone marrow cells suggested cellular multipotency. To ascertain their potency, they were transplanted into mouse blastocysts. Adult CD34+ stem cells from male C57Bl/2 J transgenic mice that expressed β-galactosidase, B6.129S7-Gt(ROSA)26Sor/J were cultured as previously reported [6]. When these ROSA CD34+ cells were treated with X-gal they stained blue, whereas wildtype CD34+ cells do not stain with X-gal (Fig. 2). These male ROSA CD34+ cells were transplanted into wildtype C57Bl/6 J blastocysts that do not express β-galactosidase. As a control, wildtype CD34+ cells that do not have the β-galactosidase transgene were transplanted into separate wildtype mouse blastocysts. Eight to fifteen adult ROSA or wildtype CD34+ cells were transplanted into each E 3.5 stage blastocyst. At E 3.5, each blastocyst is composed of 64 cells including both the trophoblasts that make up a single cell layer thick wall of the blastocyst that go on to become all of the extra-embryonic structures, e.g., placenta, umbilical cord, amnion and chorion, as well as the inner cell mass of embryonic stem cells that forms the embryo itself. After transplantation of the ROSA CD34+ cells, staining of the chimeric blastocyst with X-gal revealed the blue-stained ROSA CD34+ cells within the blastocoel of the unstained wildtype blastocysts (Fig. 2). Seventy-seven ROSA chimeric blastocysts then were implanted into four pseudo-pregnant mice that yielded six pups. The chimeric embryos were allowed to develop normally through birth to weaning. All chimeric mice were robust and behaved normally.
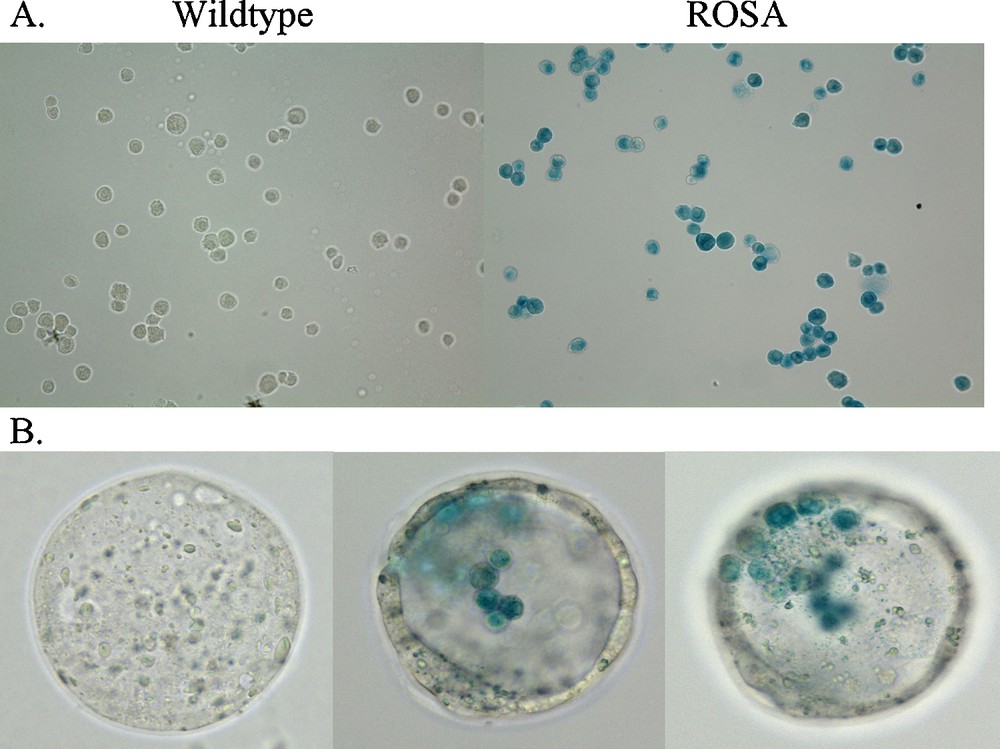
X-gal labeling of ROSA CD34+ cells in culture (A) and after injection into wildtype blastocysts (B). Adult wildtype CD34+ cells do not stain with X-gal whereas, adult transgenic ROSA CD34+ cells, that carry the β-galactosidase gene, do stain with X-gal (blue). CD34+ cells were fixed with 2% paraformaldehyde plus 0.2% glutaraldehyde for 15 min. at 4 °C then washed with PBS. X-gal staining was performed with a Stratagene In situ β-galactosidase Staining Kit (Cat. # 200384) by the manufacturer's protocol. Cells were stained for 12 hr. Similarly, sixty-four cell stage chimeric E 3.5 mouse wildtype blastocysts that contained injected adult ROSA mouse CD34+ cells were fixed and stained as above. The X-gal stained CD34+ cells were observed in different focal planes of the blastocyst.
3.2 X-gal staining of chimeric mouse organs
To determine the potency of the adult hematopoietic progenitor cells to form multiple differentiated cell types in tissues and organs from each of the embryonic germ cell layers, sections from fourteen tissues and organs from six ROSA CD34+ chimeric mice and from four wildtype CD34+ control chimeric mice were stained with X-gal to determine if the cells of any organs of the chimeric mice were derived from the adult ROSA CD34+ cells that were transplanted into the wildtype blastocysts. The adult ROSA CD34+ cells gave rise to tissues and organs of each of the three embryonic germ cell layers, e.g., ectodermal brain, dorsal root ganglia and skin, mesodermal femur bone and bone marrow and endodermal pancreas, liver, intestine and bladder (Fig. 3). We found organs containing X-gal (+) cells derived from CD34+ cells in both male and female chimeric mice. The pattern of tissues and organs derived from the adult ROSA CD34+ cells was different in each chimeric individual. (Fig. 3)
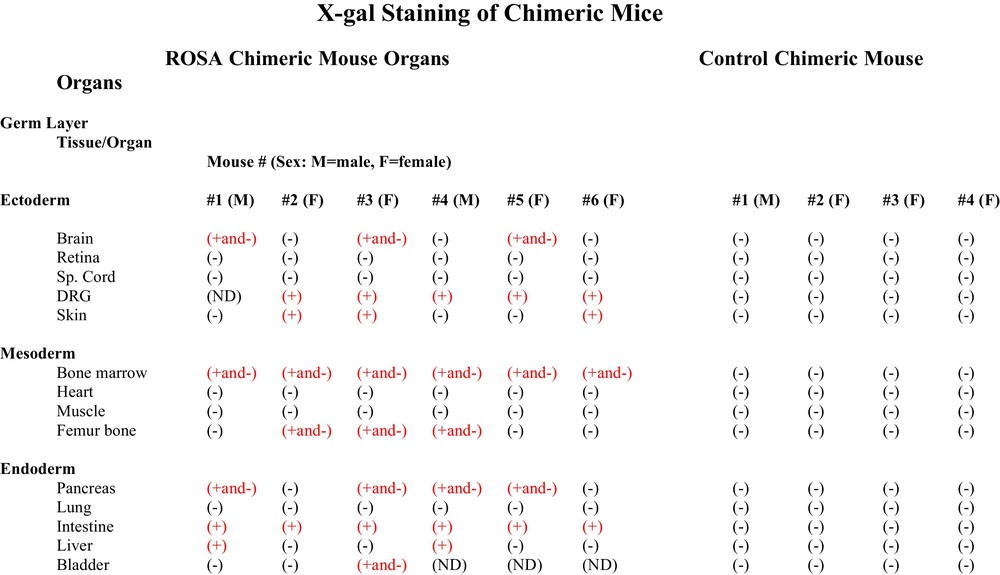
Table of organs of ROSA CD34+ chimeras and control wildtype chimeras stained with X-gal. Chimeric animals were killed and perfused with PBS followed by 2% paraformaldehyde plus 0.2% glutaraldehyde. Perfused tissues were immersed in the same fixative at 4 °C for 72 h. Fixed tissues and organs were exchanged with PBS then 30% sucrose, frozen and sectioned. X-gal staining was performed with an in situ β-galactosidase Staining Kit (Stratagene Cat. # 200384) by the manufacturer's protocol. X-gal staining was found in some organs and not others. In some organs some cell types were stained and others were not. Different ROSA chimeras had different X-gal staining patterns. Control wildtype CD34+ chimeras stained under the same conditions as ROSA CD34+ chimeras showed no X-gal staining.
3.3 Ectodermal neural cells were derived from adult hematopoietic progenitors
Three of six ROSA chimeric mice brains had neurons that stained positively for X-gal (Fig. 3). Some, but not all, neural cell types stained with X-gal. For example, hippocampal pyramidal neurons of areas CA 1-3 stained positively, as did locus ceruleus neurons and Purkinje neurons of the cerebellum, while granule neurons did not stain (Fig. 4). The cerebellar cells that stained with X-gal also stained for Purkinje calbindin but not Bergmann glial GFAP. Many but not all neural cell types stain throughout the brain (not shown). Because some neural cells in the ROSA chimeras stained with X-gal and others did not, we looked at the X-gal staining pattern in the brain of the transgenic ROSA mouse itself – the mouse from which the ROSA CD34+ cells were derived for transplantation into wildtype blastocysts. To our surprise, even though all cells of the transgenic ROSA mouse contain the β-galactosidase gene, all cells do not stain for X-gal, for example in brain astroglia, oligodendroglia and many neuronal types do not stain with X-gal. We found indistinguishable X-gal staining patterns in the ROSA chimeric mice and the ROSA transgenic mice – some cells stain but others do not. Control chimeric mouse brain derived from blastocysts injected with wildtype CD34+ cells showed no X-gal staining under parallel staining conditions used for ROSA chimera and ROSA transgenic brain (Figs. 3, 4). In the peripheral nervous system, which is also derived from ectoderm, the neural crest, dorsal root ganglion neurons stained with X-gal. Control chimeric dorsal root ganglia did not stain with X-gal. Neither ROSA chimeric nor control chimeric spinal cord stained with X-gal.
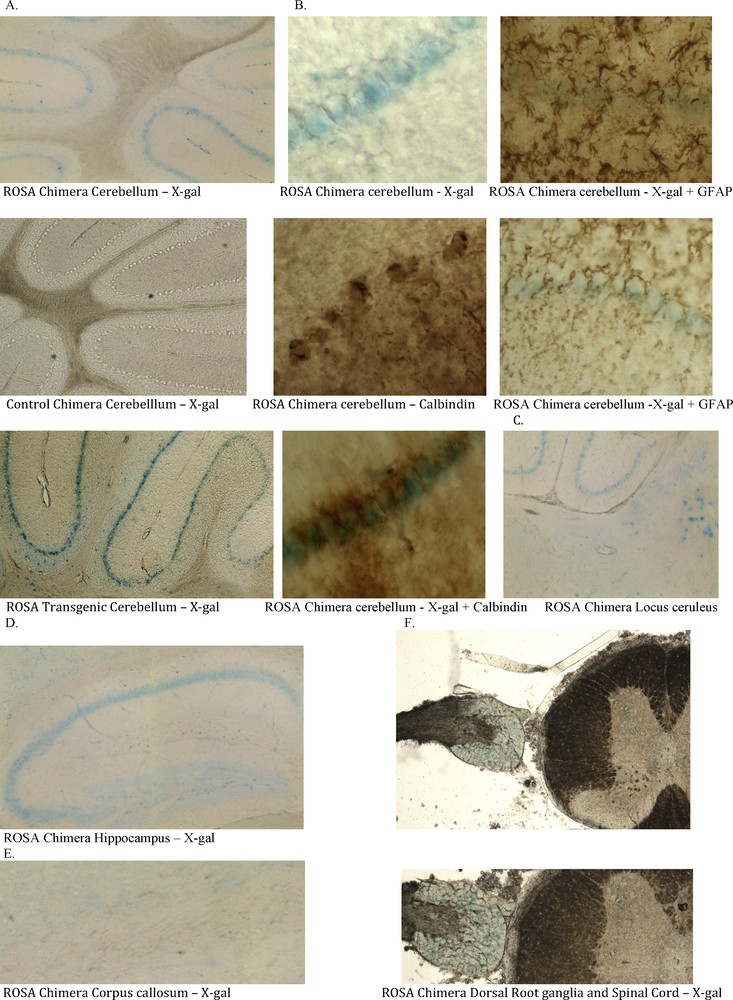
Ectodermal tissues in ROSA chimeric mice are derived from adult ROSA CD34+ stem cells. ROSA chimeric cerebellum exhibits X-gal stained cells (blue) that originate from the adult ROSA CD34+ cells that were injected into the early wildtype blastocyst. Chimeric animals were processed as in Fig. 3. A. Cells in the Purkinje cell layer of some ROSA chimera brains stained with X-gal (blue). Control wildtype CD34+ chimeras did not stain. ROSA transgenic cerebellum showed the same X-gal staining pattern as did the ROSA chimeras. Some cell types stain with X-gal whereas others, e.g. astroglia, oligodendroglia and granular neurons do not. B. The X-gal positive cells in cerebellum co-stain for calbindin (brown stain). Both the cell bodies and the dendritic arborizations (lower right) stained for calbindin. Astroglia and Bergmann glia stained for GFAP but the stain did not co-localize with X-gal. Thus, the X-gal stained cells in cerebellum are Purkinje cells. C. Locus ceruleus and D. hippocampal CA 1-3 neurons also stain with X-gal. E. No cells in Corpus callosum were stained with X-gal. F. Peripheral nervous system neurons of the dorsal root ganglion, derived from neural crest cells stained with X-gal. These blue cells in central and peripheral nervous system indicate that they were derived from the ROSA CD34+ cells originally transplanted into the early blastocysts.
3.4 Mesodermal bone and bone marrow cells were derived from adult hematopoietic progenitors
A band of bone marrow cells at the interface with femur bone stained with X-gal, whereas cells in more central marrow did not stain in all six ROSA chimeric mice. Three of six ROSA chimeras elicited X-gal staining in femur bone (Fig. 3); femur cartilage cells stained with X-gal, as did cells in the trabeculae of spongiform areas of femur (Fig. 5). Some cells in ROSA chimera bone stained with X-gal and others did not. Similarly, some cells of ROSA transgenic bone stained with X-gal and others did not. The femurs and bone marrows of control chimeric mice were unstained with X-gal when stained under the identical X-gal staining protocol used for ROSA chimeras (Fig. 3). None of the hearts from ROSA chimeric mice had X-gal (+) cells that were detected. Similarly, transgenic ROSA hearts were X-gal (-).
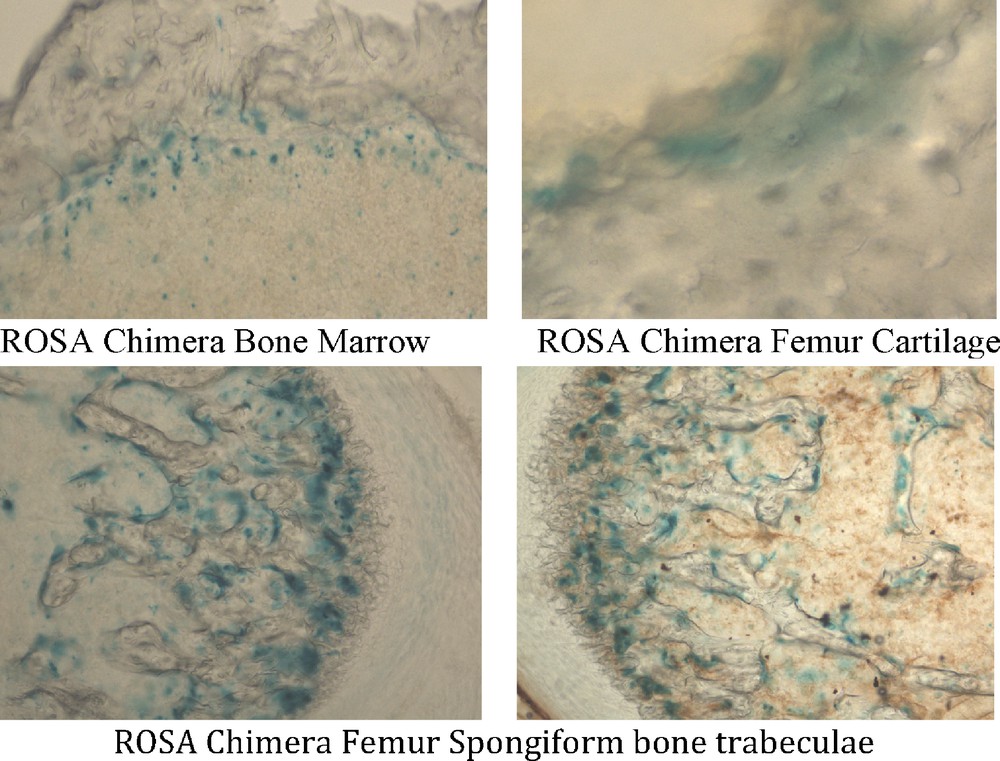
Mesodermal tissues in ROSA chimeric mice are derived from adult ROSA CD34+ stem cells. Tissues were processed as in Fig. 3. ROSA chimeric mouse femur bone and bone marrow were derived from ROSA CD34+ stem cells. Bone marrow cells in a band adjacent to the femur bone itself stained with X-gal (blue). Femur cartilage and spongiform bone trabeculae stained with X-gal. Thus, mesodermal derivatives formed from adult ROSA CD34+ cells.
3.5 Endodermal intestine, pancreas and liver cells were derived from adult hematopoietic progenitors
The intestine of all six ROSA chimeras stained with X-gal apparently in their entirety. (Figs. 3, 6). Four of six ROSA chimeric mouse pancreases stained with X-gal (Figs. 3, 6). And two of six ROSA chimeras showed X-gal staining of liver (Figs. 3, 6). The X-gal staining pattern in ROSA transgenic mouse intestine, pancreas and liver was similar to ROSA chimeras. Control chimera intestine, pancreas and liver did not stain with X-gal under the same staining conditions used for the ROSA chimeras (Fig. 3).
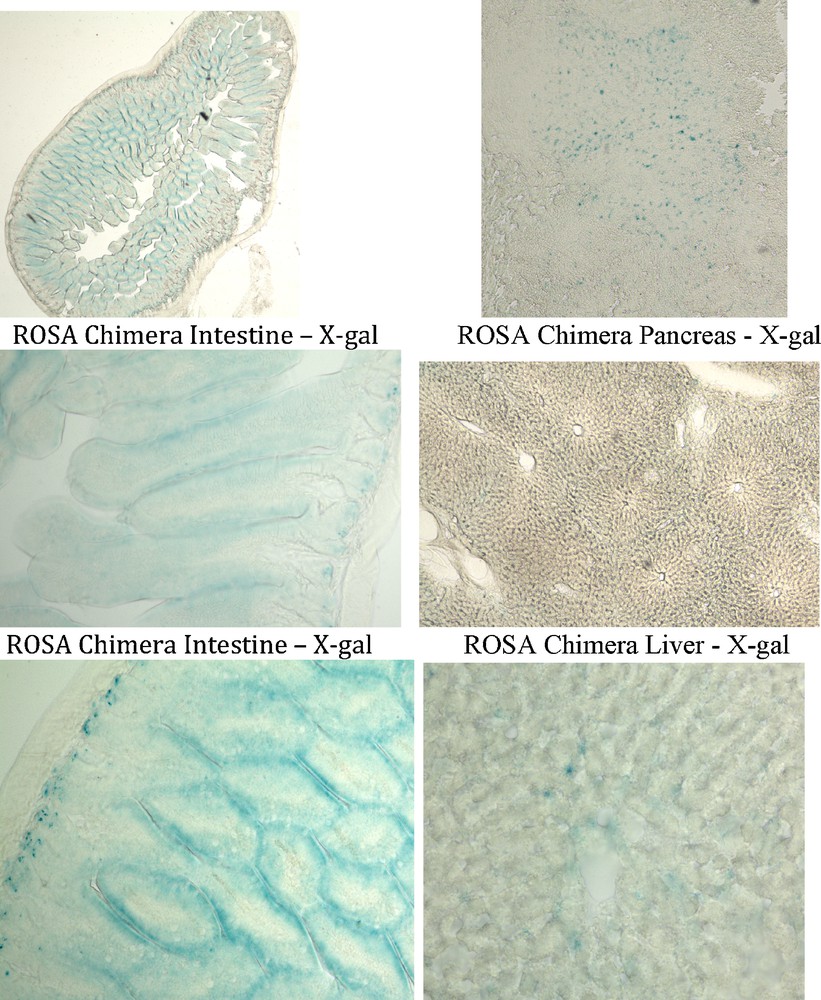
Endodermal tissues in ROSA chimeric mice are derived from adult ROSA CD34+ stem cells. Tissues were processed as in Fig. 3. ROSA chimera intestine, pancreas and liver cells were stained with X-gal (blue) indicating that these endodermal tissues were derived from the adult ROSA CD34+ cells transplanted into the wildtype blastocysts.
3.6 X,Y Chromosome Fluorescence In Situ Hybridization (FISH)
Both ROSA CD34+ chimeric mice and transgenic ROSA mice had organs with the same pattern of X-gal (+) and X-gal (–) cells. Thus, the question arose whether the X-gal (–) cells in the ROSA chimeric mice were derived from the inner cell mass of the blastocyst or whether they derive from the male ROSA CD34+ cells that give rise to cell types that are under regulatory control like those X-gal (–) cells in the ROSA transgenic mice. The Y chromosome in male CD34+ cells was used to identify progeny of those CD34+ cells in female chimeric mice. We used XY-FISH to determine whether X-gal (–) cells in chimeric mouse organs were derived from male ROSA CD34+ cells in the blastocysts. Organs from anatomically female chimeric mice were examined for X,Y and X,X chromosome ratios. Images of X,Y chromosome staining are shown in Fig. 7A. X,Y chromosome counts were done on random fields of brain, heart, pancreas and liver (Fig. 7B). Brains of female chimeric mice #3 and #5, that have X-gal(+ and -) cells, had X:Y ratios of 1:1 in all cells examined, meaning male brains. Also, chimeric brain #1,which contained both X-gal(+ and –) cells in a male mouse, had an X:Y ratio of 1:1, indicating a male brain. Therefore, the absence of X-gal labeling of brain cells does not preclude that they were derived from male CD34 + cells. In contrast, female brain #6, that had no X-gal + cells, also had no Y chromosomes detected, indicating a female brain. Futhermore, regions of mouse brain like the corpus callosum, which is devoid of neurons but contains glial cells that had no X-gal (+) cells, had an X:Y ratio of 1:1 showing that they are male. Thus, in glial cells also, the absence of X-gal does not preclude that the X-gal (–) cells were derived from the male ROSA CD34+ cells. As reported above, none of the hearts from chimeric mice had X-gal (+) cells that were detected. Similarly, transgenic ROSA hearts were X-gal (–). Female chimeric mouse #3 heart had a X:Y ratio of 1:1, indicating that it was a male heart derived from the male ROSA CD34+ bone marrow stem cells. In contrast, female chimeric mouse #5 had no detectable Y chromosomes in heart meaning a female heart. Pancreas from both female chimeric mice # 3 and 5, with X-gal (+ and –) cells, had a X:Y ratio of 1:1, i.e., that of male pancreas from male CD34+ cells. Chimeric mouse #3 liver, that did not exhibit X-gal (+) cells, also had no Y chromosomes. Therefore, the Y chromosome marker showed that chimeric mouse organs that had X-gal (+ and -) cells, and in the case of heart, which had no X-gal (+) cells, were wholly derived from male ROSA CD34+ cells.
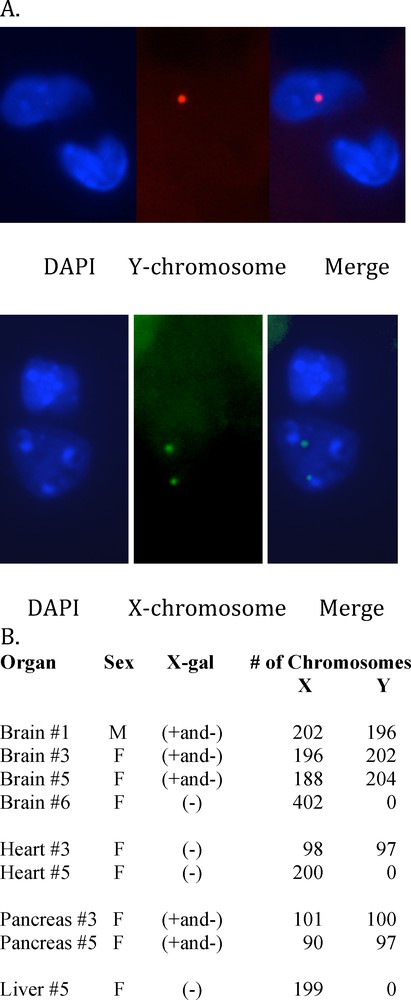
XY chromosome Fluorescence In Situ Hybridization was performed by Empire Genomics, Albany, NY, using green 5-Fluroescein dUTP mouse X chromosome probe (Cat. # CLN-10048) and red 5-ROX dUTP mouse Y chromosome probe (Cat. # CLN-100920) on 4 μm-thick sections of chimeric mouse tissues. A. Fluorescent micrograph of mouse #3 brain cell nucleus showing one Y chromosome (upper row) and mouse #6 brain cell nucleus showing two X chromosomes (lower row). B. Organs were scored for X (green)/Y (red) chromosome ratios of DAPI stained tissue sections of the chimeric mouse organs by fluorescence microscopy. The total number of X and Y chromosomes scored for each organ are shown. A ratio of 1:1 X:Y chromosomes indicated a male organ and a X:Y ratio of 2:0 indicated a female organ.
Another question addressed by the X,Y-FISH is whether the X-gal (+) containing organs were derived from the fusion of a male ROSA CD34+ cell with a wildtype cell of the inner cell mass in the early blastocyst. We found no XXXY cells in the chimeric organs examined and the total X:Y ratios for all organs were that of an XY male or a XX female, indicating no cell fusion.
4 Discussion
The quest for a versatile multipotent stem cell capable of differentiating into various cell types and therefore eventually repair damaged tissues and organs is the subject of intense investigation, which has led to very promising results. However, a major question, which has yet to be determined, is whether such a multipotent cell type is present in the normal adult mammal. Here we show, as a proof of principle, that a bone marrow cell sub-population present in normal adult mice and which expresses both an array of ESC genes and germ layer lineage genes can give rise to many somatic tissues of an apparently normal adult mouse.
A characteristic of embryonic stem cells in culture is that they manifest unlimited self-renewal. Although the adult bone marrow CD34+ cells investigated here express ESC genes [17] and have the potential for multipotency, they do not exhibit the unlimited proliferation of ESCs. The CD34+ cells have a limited lifespan since they stop dividing and plateau after 30 to 40 doublings like diploid somatic cells [6]. These CD34+ cells do not form teratomas when injected into a host [6] in sharp contrast to ESCs and iPSCs.
Chimeric mice obtained from blastocysts inoculated with CD34+ cells differ from chimeric mice generated from ESCs or iPSCs where the whole individual is derived from the inoculated stem cells [18,19] as opposed to multiple, but apparently not all organs derived from the CD34+ cells. One possible explanation is that ESCs and iPSCs are totipotent and these CD34+ cells may be only multipotent.
The pattern of organs derived from the transplanted male ROSA CD34+ bone marrow stem cells was different for each chimeric mouse. This is most likely due to the number and site of implant of the adult bone marrow stem cells. As mentioned in the Results section, 8 to 15 CD34+ cells were injected into each 64-cell stage blastocyst. Contingent on how many CD34+ cells implanted in the early inner cell mass, which most likely contains 30 or fewer cells, and depending on cell migration and the site of implant and the time of implant during ongoing development, the transplanted CD34+ cells differentiated into distinct cell types.
Previous reports have indicated the presence, in wildtype mice, of endogenous expression of X-gal staining in some tissues and organs depending on technical conditions [20–23]. Here, we have used the staining protocol for bacterial β-galactosidase supplied by the manufacturer and we have used identical staining conditions for ROSA CD34+ chimera, ROSA transgenic and wildtype CD34+ chimera organ staining. Under these staining conditions we did not see X-gal staining in the control wildtype CD34+ chimera organs tested and we found the same patterns of X-gal+ stained cells in both the ROSA CD34+ chimeras and ROSA transgenic mice from which the ROSA CD34+ cells were grown. To our surprise some cell types in an organ, like brain, of the adult ROSA transgenic mouse stained with X-gal and other cell types did not stain. Furthermore, XY chromosome analysis showed that the X-gal (–) cells in ROSA chimeric organs that contained X-gal (+) cells were male cells derived from the male ROSA CD34+ cells. In addition, one X-gal (–) heart in a female ROSA CD34+ chimera had an XY ratio of 1:1 indicating a male heart derived from the male CD34+ cells. In all cases the X-gal staining plus the XY chromosome analysis indicated that all cell types (both X-gal+ and X-gal-) of the entire organ were derived from the CD34+ cells implanted in the blastocyst.
A possible role of these CD34+ cells from bone marrow or circulating blood in physiological or pathological processes should be considered. Indeed it has been reported that post-mortem brain samples from women who had received bone marrow transplants from male donors did contain male cells. The two main regions examined were cerebellum and hippocampus. The donor derived cells were identified by the presence of a Y chromosome. The proportion of male neurons in the female recipients varied between 1:100 and 1:1000. In addition to neurons, glial and mesenchymal cells were also labeled with the Y marker, often in clusters, suggesting a clonal origin [24–26]. Interestingly enough, skin biopsies of female patients following allogenic male bone marrow transplantation show the presence of donor derived keratinocytes [27]. In these studies the bone marrow cells that eventually gave rise to neural cells containing a Y chromosome have not been identified. Since we have shown here that a subset of bone marrow CD34+ cells can generate most if not all cells present in the brain, it is tempting to speculate that bone marrow cells that differentiated into various cell types may be the subset of multipotent stem cells we have identified.
Lately, many investigations have been carried out on iPSCs derived from individuals bearing various genetic defects to explore their mechanisms as well as to try to correct them [28,29]. It is reasonable to assume that the sub-population of CD34+ stem cells should carry the same genetic defect as the iPSCs derived from their peripheral tissues such as fibroblasts or neurons.
The growth and differentiation patterns of the adult CD34+ stem cells are reminiscent of adult Urodele Amphibian newt or salamander blastema cells or mature Planarian blastema cells that replace the cells and tissues that are needed to reform the missing limb or body part [30–33].
Taken together, these readily available adult bone marrow CD34+ stem cells, are multipotent and constitute a potential source for cell replacement therapy in multiple tissues and organs.
Disclosure of interest
BP, CB and DT hold USA (10/982,381) and International (PTC/US04/37122) Patents Pending.
Acknowledgements
The authors would like to thank David Ford and Deborah Yarnell for technical assistance in this project, Valerie Stewart of the University of Maryland School of Medicine Transgenic Core Facility and Jonathan Bradley for helpful discussions. This work was supported for PSF (VA REAP), CTB (VA Merit) and DT (Abraxis Biosci. and VA REAP).


