1 The environmental soil pollution by xenoestrogens: the case of manure and nonylphenol
Many substances poured in the environment mimic the effects of animal endogenous hormones interfering with the endocrine system [1], in particular with the male reproductive physiology [2,3]. These substances, known as endocrine disruptor chemicals (EDCs), originate from a variety of sources. Today, a natural and plentiful source of EDCs is given by the metabolites of steroid hormones in the manure used for the fertilization of the soils dedicated to organic farming [1,4,5], whereas an artificial source is the presence in pesticides of substances such as the alkylphenol polyethoxylates.
To date, little attention has been paid to the steroid hormones and to the products of their metabolism in manure. The organic farming is exponentially growing and, along with it, the use of manure as the principal choice for soil fertility. Indeed, manure ensures a proper recycling of biological by-products. However, it must be not overlooked that organic manure of animal origin contributes to a significant load of estrogen hormones in the natural environment [6], since farm animals excrete conjugated steroid hormones that persist in the manure for several months [7]. It has been estimated that pregnant cows can excrete from 700 to 17,000 mg/day of estrone in urine; in the dairy cattle, a combined (faecal + urinary) excretion of 384 mg of 17ß-estradiol was calculated [8]. In addition, the conjugated and biologically inactive forms of excreted hormones can be easily converted into free steroids by soil microorganisms as Escherichia coli [9–11]. Bioassays of estrogenicity on water samples from streams across the United States revealed that higher estrogenic activity was frequently associated with manure application to the cultivated fields in the neighbourhood [12].
As regards EDCs of synthetic origin, nonylphenol (NP) is the estrogen-like compound produced in larger quantities. NP is persistent in sediments and in aquatic environment, bioaccumulable, extremely toxic and it is used primarily to produce surfactants for a wide variety of applications and consumer products [1]. NP is commonly used as a co-formulant in pesticides.
NP is a clear-to-pale yellow viscous liquid at room temperature [13–15]. The air concentrations of NP are generally expected to be low [14], but a research has shown that in some circumstances there may be water-to-air volatilization that results in significant atmospheric concentrations of NP substances [16]. NP has been detected in many different habitats, such as groundwater, sediment, soil, freshwater, saltwater.
The human exposure is a result of the presence of NP in detergents, cleaners, agricultural and indoor pesticides, food packaging and cosmetics and it has been confirmed by biomonitoring data from breast milk [17], umbilical cord blood [18] and urine [19]. The maximum level of NP found in the breast milk was 56.3 μg/L, leading to an estimated maximum dose for an infant of 3.9 μg/kg/day [17].
Due to its structural similarity with estradiol-17β, NP is able to bind the estrogen receptors stimulating the transcription of the downstream genes (as the estrogen receptors itself or vitellogenin) [20] interfering with the reproductive and developmental events, in particular.
Most of the research on NP in the environment was carried out in ecosystems where the human impact is predominant, as in rivers in which the contribution of wastewater effluents to flow is significant [21]. Nevertheless, agricultural ecosystems can potentially be contaminated by spraying of pesticides containing NP as co-formulates, landfilling of sludge or by the application of sewage sludge or pulp and paper mill sludge [22,23]. In addition, NP remains in the soil, thus accumulating moderately [24].
2 Vitellogenin in extrahepatic tissues of vertebrates
Vitellogenin (VTG), the major precursor of the yolk proteins, is an estrogen-dependent and sex-specific protein naturally synthesized in the liver of the females of oviparous vertebrates during the reproductive period [25,26]. In males, VTG gene is silent, but it may be activated by estrogenic exposure [27–29]. For this reason, the finding of VTG in the liver and/or in plasma of males is considered a good biomarker of xeno-estrogenic pollution.
A huge amount of studies was performed on males of aquatic organisms, naturally or experimentally exposed to estrogen-like substances and were mainly aimed to highlight the hepatic induction of VTG [27–35].
A possible extrahepatic synthesis of VTG was suggested by Wallace [26], according to which in females 5–10% of the total VTG could be produced by the oocyte itself; this process was referred to as endogenous or ectopic vitellogenesis.
Over the years, many studies resumed the possibility of an extrahepatic origin for VTG protein by using biochemical and molecular approaches (Table 1). Investigations carried out on oviparous fish suggest that estrogenic hormones and estrogen-like compounds are able to induce the expression of the VTG gene in both hepatic and extrahepatic tissues [36–38]. It has been demonstrated that in male zebrafish 17α-ethinylestradiol induces VTG expression and synthesis in dose- and time-dependent pattern in skin and eye [39] and estradiol-17β or EDCs are able to determine the VTG synthesis in the heart, cerebral tissue [40], epidermis [41], gills [42,43], white adipose tissue [44], intestine and muscle tissue [38].
Estrogen-induced expression of extrahepatic VTG in male vertebrates.
| Species | Treatment | Tissue | References |
| Danio rerio | 17α-ethinylestradiol | Skin | Zhang et al., 2014 [39] |
| Eye | |||
| Estradiol 17-β/EDC | Heart | Yin et al., 2009 [66] | |
| Cerebral tissue | |||
| Epidermis | Jin et al., 2008 [67] | ||
| Gills | Islinger et al., 2003 [42] | ||
| Wang et al., 2010 [43] | |||
| White adipose tissue | Tingaud et al., 2012 [44] | ||
| Intestine | Wang et al., 2005 [38] | ||
| Muscle tissue | |||
| Testis | |||
| Acipenser transmontanus | Estradiol 17-β | Testis | Bidwell et al., 1995 [36] |
| Oryzias latipes | Estradiol 17-β/NP | Spermatocytes | Kobayashi et al., 2005 [37] |
| Koger et al., 1999 [45] | |||
| Melanotaenia fluviatilis | Estradiol 17-β | Seminiferous epithelium | Shanthanagouda et al., 2013 [46] |
| Woods et al., 2009 [47] | |||
| Tanichthys albonube | Estradiol 17-β | Testis | Wang et al., 2010 [43] |
| Torpedo marmorata | Estradiol 17-β/NP | Testis | Del Giudice et al., 2011; 2012 [48,49] |
| Kidney | |||
| Podarcis sicula | Estradiol 17-β/NP/manure | Testis | Verderame et al., 2016 [55] |
| Verderame, 2016 [63] |
Many studies focused on gonads of male fish experimentally exposed to E2 or estrogen-like substances. In Acipenser transmontanus, VTG transcripts were detected in the testis of the E2-treated samples [36]; a same result was obtained also in zebrafish [38]. In the testis of Oryzias latipes treated with E2 or NP, spermatocytes are able to transcribe the VTG gene [37,45]. In specimens of Melanotaenia fluviatilis experimentally exposed to E2, VTG-mRNA was found in the seminiferous epithelium [46], whereas the VTG protein was localized in the cellular spaces surrounding the spermatids [47]. Gene expression analysis demonstrated the presence of VTG-mRNA in the testes of Tanichthys albonube treated with E2 [43]. Again, the male germ cells of medaka incorporate and accumulate VTG after treatment with E2 or estrogen-like substances [37].
Among the cartilaginous fish, experiments carried out on Torpedo marmorata demonstrated the VTG expression and synthesis in testis and kidney following intraperitoneal injection of E2 or NP [48,49]. Interestingly enough, in this species the VTG synthesis has been found to occur also within the ovarian follicle cells in both previtellogenic and vitellogenic phases [50].
3 Expression and synthesis of VTG in the terrestrial oviparous vertebrate Podarcis sicula
In the literature, until 2015 no data are present on the extrahepatic expression and/or synthesis of VTG in terrestrial oviparous vertebrates. In addition, studies on the reproductive status of wildlife exposed to manure-fertilized cropland are very limited, so we decided to investigate the presence of endogenous VTG in testis of the lizard Podarcis sicula caught in areas devoted to organic farming and in males experimentally fed with NP-polluted food.
The Italian wall lizard Podarcis sicula is a small lacertid, considered an excellent pollution bioindicator model for ecotoxicological studies [51–56]. It is widespread in the countryside and lives in narrow ranges sheltering under the ground and the rocks [57]. As prey for many small mammals and birds, it fills an important role also as soil top predator contributing, in several areas, to the control of agricultural pests.
To make this animal a good model organism for studies on vitellogenesis mechanisms is also the in-depth knowledge of its reproductive cycle in both male and female [58–62]. In this oviparous species, VTG synthesis occurs in female liver during the breeding season, under the control of the estrogen receptor alpha (ERα) [59]. The protein is also detected in the liver and the plasma of males experimentally treated with E2 [59] or exposed to NP-contaminated food [51,54]. VTG expression in the P. sicula male liver is also accompanied by the expression of the silent ERα gene [51,54]. These estrogen-contaminated animals show also a sharp slowdown of spermatogenesis, a condition that seriously threatens the continuity of this species. More recently, it has been demonstrated expression and synthesis of VTG also in the liver of P. sicula collected in cultivated fields devoted to organic farming, fertilized only with manure [56]. These animals, however, did not show any impairment of spermatogenesis [56].
We decided to verify the ability of P. sicula testis to express and synthesize VTG following exposure to estradiol-17β. Sexually mature males in the mating period were intraperitoneally injected with E2 at the same concentration able to stimulate in these males the hepatic synthesis of VTG [59]. Immunohistochemical and in situ hybridization analyses demonstrate the presence of both VTG transcript and protein in all the germ cells forming the seminiferous tubule (Fig. 1) [55].
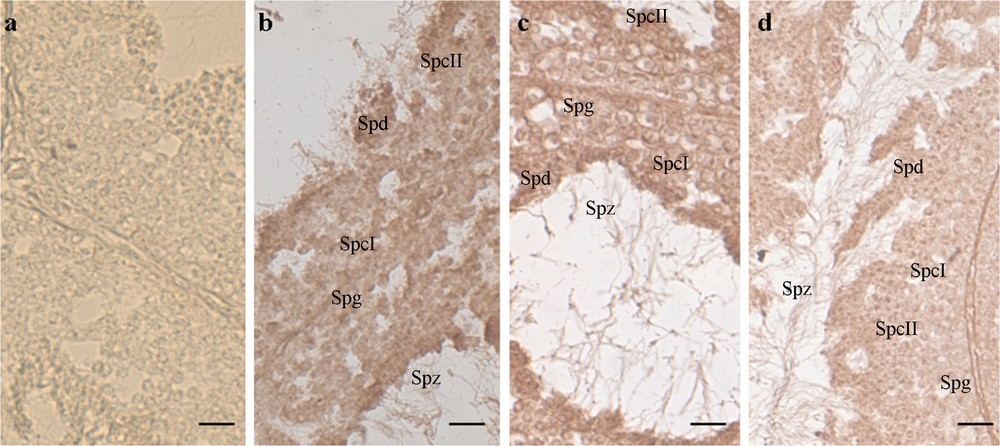
In situ hybridization on Podarcis sicula testis sections, incubated with DIG-probe to detect VTG-mRNA. The brown hybridization signal was absent in the testis of wildlife males collected on uncultivated, rural areas [a]. In the samples treated with E2 [b], fed with NP-polluted food [c] or housing in manure treated soil [d], the signal was evident in the cytoplasm of all the germinal cells of the seminiferous epithelium, i.e. spermatogonia (Spg), primary spermatocytes (SpcI), secondary spermatocytes (SpcII), spermatids (Spt), and spermatozoa (Spz). See [55] for details on methods. Bar: 30 μm. Masquer
In situ hybridization on Podarcis sicula testis sections, incubated with DIG-probe to detect VTG-mRNA. The brown hybridization signal was absent in the testis of wildlife males collected on uncultivated, rural areas [a]. In the samples treated with E2 [b], ... Lire la suite
Once experimentally assessed the estrogen-induced synthesis of VTG in the testis, we investigate the induction of testicular VTG in two possible environmentally estrogenic exposures derived from:
- • estrogen metabolites naturally present in animal manure;
- • NP-polluted food and water.
Biochemical and biomolecular investigations demonstrated that both estrogenic exposures, i.e. animals caught in a field where the only fertilizer used was manure or in the animals experimentally exposed to NP-polluted diet, are able to switch on the VTG gene in the germ cells of the seminiferous epithelium (Fig. 1) [63].
While there is no doubt on the endogenous origin of VTG transcripts, the protein observed in lizard testis could have a dual origin. It is conceivable that VTG protein could be synthesized from the transcripts present in the same cells, but it could not be excluded that, as occurs in the ovary during the oocyte vitellogenesis [64], the hepatic VTG protein, synthesized under estrogenic stimulation and released into the bloodstream, could be taken up by receptor-mediated endocytosis in the testis. The failure to detect VTGR transcripts in testis of lizards environmentally or experimentally contaminated with EDCs [56] strongly suggests that the VTG protein detected in this tissue derives entirely from the biosynthetic process locally activated by the estrogenic contamination (Fig. 2).
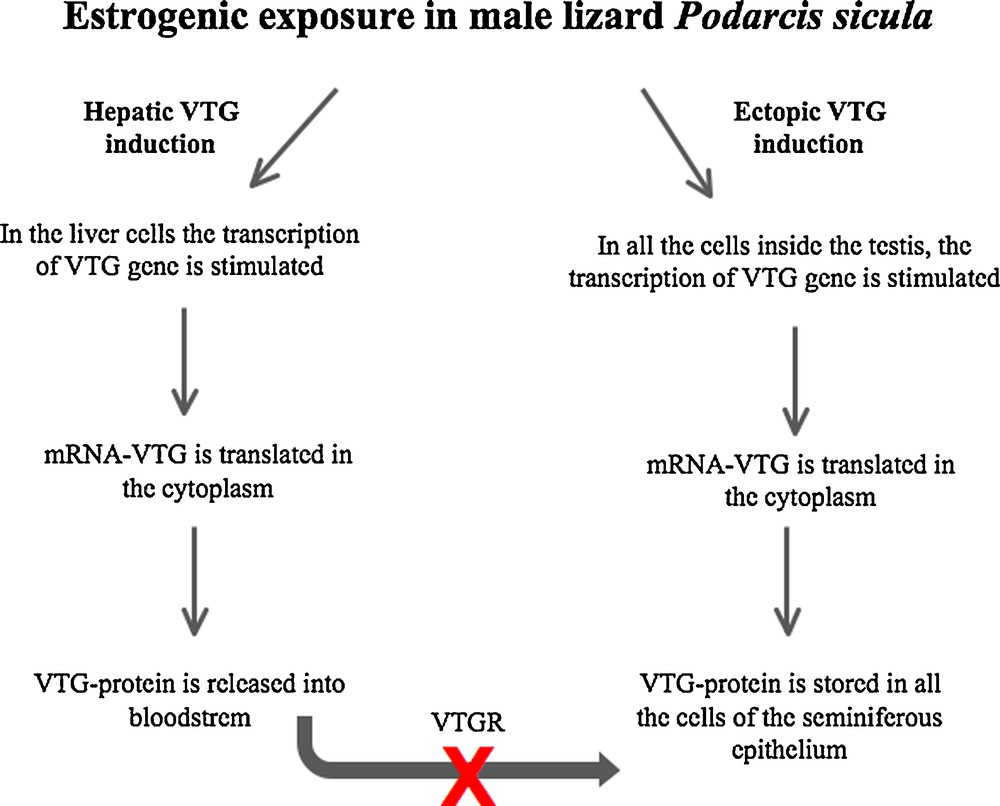
Schematic illustration of hepatic and extrahepatic synthesis of VTG in male lizard Podarcis sicula exposed to estrogenic environment.
4 Concluding remarks
Taken together, these data demonstrate the ability of both aquatic and terrestrial oviparous vertebrates to synthesize VTG in the male gonad. The presence of VTG in the gonads opens a new scenario on the possibility of using this protein as a biomarker of environmental estrogen pollution, as well as on its role in the impairment of reproduction observed in estrogen contaminated males. Our data also demonstrate the estrogenic nature of manure and this sheds new light on the use of animal manure as a fertilizer. Indeed, the excessive and uncontrolled use of manure as a fertilizer in organic farming could endanger the reproduction and survival of soil organisms. Considering that in the last past years there has been a huge increase in organic farming, estimating to grow by 8.9% per year [65], soil contamination by estrogen metabolites could soon represent a serious risk for wildlife.
Finally, the result opens new scenarios and questions on the role of VTG in cells other than oocytes.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgement
The authors gratefully acknowledge Prof. Ermelinda Limatola for her critical review of the manuscript.


