1 Introduction
Self-incompatibility (SI) in homomorphic flowers is governed by one locus (The S-Locus) that directs two different mechanisms (Fig. 1) in plants. The S-locus carries several genes of unknown function. In species of the gametophytic type, the pollen grain harbors only one determinant, either the Sx or Sy allele; this explains why it was named gametophytic S-I (GSI). The S-locus encodes an S-RNAse that is released into the style in Solanaceae, Papilionaceae and Rosaceae [1]. This enzyme penetrates from the style into the pollen tube whatever the compatibility alleles and triggers a cascade of events leading to programmed-cell death (PCD) only if the pollen is incompatible [1,2]. GSI has a variant in Papaver without S-RNAse expressed in the pollen tube [3]. In the other functional mechanism, the pollen grain harbors the two determinants SxSy. It functions in Brassicaceae and Asteraceae [4]. Thus, it is called sporophytic S-I (SSI), that enables dominance relationships (as an example, Sy > Sx) leading to ♀SxSy × ♂SxSw being inter-incompatible (IIC) and ♀SxSw × ♂SxSy being inter-compatible (ICO) in only this direction for a pair-wise combination of cultivars [5] (Fig. 1). For Brassicaceae, an incompatible pollen grain that lands on the style triggers a cascade of events leading to PCD that involved a SRK motif protein kinase. Several hundreds of protein kinase exists in the plant genomes, but only one is involved in the events of the SSI cascade [6]. The number of S-alleles in one species with GSI mechanism has been found to be 40 to 60 in several Prunus species [7], as in Senecio with an SSI mechanism [8], with, for consequence in a population, that the mating availability–the number of individuals that are fertilized/the total number of individuals–is relatively high [7,8].

Model for gametophytic S-I (GSI) and sporophytic S-I (SSI) mechanisms of self-sterility and inter-compatibility. > means dominant upon; < s recessive upon; 00 means no possible progenies. The difficult case are only represented in this figure.
Most olive (Olea europaea Subsp. europaea Var. europaea) varieties are more or less self-incompatible (S-I), they produce little or no fruit in pure orchards, and even for those partially self-fertile (S-F), cross-pollination with pollinisers favors higher and more regular yields. Although S-I is an important trait for production of the olive tree, the mode of inheritance of the S-I trait is, as yet, unknown for this species. However, looking for pollinisers–pollinator is devoted to an insect that carries pollen–is not rational and thus in most orchards irregular yields and alternate production are the major concerns for olive growers as well as for economists and market forecasts. In the olive tree, several authors have considered it is of the gametophytic (GSI) type [9–11]. The genetic structure for most varieties is the clone [12], which means all individuals are identical for all alleles, and consequently, if the two S-alleles are leading to SI, the orchard will produce no, or little, fruits. To give efficient pollen grains, olive growers add polliniser trees. Historically, each variety fits one or several traditional pollinisers, but researchers have developed diallel designs in most countries to look for more efficient pollinisers than the traditional ones, which pollinate one of the varieties of the countries as Mission from USA [13], Manzanillo in Spain and Israel [14,15], Koroneiki from Greece [16], and Lucques and Olivière in France [17–20].
2 Determination of self-incompatibility/self-fertile and inter-incompatible/inter-compatible
Practically, pollen tube progression onto the style to the ovary is the method to determine whether a plant is S-I/S-F and whether a cross is ICO or IIC. For a species, such as the olive, this method has been used [9,21,22], but it is time-consuming and thus expensive. Moreover, the germination of a pollen grain appeared a quantitative trait and authors also considered the fruit set that is easier to determine on a large scale by surrounding some branches with pollen-proof paper bags [9]. However, fruit set under the bag may be not well correlated to pollen grain germination due to other events that may affect the set up. Finally, following pollen tube growth in the style has a limit in practice and most researchers prefer to record final fruit sets to estimate S-I, even though it can be affected by other events than S-I status.
S-I is estimated by the self-incompatibility index (S-II), which is the ratio of number of fruits for a branch carrying about 200 flowers under a bag, which enables self-pollination, to the number of fruits for 200 flowers left to free pollination without the bag [14,22]. This ratio varies from 0 (strictly S-I) to 1 (S-F), behind the threshold (10–30 fruits), a variety is S-I, and above, it is given as S-F, and for most varieties S-II is about 0.1–0.3. However, S-II was found up to 5, that indicates that most olive varieties are somewhat partially self-incompatible, and moreover, an adequate peculiar polliniser may be more efficient than free pollination. Thus, looking for such pollinisers may be attractive to enhance fruit yield [23–25].
2.1 Environment effects on self-incompatibility and flower structure
In the olive tree, some flowers have no pistil (staminate flower) and some have no stamen (pistillate flower). The proportion of perfect/complete (hermaphrodite) flowers is variety dependent. Moreover, most fruits may fall after set up and in fine only 3 to 5% of the complete flowers will produce fruits that average one fruit per inflorescence. Consequently, for most S-I studies, the fruit set in controlled cross-pollination is compared to the fruit set in open pollination. S-II appeared a quantitative trait that requires to choose a threshold to estimate S-I or S-F. Depending on the number of flowers under the bag (20 to 200) the estimation is more or less accurate, on the threshold chosen 0.1 to 0.3, a cultivar with S-II = 0.2 will change its status from S-I to S-F [17–25]. Another concern with the olive pollination comes from environmental effects on cultivar behaviors. Temperature could be the cause of this change [9]. Some are given as S-F in their countries (Manzanilla, Spain) or region (Aglandau, Haute-Provence, Vaucluse, and Var; Salonenque, Les Baux and La Crau), but are S-I in other regions as for Manzanilla [9–12]. However, the molecular characterization of varieties is not routine and based on morphology many synonymies may be the source of such variation [26]. Consequently, the literature displays heterogeneous results about the classification of cultivars in S-F or S-I. Moreover, ambiguities may also come from methods that estimated S-I/S-F by determining pollen grain germination, fertilization of the ovaries, or fruit set, and so on. Nevertheless, whatever the methods used to examine cultivars, most cultivars are at least partially S-I and conversely, some are partially S-F. Indeed, most olive cultivars will usually set a better crop with cross-pollination especially under adverse weather conditions. Most are declared S-F varieties even although they require a pollen source from another variety [13–25].
However, olive tree pollination in orchards is the main concern for olive growers and for researchers to ensure a regular production. Experimental designs to test IIC versus ICO for varieties are varied due to many constraints in a long shelve tree species. Some authors have tried to plant trees in containers to move them easily into the neighbourhood of the female trees or vice versa, to experiment pair-wise combinations of cultivars, so as to check other factors such as the effect of temperature during pollination [9]. Most researchers have preferred to move onto the female the pollen either in paper bag once harvested or to move branches that are introduced into the paper bag that surrounded the female flowers. All these experimental techniques suffer from drawbacks due to the pollen conservation methods and to possible contaminant pollen grains that are dispersed by wind. The pollen can be trapped onto filters and its density enables one to forecast olive yields on a large scale [27,28]–during full blossoming of olive trees in an orchard, the opening of a bag presents a risk difficult to estimate, but that can make results inconsistent on a year-to-year basis, depending on the contamination rate.
2.2 Choice of the data set
With all these constraints, to carry out a new diallel design to estimate S-I/S-F as IIC/ICO on a set of varieties appeared unrealistic. Furthermore, the literature contains enough data providing fruit sets on crosses between pair-wise combinations of varieties, frequently in both directions of crosses, with all possible drawbacks listed above when the trees are not in the same orchard, that infers pollen should be displaced. However, data are relatively consistent between different authors and over years for some varieties. We attempted unraveling their differential composition in S-alleles to explain inter-compatibility and inter-incompatibility in the frame of the gametophytic and the sporophytic models to determine the one to which the olive may belong.
We retained IIC and ICO data from varieties of the crosses controlled by Moutier's team [17–20] and some from crosses controlled, Villemur's team [21,22,29]; both have provided consistent data over the years at the threshold of S-II at thresholds 0.3, above which the variety is S-F. Both teams have looked for pollinisers of Lucques and Olivière in the Languedoc-Roussillon region (South France) and the varieties they used have been widely described (Supplementary material, Table S1). Moutier's team has used the paper bag method, whereas Musho [17,18] has used pollen tube germination, and interestingly they used the same varieties between 1976–1977 and 2002–2009 as Moutier's team. The polliniser is declared IIC with the recipient variety when in a cross; S-II is below 0.3 for the female, and above 0.6 the polliniser is considered as very efficient. Between 0.3 and 0.6 SII may be advocated IIC or ICO depending on the case study. In self-pollination trials, the same threshold leads to S-S or to S-F. We looked for a method to convert + and 0 from Moutier's team and Musho into S-alleles [16–18,22–24]. However, several authors have reported, based on fruit set, strong differences–even opposite results–in reciprocal crosses for several plant species [5] and for the olive ([17] and Fig. 2). Gerstel's [5] findings on Guayule (Parthenium argentatum Gray, Asteraceae) suggested that reciprocal differences should be the best way to start the conversion for two varieties carrying four different alleles (R1 to R4) due to one variety having a unique polliniser among the five possible (Fig. 3, Table 1).
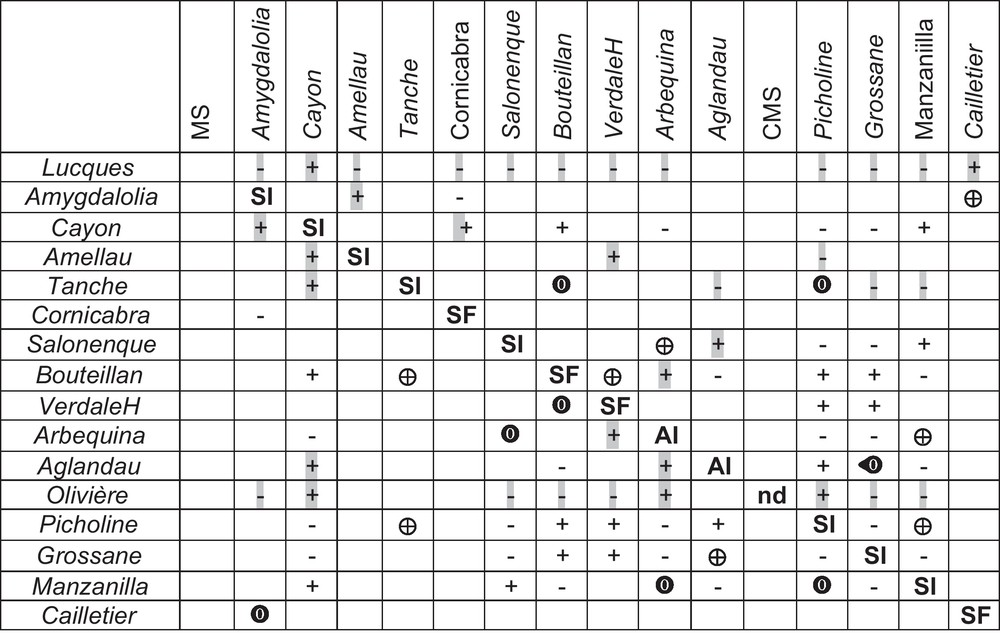
Rewritten from Moutier et al. [6] with symmetric lines (female) and columns (male). All +/– were converted into +. Cross in one direction only+ –; , : Crosses in two directions but dissymmetric fruit sets; Symmetric fruit sets – –; or + + MS: male sterile; CMS: cytoplasmic male sterile.
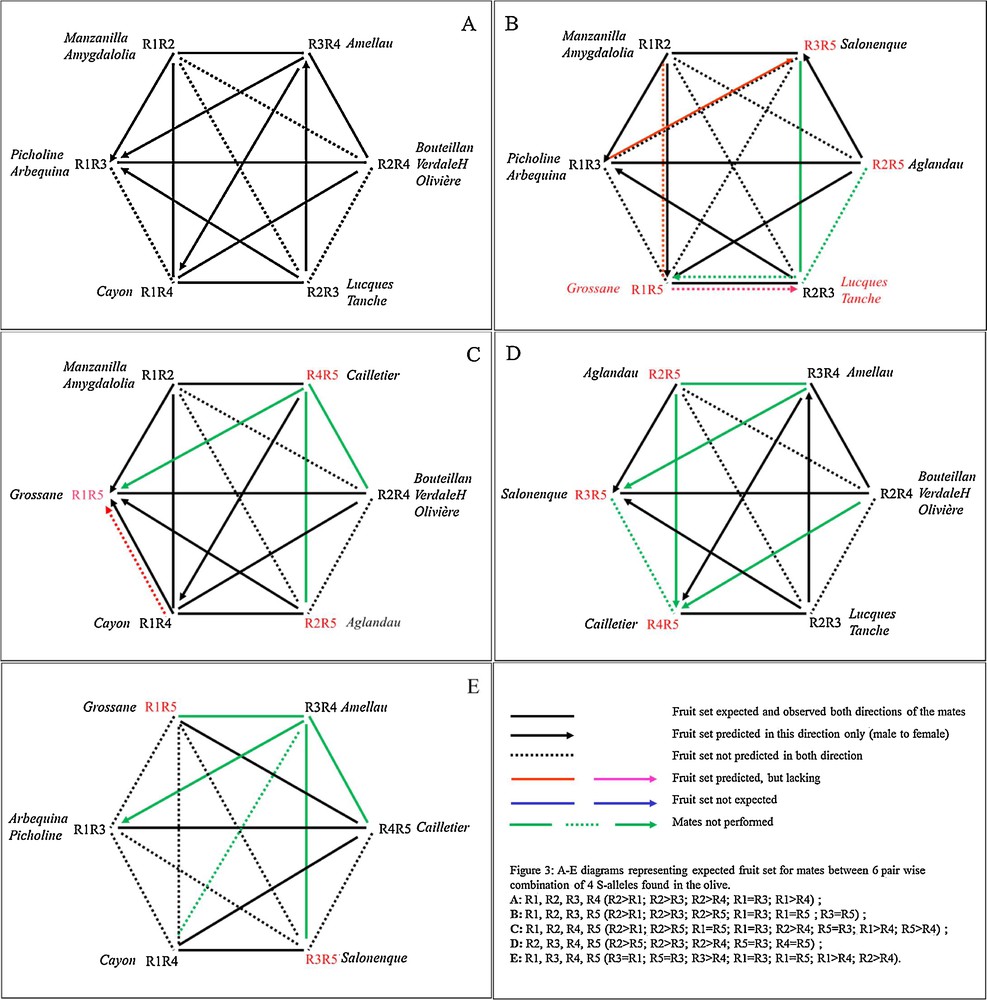
A–E diagrams representing expected fruit set for mates between six pair wise combination of four S-alleles found in the olive.
Variety pair-wise combinations leading to symmetric fruit sets (left) and dissymmetric fruit sets (right) that have enabled to decipher S-alleles attribution and enabling to construct Fig. 4. Alleles responsible of the symmetry and the dissymmetry are indicated with those involved in the reject or in dissymmetry. cross in one and reverse direction. Key pair-wise combination of crosses with reciprocal differences, compatibility (ICO) and incompatibility (IIC) in both directions. and represent crosses in two directions but dissymmetric fruit sets.
| Some key symmetric crosses | Dissymmetric crosses | ||||||
| Bouteillan | Cayon | + | + | Arbequina | Manzanilla | ||
| Bouteillan | Picholine | + | + | Bouteillan | VerdaleH | ||
| Picholine | VerdaleH | + | + | Picholine | Manzanilla | ||
| Cayon | Manzanilla | + | + | Picholine | Tanche | ||
| Picholine | Cayon | – | – | Bouteillan | Tanche | ||
| Manzanilla | Tanche | – | – | ||||
| Picholine | Arbequina | – | – | Cailletier | Aglandau | ||
| Manzanilla | Belgentier | ||||||
| Grossane | Bouteillan | + | + | Salonenque | Arbequina | ||
| Manzanilla | Salonenque | + | + | Aglandau | Grossane | ||
| Grossane | VerdaleH | + | + | Cornicabra | Amygdalolia | ||
| Aglandau | Picholine | + | + | ||||
| Aglandau | Manzanilla | – | – | ||||
| Picholine | Grossane | – | – | ||||
| Cayon | Grossane | – | – | ||||
| Manzanilla | Grossane | – | – | ||||
| Arbequina | Grossane | – | – | ||||
| Salonenque | Grossane | – | – | ||||
| Amygdalolia | Cornicabra | – | – |
3 Results
For each olive tree carrying two S-alleles (SxSy), without dominance between the S-alleles, thus both alleles are expressed in the pollen (coded Sx = Sy) it cannot self-pollinate. This is true both in the GSI and SSI model frame. However, when Sx is dominant over Sy (Sx > Sy) then self-pollination is possible, Sy is hinted by Sx, and may lead to homozygous SySy individuals that are strictly self-incompatible. This feature has two main consequences:
- • a variety displays self-fertility when dominance relationships exists between the two alleles, or, in other words, one self-compatible olive variety may not carry a self-compatible allele as should be inferred with the GSI model;
- • a variety should therefore display reciprocal differences when crossed with a male that carries the same recessive allele when this latter is hidden by a dominant one.
Consequently, we focused the model for the olive on an allelic series of S-alleles with dominance relationships. These features are detailed for several case studies Fig. 4A–F. Fig. 4A–F show that dominance relationships between four alleles make radical changes in expected fruit sets. As for R1 = R2, R3 = R4 most crosses are IIC, Fig. 4A,B, and we introduced dominance relationships between the four alleles.
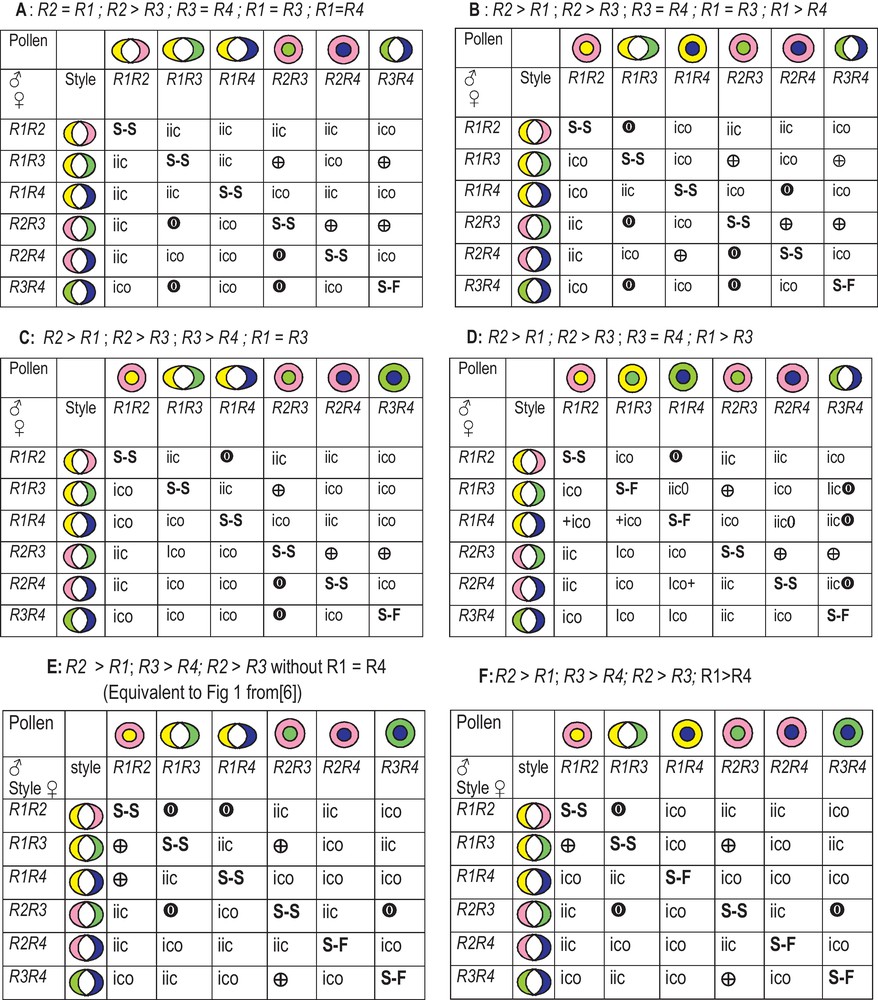
Key to explain reciprocal differences in the olives constructed based on D.U. Gerstel [6] for Guayule. For the male double circle means the inner allele is hinted in the male due to dominance. Double quarter moon means the two alleles are equally expressed. In the female there is no dominance and all the alleles are equally expressed. When the colour hinted in the male is found in the female the cross is ICO whatever the surrounding colour. When the same colour can contact the cross is IIC. , dissymmetric crosses (six pair wise combinations of genotypes). Self-pollination result is given as S-S or S-F and discussed in the text.
3.1 To decipher S-alleles in sets 1 and 2
Consequently, to decipher S-alleles we first considered Lucques, which is very difficult to cross with an efficient male. We supposed, based on Gerstel’ hypotheses [4], that it should carry two dominant alleles named R2 and R3 due to the fact that it can be pollinated only by a variety carrying R1R4 (Fig. 3A; Fig. 4E); the scheme was borrowed from Gerstel [4] to explain fruit set in the pair-wise combination of the set1 of varieties Manzanilla, Picholine, Arbequina, VerdaleH, Tanche and Cayon. Consequently, all varieties that are ICO with Lucques cannot carry both R2R3, or either R2 or R3 (Fig. 3A, Fig. 4A). In contrast, all pair-wise combination of varieties that are IIC with Lucques will share one or two of the alleles R2R3. Lucques and Tanche carry the same set of S-alleles R2R3 (Fig. 3A). Some of these varieties cannot cross Tanche and Lucques and they displayed reciprocal differences in fruit set, for some pair-wise combinations (Table 1), but not Lucques that is male sterile and the cross was unidirectional [30]. We attributed the S-alleles to the set1 of varieties to fit Fig. 4A. Thus, Picholine is R1R3 and Manzanilla is R1R2, that infers that Manzanilla can cross Picholine due to R2 > R1, whereas the cross is not possible in the reverse direction. Arbequina can cross Cayon, but not Picholine and Lucques; consequently it is R1R3.
The set two of varieties included Olivière (also male sterile) [12], Cayon mated Olivière, as Picholine and Arbequina can, whereas Manzanilla cannot. Consequently, Olivière carries the same S-alleles as VerdaleH (R2R4), but, as it is male sterile, we cannot verify it crossed Arbequina, but VerdaleH can cross Arbequina in both directions. Bouteillan behaves as Olivière as female (four crosses) except with Grossane and VerdaleH. Bouteillan should carry the same alleles as VerdaleH and Olivière. Consequently, Grossane should cross Olivière. This is the first misfit between the data and the model. However, more recent data obtained on a large scale verified that Grossane crossed Olivière [J. Vaisse, Pers Comm]. Thus, the misfit is eliminated.
For Aglandau we constructed the Fig. 4C, D with R1, R2, R3 and R5 (Fig. 3B, C) to verify that Aglandau mated Manzanilla, Tanche, Lucques and Picholine. With R5 > R3 Aglandau is ICO with Picholine in both directions and Aglandau cannot cross Tanche; thus, it carries R2 and the R5 S-allele. Salonenque cannot cross Lucques and Picholine and it carries R5, with R5 > R3. Grossane behaves as Picholine for all varieties except with Olivière (R2R4); it thus carries R1, and, since as Cailletier it gives reciprocal differences with Aglandau, it carries R5. Thus, Aglandau is R2R5, Grossane is R1R5 and Cailletier is R4R5. However, Grossane cannot cross with Cayon, that does not agree with the model. Cailletier should carry R4 as it crosses Lucques, but considering it cannot cross with Aglandau (the reverse is possible) it should carry R5. Aglandau and Cailletier are predicted S-F. Amellau is R3R4 and Cornicabra is R1R2. Corniale (Coil) is ICO with Lucques (R2R3) and VerdaleH is R2R4, and thus are as efficient pollinisers as Cayon. Salonenque fits R3R5 and is ICO with Arbequina. Salonenque mates Manzanilla whereas Grossane cannot; thus this sustains the theory that Salonenque should carry R3R5. Villemur's team [22] has shown that Manzanilla can be crossed by Belgentier whereas the reverse was not possible; that infers Belgentier should carry another R6 R-allele with R6 > R2. R6 was also present in Ascolano, but cannot be deciphered in this cross [9] due to not enough mates. From crosses performed in Australia [10], Frantoio mates Manzanilla (R1R2), so it is R3R4, whereas the other mates lead to infer Kalamata correspond to R2R4, Pendolino and Picual are R1R2. Thus, the list of varieties with attributed S-alleles could increase rapidly.
3.2 Explanation of variation in the self-fertility rate
Moreover, due to the hierarchy in dominance relationships, we suggest that the more dominant allele paired with the more recessive allele will lead to the higher S-F rate: namely R2R4. A given pair of R-alleles enables one thus to predict whether the variety is S-F or S-S. R1R2 (Manzanilla), R3R4 (Amellau), R2R4 (VerdaleH, Bouteillan), are predicted to be more or less S-F, whereas R1R4 (Cayon), R1R3 (Picholine, Arbequina), and Tanche (R2R3) are predicted to be strictly S-S. This prediction matches the Bouteillan and VerdaleH pair. Then, we expected R3R4 less S-F than R1R2 pair. The most S-S pair should be R2R3 both highly dominant. R1R3 is expected S-S, there is no dominance relationships in the pair. Still, we propose a logical explanation for variation of self-fertility levels. In this set it fits data, but it can be widely verified with data from other teams, and if it fails it must be rejected.
3.3 The whole model
The whole possible pair-wise combination for 15 cultivars displays, 225 (15 × 15) possibilities, but because some share the same S-allele pair, we considered 5 S-alleles. However, Manzanilla in the model displays self-fertility that may explain that Cayon to Manzanilla was noted ICO by confusion of self-fruit set with cross-fruit set, but in the model it is IIC. Consequently, the data from Moutier should be reconsidered with the deduction of the S-F rate, when the model predicts self-fertility. The two crosses Manzanilla × VerdaleH in both directions were not performed, but Manzanilla IIC with Olivière was verified. Except with this misfit and the two lacking crosses all other pair-wise combinations of fruit set matched model prediction. Consequently, the probability that six crosses in each direction fitted the model due to chance only, is weak. We verified dominance relationships between R1, R2, R3, and R4 in 28 of the 30 occurrences (Fig. 3A), but we estimated that we have enough crosses that involved R5 (as male and as female on 30 occurrences) to verify the correct dominance relationships with all other S-alleles.
4 Discussion
When considering confidence in the model, obviously the interest of Fig. 5 is to predict cross results and to introduce logic into cross results that could appear erratic. Nevertheless, the results could serve to other varieties if researchers refer in the same testers. The varieties conserved at Inra have been characterized with molecular markers [26] and are available. Probably, there exists several clones for Manzanilla, the provenance from Montpellier has been introduced by G. Piquemal, P. Villemur (Pers. Comm.) and is referenced as Manzanilla dos Hermano [26]. For further enlargement of Table 2 with more S-alleles some crosses appeared strategic to attribute S-alleles to a cultivar as a male. With Lucques if VarX is ICO, this eliminates R2 and R3. Thus, VarX could be R1R4 or R1Rx, or R4Ry. VarX mates Cayon and Picholine, which enable one to attribute the correct R1 and R4 alleles, but Rx and Ry will remain to be identified. The literature frequently reported crosses with Picholine as male. Fig. 3 shows that if the cross is ICO, the variety has to be R2R4, R2Rx, R4Ry or RxRy.
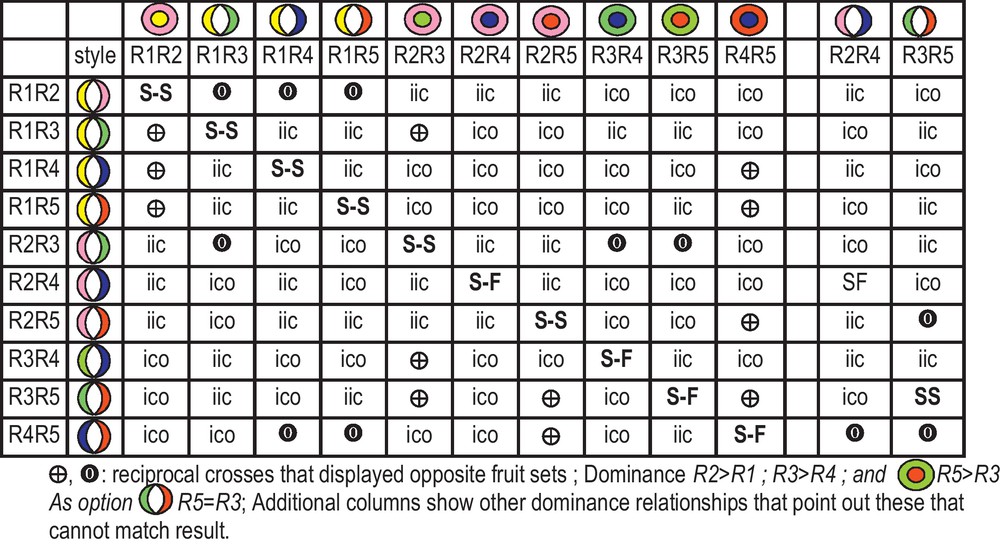
Key to determine inter-incompatibility (0) and inter-compatibility (+) for pair-wise combination of genotypes with parsimony approach up to 5 S-allele assuming dominance relationships.
Summary of S-alleles attributed to olive cultivars based on Moutier et al. [17,21,22]; Villemur et al. [18], Musho [9].
| S-S/S-F alleles at the S-locus | Best polliniser | ||||||||||
| Cultivars | R1 | R2 | R3 | R4 | R5 | R6 | R7 | ||||
| Manzanilla, Amygdalolia, Cornicabra | S-S | R1 | R2 | R4R4 | R5R5 | ||||||
| Bouteillan, VerdaleH, Olivière | S-F | R2 | R4 | R1R1 | R5R5 | ||||||
| Picholine, Arbequina | S-S | R1 | R3 | R4R4 | |||||||
| Cayon | S-F | R1 | R4 | R5R5 | |||||||
| Cailletier | S-F | R4 | R5 | R1R1 | |||||||
| Salonenque | S-S | R3 | R5 | R1R1 | R4R4 | ||||||
| Grossane | R1 | R5 | R4R4 | ||||||||
| Lucques,Tanche | S-S | R2 | R3 | R1R1 | R4R4 | R5R5 | |||||
| Amellau | S-S | R3 | R4 | R1R1 | R5R5 | ||||||
| Aglandau | S-S | R2 | R5 | R1R1 | R4R4 | ||||||
| Belgentier [17] | R2 | R6 | R1R1 | R4R4 | R5R5 |
Thus, the results from Moutier's team that Grossane was not ICO with Cayon appeared illogical. When a cross is found IIC, whereas theoretically it is ICO, this suggests that there is another reason that S-I prevents pollination to occur, such as the coincidence of blossoming between the two varieties–the state of the pollen brought in the bag, the late stage of the female flower–a too hot temperature in the bag, and so on. In contrast, if the cross is found ICO when expected to be IIC, this means an error has occurred. The main sources of errors should be:
- • self-pollination that may be enhanced by mentor effect of the foreign pollen under the bag;
- • contamination by surrounding pollen that may prevail over self-incompatible pollen.
Several features merge from controlled crosses in the olive:
- • self-pollination in a bag leads to weak fruit set for most cultivars;
- • year-on-year results may appear quite variable due to temperature variation (consensus in literature, not shown here);
- • free pollination for all cultivar ensures an increase in fruit setting;
- • pair-wise combination of cultivars may in some case increase the fruit set by 500%;
- • in 18% reciprocal fruit set is opposite (±) for pair-wise combinations of cultivars (Table 2).
Villemur et al. [21] have indicated that Lucques ovule longevity has the shortest viability during 3 to 4 days compared to 6 to 7 days for other cultivars that may explain no coincidence between blossoming of Lucques, which is very early, and its pollinisers. Considering 122 pair-wise combinations of olive cultivars checked by Moutier's team [17–20] and Villemur [21,22], the good coincidence obtained with our model sustains it may run. Most crosses match our model except some exceptions that could be due to self-pollination of the female cultivar that blurred results with the IIC variety. Our prediction agrees with most results in the literature, that suggests examining again the basic data to check whether they have been misinterpreted, or whether it could be justified to move the threshold [17,21]. Many heterogeneous results are given for Manzanilla [9,13,15]. We maintained these allele compositions, but more data should be examined.
In most of the situations Vossen [13] has reported self-pollination of the male parent, whereas Moutier [17–21] did not report such results. In fact, Vossen [13] has probably taken into account low S-II (about 0.1–0.2), whereas Moutier's team did not, because they chose 0.3 as threshold. Consequently, our prediction was formerly correct, but anyway we can say that even if self-pollination is possible, cross-pollination will enhance fruit sets. This is due to the position of the threshold for S-II.
Some of those combinations of cultivars that have never been attempted are given as predictions through our model. We indicated for each cross why it failed–presence of the same S-allele or why it succeeded–dominance of one allele that enabled to mask a recessive one, even it was present in the female side and therefore the cross will lead to fruit sets (Fig. 5, Supplementary material, Table S1).
4.1 Same S-alleles from different regions
These allelic compositions show that the olive displays a range of S-alleles, but their pair-wise combinations are not wide, leading to difficulties to find adequate pollinisers for each genotype. We have here examined cultivars from different origins: France, Spain, and Greece; moreover, the history of these cultivars rooted them in the Near-East and in North Africa [31], that suggests the S-allele set is common to the olive in the whole Mediterranean basin. Crosses of cultivar made with oleaster trees will verify possible gene flow between the wild and the crop that is of importance not only for fruit production, but also for consideration of the evolution of the mating system of both forms.
It is probably useful for olive growers to handle a key to predict whether a cross will be IIC or it will be ICO in a pair-wise combination of cultivars. Here, we do not give all the details, but just a glance on the way to construct the table following the rule that reciprocal cross differences are due to dominance of S-alleles in the male only (Table 1). We stressed already that moving the threshold may change the results, but it seems realistic to keep a high threshold for SII (0.3–0.4) to avoid insufficient pollination in case pollination coincides with a rainy period or high temperature, since these are known to decrease olive yields on the whole. Probably, other physiological troubles occur, affecting fruit sets with Lucques and Olivière that may disturb SSI results and consequently examination of pollen tube growth is required [9,19] for control, but it involves much work. This model will undergo probably some adjustments due to the low numbers of cultivars (#13) with enough crosses and four with a few informative crosses. Data on systematic cultivar pair-wise combinations from other countries could make the model more efficient, for the benefit of the olive community. Moreover, other teams in most countries have plenty of unpublished data and could fulfill diallel tables based on our model. Furthermore, private or rare alleles as R6 and R5 could be informative on the origin of the cultivars as for Aglandau, Grossane, Salonenque and Cailletier, all from Provence.
4.2 Sporophytic self-incompatible prevalent in Oleaceae
The last point deals with the question of SSI in Oleaceae although many reports have favoured GSI. Jasmineaea primitive section of Oleaceae displays heterostyly, an archaic form of SSI [32]. Recently, in the Oleae section, Phillyrea, a genus close to Olea, Saumitou-Laprade et al. [33] have clearly identified androdioecy due to two S-alleles that enable one to maintain male and hermaphrodite trees. Our results suggest that for the olive a SSI mating agrees with works in other genera of the Oleaceae.
In conclusion, we have proposed a model to attribute S-alleles to some olive cultivars. Data are not fitting the GSI model. We verified that for almost the whole data the SSI model is accepted. There are still a few inadequacies between data and model, but most predictions are novel and could be verified rapidly to sustain or reject the model. We faced concerns with thresholds to rank cultivars as S-F or S-I as well as IIC or ICO. It is likely that data from other teams will fulfill our model to the benefit of olive growers. Cailletier (called Taggiasca in Italy) is as efficient as Cayon as polliniser and should not be neglected. Moreover, we suggest to create homozygous pollinisers to ensure efficient pollination, having R1R1, R4R4 and R5R5 that could each pollinate a wide set of varieties (Table 2). Probably, at the field level some pair-wise combinations of varieties do not occur or are rare due to lack of coincidence of these blossoming. Temperature may shift the blossoming period and thus makes coincidence more or less extended. Anyhow, the main feature in this study stresses that some varieties to be pollinated such as Tanche and Lucques can accept only R1 pollen grains from Cayon, Corniale and Cailletier. In the pollen cloud above olive orchards such pollen grains are at low frequency, whereas R1 pollen grains from Picholine, Manzanilla, and Amygdalolia are certainly much more prevalent. R4 pollen grains from Cayon and Cailletier will be also at low frequency in comparison to those (non efficient) from VerdaleH, Bouteillan, and Arbequina. If competition may occur on the stigma for pollen germination, it is now clear that olive growers should take into consideration our model to improve fruit setting in the olive, and modify the design of varieties in orchards to ensure better pollination efficiency.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
Thanks are due to Nathalie Moutier to enabling us to consult her data sets already published in several journals and we regret that she was not allowed to contribute as co-author. Pierre Villemur and Nathalie Moutier are acknowledged for helpful discussions. This work was supported by ANR-PATERMED “Paysages terroirs méditerranéens” coordinated by Stéphane Angles.


