1 Introduction
Among heavy metals, lead is one of the most phytotoxic agents. The physiological effects of lead have been extensively studied with various plants, particularly with regard to the effect on growth and development, photosynthesis, respiration, membrane transport and others (reviewed in [1,2]). In spite of the abundant literature on the harmful effects of lead on plants, the mechanism of its toxic action is still not sufficiently explained. This is especially true of organic lead derivatives, which are characterized by a very high toxicity towards living organisms [3–9]. Toxicity of organolead compounds depends on various factors, among them lipophilicity seems to be the most important [10–14]. There are two main sources of organolead compounds which contribute to Pb pollution in the environment. They may be introduced to the soil as pesticides or originate from additives in gasoline [11–17]. Although organolead as a gasoline additive is banned in most countries, contamination by organolead compounds is still a real menace to all living organisms. After entering the cytoplasm (for transport of Pb see [18]) Pb ions influence, among others, the vacuole, which in most plant cells constitutes 80 to 90% of the cellular volume. The vacuole plays an important role in homeostasis, pH regulation, Ca2+ signaling and other physiological functions (reviewed in [19]).
Data accumulated in patch-clamp studies revealed that in the vacuolar membrane (tonoplast) there are two main classes of tonoplast non-selective cation channels (NSCCs): slow-activating vacuolar channels (SV channels) and fast-activating vacuolar channels (FV channels) (reviewed in [19,20]). These two channels and the ATP-dependent proton pump constitute the major conductances of the vacuolar membrane (reviewed in [21]). The SV channel was the first channel to be characterized in vacuolar membrane [22,23]. The SV channel is ubiquitous in all higher plants tested so far and in recent years, the understanding of the mechanisms that account for its activation or inhibition has increased substantially (reviewed in [21,24]). It has previously been shown that SV channels are regulated by heavy metals, such as zinc [25], nickel [26,27], copper [28] and cadmium [29].
To our knowledge, in the literature are lacking systematic data concerning the effects of organic compounds of heavy metals on SV channels. In our recent theoretical studies [30], where trimethyllead chloride was used for analysis of the ion current distribution function, it was shown that Met3PbCl influences the variance of the open-state ion current but does not alter the probability distribution function of the closed-state ion current. Taking into account our theoretical studies and also the fact that organolead compounds influence model and biological membranes [31–35], we have undertaken systematic studies on the effect of trimethyllead chloride (Met3PbCl) on SV channels in vacuoles isolated from red beet (Beta vulgaris L.) taproots.
2 Materials and methods
Red beet (Beta vulgaris L.) vacuoles were mechanically isolated according to the method described previously by Coyaud et al. [36]. Briefly, the vacuoles were directly extruded into the recording chamber (about 1 ml in volume) by cutting a slice of fresh storage tissue and rinsing the surface with bathing solution. The control bath solution was: 100 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, 5 mM MES, 5 mM Tris and 400 mM sorbitol, pH 7.5 (adjusted with 0.1 N NaOH), osmolarity 656 mOsm. The standard pipette solution was: 100 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 5 mM MES, 5 mM Tris and 340 mM sorbitol, pH 5.5, osmolarity 587 mOsm. Patch-clamp experiments were performed on the whole-vacuole and excised cytosolic side-out patch configurations using an EPC-7 Plus amplifier (List-Medical-Electronic, Darmstadt, Germany). The current and voltage convention was in accordance with Bertl et al. [37], i.e. the sign of voltage refers to the cytosolic side, and positive (outward) currents represent an efflux of cations into the vacuole. The experimental results were stored using Patch-Master software (HEKA Electronic, Lambrecht, Germany). Signal was filtered by a five-pole Bessel filter and recorded on hard disk with sampling frequency of 1 to 100 kHz. The Bessel filter was an integral part of the EPC-7 Plus amplifier. Patch pipettes were pulled from Kimax-51 (Kimble Products, Toledo, Ohio, USA) using two-stage pipette puller (model L/M-3-PA, List Medical, Germany), fire-polished with a microforge CPZ 101 (List Medical, Germany) and coated with Sylgard (Dow Corning, Midland, MI, USA). The resistance of patch electrodes filled with pipette solution was 2-4 MΩ; seal resistance was in the range 5-21 GΩ. Access to the vacuole interior was gained by breaking the membrane under the pipette with a voltage pulse in of 200 to 800 mV. Effect of Met3PbCl on SV channels was studied. In the experiments the control bath was changed for a new one of the same salt composition, containing additionally Met3PbCl at a final concentration of 0.1 to 100 μM. The exchange of bath solution was carried out by continuous perfusion of the measuring chamber using an infusion pump model SP200 (World Precision Instruments, USA). All experiments were carried out at room temperature (22 ± 1 °C).
Data analysis was carried out using the FitMaster (HEKA Electronic, Lambrecht, Germany) and computer software Statistical for Windows (StatSoft 2010; STATISTICA data analysis software system, version 9.1. http://www.statsoft.com, USA).
3 Results
Patch-clamp experiments performed in the whole-vacuole configuration revealed a typical SV-type channel activity in vacuoles isolated from Beta vulgaris L. taproot. Macroscopic currents recorded in symmetrical 100 mM KCl and Ca2+ gradient (0.1 mM in the bath and 1 mM in the pipette) showed slow activation (Fig. 1A, control) and strong outward rectification of the steady-state currents (Fig. 1 C, control). SV channels were closed at negative potentials and activated at voltages more positive than +10 mV (Fig. 1A, C). The addition of Met3PbCl to the bath solution blocked, in a concentration-dependent manner (Fig. 2), SV currents in red beet vacuoles (see also Fig. 1B, C). When Met3PbCl at a final concentration of 1.0, 10 and 100 μM was added to the bath solution, SV currents decreased at all potentials between +40 and +100 mV (Fig. 2, insert). For example, in the whole-vacuole configuration addition of 100 μM Met3PbCl resulted in a 60% decrease of current amplitudes (Fig. 2, insert). Fig. 3 shows the time constant τ of monoexponential function as a function of membrane voltage in control solution and in the presence of trimethyllead chloride (although activation of the channels is biphasic, we chose a monoexponetial function because the fit is satisfactory and includes a smaller number of parameters). As Fig. 3 indicates, the time constant τ increased several times in the presence of 100 μM Met3PbCl, as compared to the control, at all voltages tested. This means that with organolead the ensemble of channels relax slowly, because the number of the transitions closed-open is smaller compared to control solution; on the macroscopic level, this may reflect a decrease in the open probability of the SV channels in the presence of trimethyllead chloride.
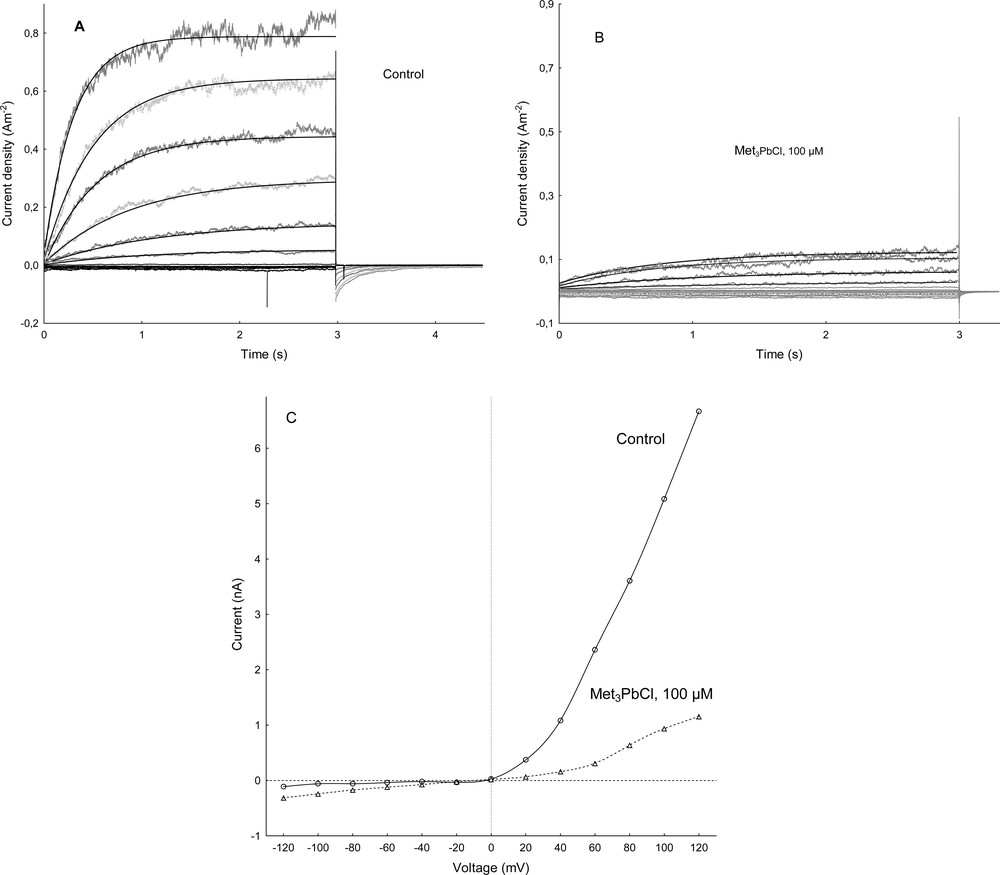
Cytosolic Met3PbCl effects on the slow vacuolar (SV) channels in red beet (Beta vulgaris L.) taproot vacuoles. (A) An example of SV current recording in control bath on single vacuole. SV currents elicited by a series of voltage steps ranging from –120 to +120 mV in 20 mV steps; holding potential 0 mV. (B) The same vacuole as in A treated with Met3PbCl at a final concentration of 100 μM. Current traces were fitted with the exponential function i(t) = io + i1 exp(-t/τ), where io is steady-state current, t – time, io + i1 – current at t = 0 and τ – time constant. (C) Current-voltage characteristics (from A and B) obtained by plotting the steady-state currents as a function of the applied test potential (the same vacuole as in A and B). Masquer
Cytosolic Met3PbCl effects on the slow vacuolar (SV) channels in red beet (Beta vulgaris L.) taproot vacuoles. (A) An example of SV current recording in control bath on single vacuole. SV currents elicited by a series of voltage steps ... Lire la suite
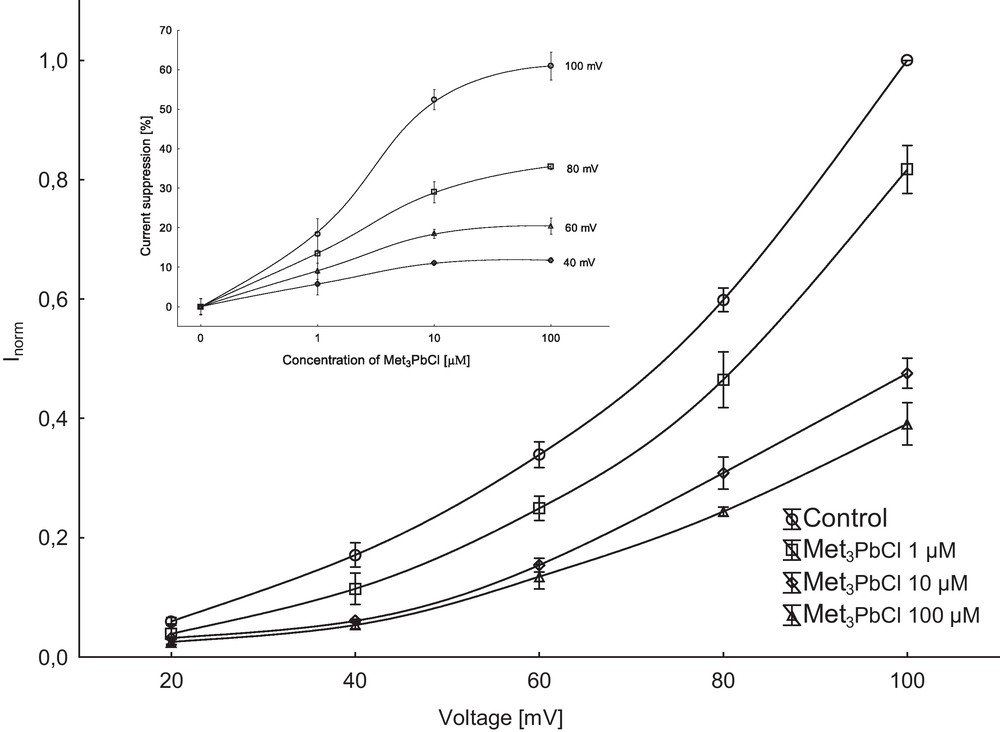
Suppression of macroscopic currents by Met3PbCl. Steady-state current (normalized to the current amplitude determined at +100 mV under control conditions) were determined in the absence or presence of 1, 10 and 100 μM Met3PbCl. Data points represent means (± SE) from seven experiments performed with different vacuoles. The inset represents percent of current suppression vs. trimethyllead concentration at different voltages.
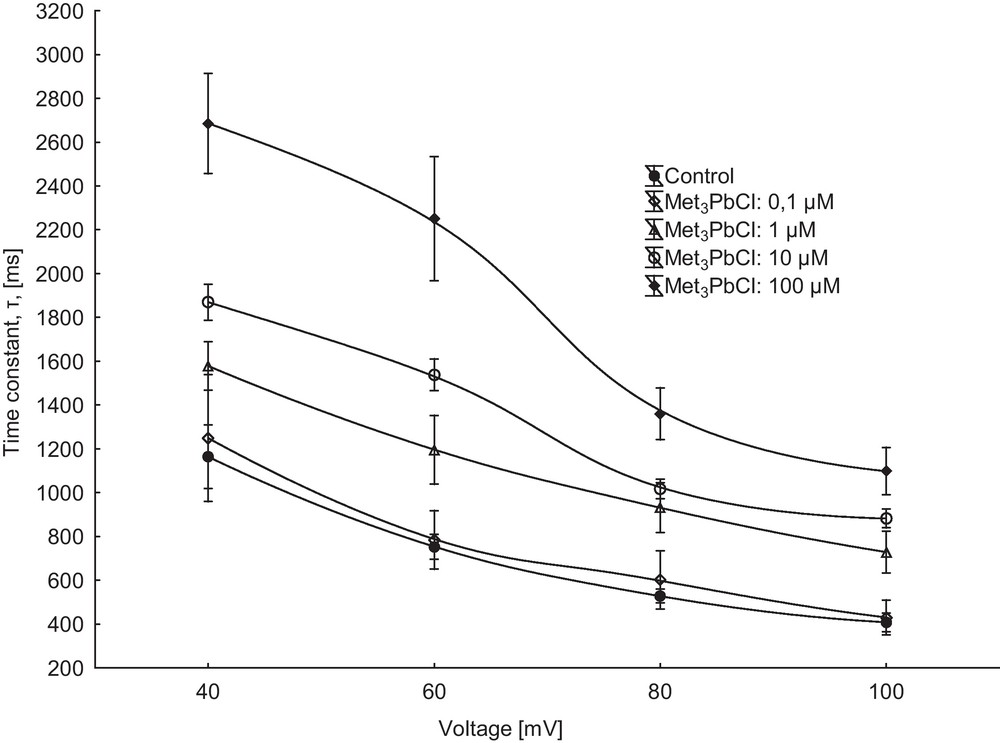
Effect of 100 μM Met3PbCl on activation of slow vacuolar (SV) currents. Mean (± SE, n = 7) values of characteristic activation time τ as a function of voltage show that addition of Met3PbCl (0.1, 1.0, 10 and 100 μM) slowed the activation of SV channels significantly. Time constant τ was calculated by fitting the macroscopic SV current i(t) with an exponential function as shown in Fig. 1.
When single channel properties were analyzed (Fig. 4), only little channel activity could be recorded in the presence of 100 μM Met3PbCl. The open probability of single channels (Fig. 5) decreased significantly in the presence of 100 μM Met3PbCl. In the control solution, the open probability of single channels increased with increasing membrane voltage from +40 to +100 mV. Below 40 mV the openings, practically, were not observed in the current traces. The addition of 100 μM Met3PbCl to the bath solution decreased the open probability of single channels by about one order of magnitude as compared to the control (Fig. 5). The single channel recordings obtained in the control solution and in the presence of Met3PbCl showed that trimethyllead chloride slightly decreased the unitary conductance of single channels; in the presence and absence of 100 μM cytosolic Met3PbCl the unitary conductance was about 66 ± 4 and 75 ± 6 (n > 7) pS, respectively (Fig. 6). The ratio between the two conductances was 0.88.
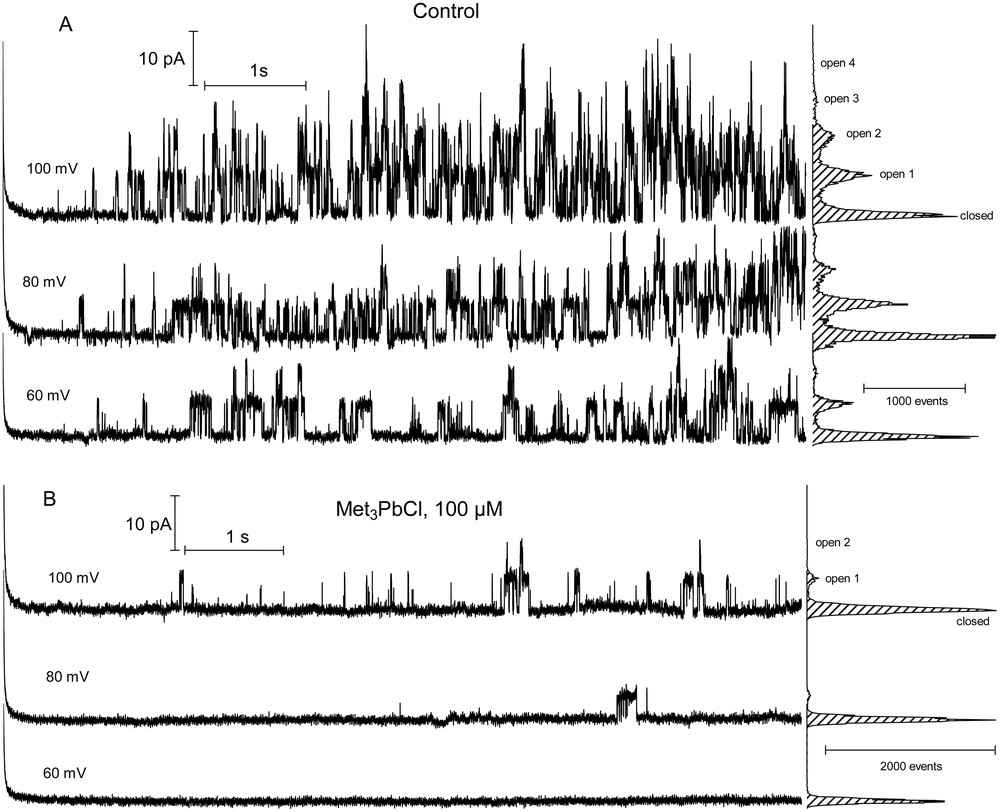
Microscopic currents traces of slow vacuolar (SV) channels. Single channel openings in control bath (A) and in the presence of 100 μM Met3PbCl (B) are shown. The experiments were performed on the same vacuole in the cytosolic side-out patch configuration. Single channel fluctuations were recorded at +60, +80 and +100 mV. The corresponding amplitude histograms are shown to the right of the current traces. The highest amplitude of the histograms obtained at each voltage means closed state. Open 1, 2, 3 and 4 give the current levels at which 1, 2, 3, or 4 SV channels were open simultaneously. Masquer
Microscopic currents traces of slow vacuolar (SV) channels. Single channel openings in control bath (A) and in the presence of 100 μM Met3PbCl (B) are shown. The experiments were performed on the same vacuole in the cytosolic side-out patch configuration. Single ... Lire la suite

Open probability of slow vacuolar (SV) channels as a function of membrane voltage. The open probability was calculated (using FitMaster software) as a sum of channels open times in the current traces normalized to total time of traces and divided by the number of active channels in the patch. Data points are means (± SE and SD) from eight independent experiments.

Current-voltage relationships for microscopic slow vacuolar (SV) currents (A – control, B – 100 μM Met3PbCl in bath solution). Values of current were obtained as differences of the maximum of current histograms, representing open and closed states of channel, respectively. Data points are means (± SE and SD) from nine independent experiments.
Using the FitMaster software, we have performed an analysis of the opening events that included the current amplitude during the open state of the channel, the level of current recording (indicating the number of SV channels in the open state at the same time) and time of opening. Fig. 7 shows the distribution of times of different states of the ensemble of four active channels recorded in control solution as a function of current level. The same dependence determined in the bath solution with 100 μM Met3PbCl clearly showed (Fig. 8) that trimethyllead chloride decreased significantly the number of openings, as compared to control solution. However, as can be seen in Fig. 7 (insert) and Fig. 8 (insert) trimethyllead chloride did not change the opening times of the channels; the small differences between the opening times found in control solution and in the presence of 100 μM Met3PbCl are close to statistical errors.
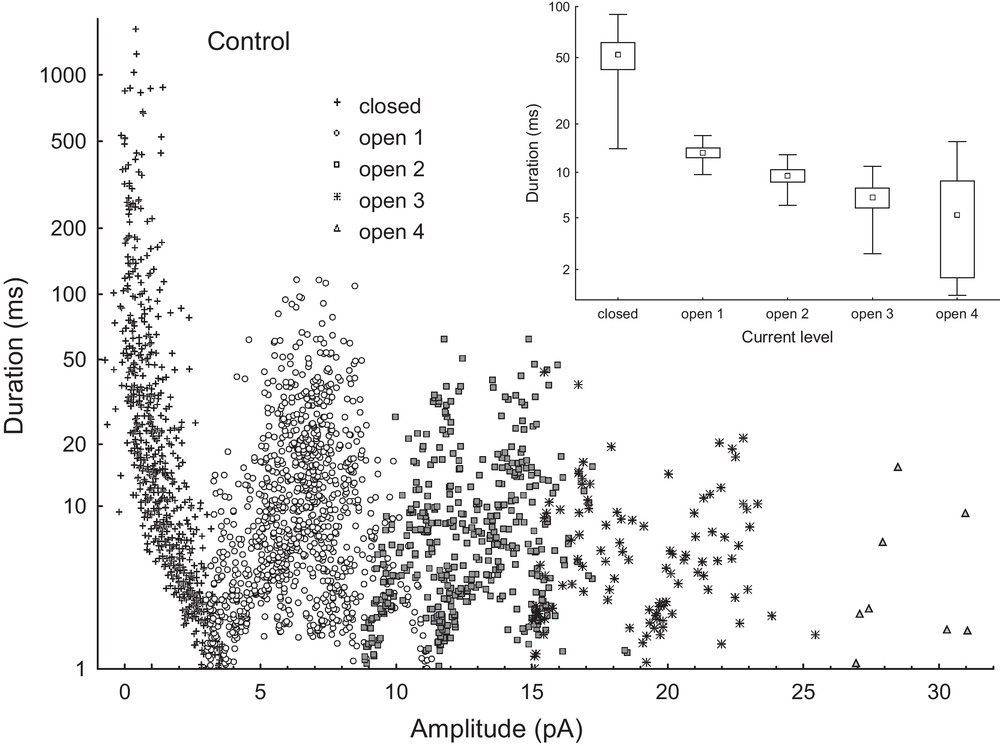
Distribution of times of different current states events (closed, open 1, 2, 3 and 4) as a function of amplitude of current events (i.e. its average value during event) in the control solution. All events were collected from six current traces (each of 8 sec duration) recorded on the same patch at membrane voltage of +100 mV. The inset on the right side shows average values of times of current events versus current level. The points are means ± SE and SD. FitMaster software was used to analyse of opening events. Masquer
Distribution of times of different current states events (closed, open 1, 2, 3 and 4) as a function of amplitude of current events (i.e. its average value during event) in the control solution. All events were collected from six current ... Lire la suite
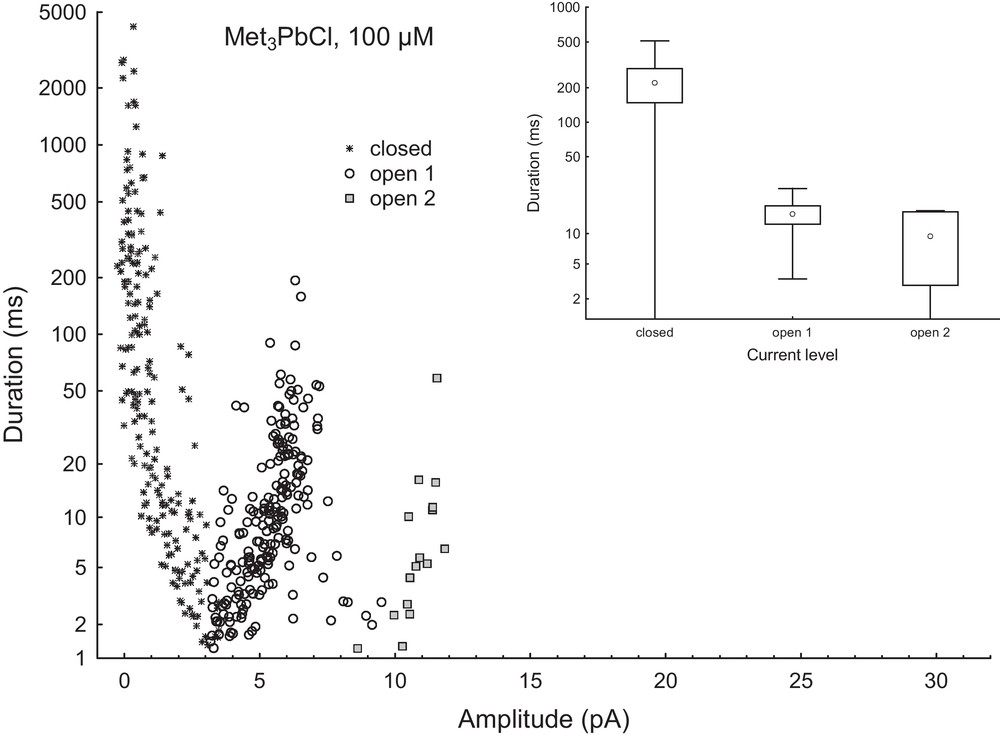
Distribution of times of different current states events (closed, open 1, 2, 3 and 4) as a function of amplitude of current events (i.e. its average value during event) in the presence of 100 μM Met3PbCl. Number of recorded traces was the same as in Fig. 7. The inset on the right side shows the average values of times of current events versus current level. The points are means ± SE and SD. FitMaster software was used to analyse of opening events. Masquer
Distribution of times of different current states events (closed, open 1, 2, 3 and 4) as a function of amplitude of current events (i.e. its average value during event) in the presence of 100 μM Met3PbCl. Number of recorded traces was ... Lire la suite
4 Discussion
The slow-activating vacuolar (SV) channel is a non-selective cation channel that is activated by cytosol-positive voltages and elevated cytosolic Ca2+ concentrations [23,38]. The SV channel shows a characteristically slow activation and its current is predominantly outward (i.e. directed out of cytoplasm), apart from conditions when the K+ gradient is directed out of the vacuole into the cytosol [39–41]. Elevation of the cytosolic Ca2+ concentration has a dual effect on SV channel activity: an increase in the number of open SV channels at high positive potentials and a shift of the voltage dependence to less positive potentials [21,25,39 for review].
Despite extensive research over the past decade, the physiological function of SV channels is still not clear. Here, we demonstrate that under the experimental conditions of this study the vacuoles isolated from the red beet taproot are characterized by SV channels whose electrical properties, such as slow activation, outward rectification and unitary conductance, are close to those previously described under similar conditions in Beta vulgaris taproots [23,25,42]. The addition of Met3PbCl to the bath solution blocked, in a concentration-dependent manner, SV currents in red beet vacuoles (Fig. 2). The time constant τ obtained with fitting the macroscopic SV currents by a monoexponential function, showed that addition of Met3PbCl slowed the activation of SV channels significantly, suggesting (on macroscopic level) a decrease in the open probability of SV channels in the presence of trimethyllead chloride (Fig. 3). However, the open probability of single channels determined in cytosolic side-out configuration clearly showed that in the presence of 100 μM Met3PbCl its value is by one order of magnitude lower as compared to the control (Fig. 5). This finding suggests that Met3PbCl stabilizes the closed states of the channels. Taking into account that in the presence of 100 μM Met3PbCl the unitary conductance of single channels decreased only slightly (Fig. 6) and that the ratio between the current recorded in the presence and in the absence of cytosolic Met3PbCl did not depend on voltage in the range from +60 to +100 mV (Fig. 2), it can be suggested that trimethyllead chloride binding site is located outside the channel's selectivity filter. As mentioned above, trimethyllead acting on the whole membrane reduced the macroscopic current by about 60% (at 100 μM). However, the results obtained on the single channel level showed that the addition of the same concentration of Met3PbCl resulted in a reduction of the open probability by approximately one order of magnitude, and a reduction of the single channel current amplitude to 88%. Inconsistency of the whole-vacuole and single channel recordings may be due to two reasons: different affinity of this compound to the membrane (channel) in the whole cell configuration and configuration patches detached (e.g, because of the presence of mechanical stress of lipid phase in the system of patches), and the second reason may be the fact that SV channels operate cooperatively in the system of whole membrane, which does not exist in the patches system.
The experiments carried out on model lipid membranes [33,43] showed that lipophylic properties of organolead compounds are crucial for modification of model membranes. It is noteworthy that experiments on the influence of organolead compounds on the model and biological membrane have shown various effects: erythrocyte hemolysis [32], change in membrane fluidity and its mechanical properties [33], modification of lipids and membrane proteins [34], influence on ion channels and membrane pores [35], and others.
In conclusion, the results presented in this article suggest that the trimethyllead chloride (Met3PbCl) binding site is located outside the channel selectivity filter and that the inhibitory effect of the compound on SV channel activity probably results from Met3PbCl-induced disorder in compatibility between membrane lipids and membrane proteins. This suggestion is supported by three lines of evidence:
- • Met3PbCl only slightly decreased the unitary conductance of single channels;
- • the ratio between the current recorded with and without cytosolic Met3PbCl did not depend on the voltage in the range +60 ± 100 mV;
- • lipophilicity of organolead compounds.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
This work was supported by the Ministry of Science and Higher Education (Grant No 305336434).



Vous devez vous connecter pour continuer.
S'authentifier