1 Introduction
Choosing a new habitat that is suitable for survival and reproduction is an important step in the settlement process as it strongly influences the spatial distribution as well as dispersal strategies within populations [1]. This choice is influenced by the presence of conspecifics that can orient themselves to find suitable microhabitat conditions [2–4]. The most frequently mentioned benefits of settling near conspecifics include reduced search and settlement costs [5], use of conspecifics as indicators of habitat quality [6], and fitness increase associated with Allee effects (i.e. positive group effects) [7]. These benefits account for the conspecific attraction (or retention) found in various taxonomic groups throughout the animal kingdom [8].
The mechanisms used to assess the number of conspecifics in a given patch depend to a great extent on the type of information available to the animal [9]. In silk-spinning arthropods (e.g., spiders, caterpillars), silk can be used to select a specific location. Indeed, silken threads inform on the presence of conspecifics and thus are used as a social cue for group cohesion or for selection of a new place to live [10–15].
The two-spotted spider mite Tetranychus urticae is a widespread phytophagous mite that lives in groups [16]. Its rapid population growth (haplo-diploid way of reproduction, around 10 days per generation) [17], its polyphagy (more than 900 plant species, including field crops, greenhouse vegetables and ornamental plants) [18] and its effective dispersal strategies (i.e. ambulatory, aerial, silk-ball, phoresy) [19,20] have made this species a major pest. One of the characteristics of this mite is its abundant silk production due to a continuous silk deposit while walking [21,22]. Silk protects the mites against predators or competitors [16,23–25]. It protects eggs in extreme humid conditions [16]. It is a substrate for the sex pheromone [26,27] and physically supports locomotion and dispersal [20,21,28–30].
Although T. urticae individuals normally aggregate on their host plants and form large groups in greenhouses or laboratories, a tetranychid colony in the wild may contain only a few individuals [29]. When resources become scarce, mites need to find a new habitat suitable for living and reproducing. The selection of a new breeding site is done either alone or in small group (about five mites) thanks to their trail-following behaviour (i.e. ambulatory displacements) [29,30]. As living in a silk-covered habitat gives various advantages to the mites (i.e. locomotion, dispersal and protection), silken threads could influence individuals when choosing a new place to live (as shown in spiders) [12].
It is known that the presence of conspecifics–through the laying of silken threads–could have important implications in metapopulation dynamics by shaping the settlement behaviour of T. urticae [31]. However, until now, no population models on the growth and distribution patterns of T. urticae populations exhibiting this positive response to conspecific silk have been formulated and validated. Such models determine how silk influences individual choices during settlement, make comparisons between silk spinners belonging to a wide range of sociality levels, and finally make predictions to devise pest-control strategies.
In this study, we focus on a crucial phase during dispersal (i.e. settlement choice), as a function of the presence of silk produced by conspecifics. More precisely, we (i) compared the behaviour of five different strains of spider mites in response to the presence of conspecific silken threads. An adaptive trait such as preference for conspecific threads should have subjected to different selection pressure under different ecological conditions (e.g., temperature, predation, competition). Therefore, genetic differences in the preference are expected among T. urticae strains that were collected from different regions. We then (ii) tested whether adult females responded differently to increasing number of congeners. In this regard, mites were tested with different quantities of silk laid over a bean leaf. The perception of the amount of silken threads should allow mites to assess the density of conspecifics and hence the quality of a habitat when moving between leaves and host plants. An amplification process that results in collective settlement of spider mites requires individual's preference for larger amount of threads. Therefore, T. urticae female adults are expected to prefer leaf area with more threads. We quantified experimentally and theoretically how the probability of settling on the silk-covered area increases with the presence of silk on a microhabitat. Finally, we examined (iii) how the presence of silken threads influences larval settlement behaviour. We hypothesize that the most vulnerable stages such as larvae, which are the main beneficiaries of web protection, have developed perceptive abilities to recognize silken threads as adaptive decision-making for their settlement process. Immature stages should therefore prefer silken threads more strictly than female adults do.
2 Material and methods
2.1 Mite strain
Widely distributed species, such as T. urticae, can be made of an assemblage of strains [32,33] and exist in two different colour morphs: green and red. Strain divergence is often the case for phytophagous species living on a broad range of host plants [18] and host plant specializations have been repeatedly observed in T. urticae [34–36].
Behavioural differences do exist between strains of spider mites [32]. To test if there are any inter-strain differences in behaviour towards silken threads, five different strains of T. urticae, collected from different regions, were used in this study: URT (Louvain-la-Neuve, Belgium), LS-VL (Ghent, Belgium) [37], CHA (Louvain-la-Neuve, Belgium), CAR (Tunis, Tunisia) and JPN (Kyoto, Japan). URT was collected in October 2005 from greenhouse-grown banana leaves (Musa sp.), LS-VL was collected in October 2000 from garden-grown roses (Rosa sp.), CHA was collected in July 2009 from greenhouse-grown cannabis leaves (Cannabis sativa), CAR was collected in January 2006 from orchard-grown citrus leaves (Citrus sinensis) and JPN was collected in June 2004 from a rose garden (Rosa sp.). Individuals from the JPN strain were tested at two different times: in 2004 (after > 10 generations in the laboratory, called JPN2004) [29] and in 2009 (called JPN2009). Individuals tested in 2009 were therefore for 5 years in laboratory conditions. In laboratories (Belgium and Japan), strains were reared on beans (Phaseolus vulgaris L.), a preferred host plant [38]. Leaves were placed on moistened cotton in Petri dishes (85 mm in diameter, 13 mm deep). Stock colonies were maintained in a climate room at 26 °C, with a relative humidity of 50–60% and a photoperiod of L16:D8. All females used in this study were one-day-old adult females.
2.2 Influence of the presence of silken threads on the spatial location of mites: strain effect
Five different strains of young females were tested. The set-up described by [29,39] was used to examine whether one-day-old adult females preferred to settle on a ‘clean’ or a ‘silk-covered’ part of the bean leaf. A 10 × 20 mm flat piece of bean leaf (Fig. 1) was divided into two equally sized areas by a piece of wet paper (1 mm in width). To obtain a ‘silk-covered’ part, a female was introduced (a one-day-old ‘weaver’ female) onto a randomly selected side for 1 h (Fig. 1a). During this time, the weaver female either did not lay or produced very few feces or eggs. For our experiments, we exclusively used set-ups free of any external deposits, except the silk. The weaver female and the wet paper dividing the two sides were then removed. Five minutes later (when the leaf surface had dried), a new female (called a test female) was placed on the rectangular bean leaf section. Test females were released in the middle of the set-up where the wet paper (barrier) was positioned (lower edge of the bean square, Fig. 1b).
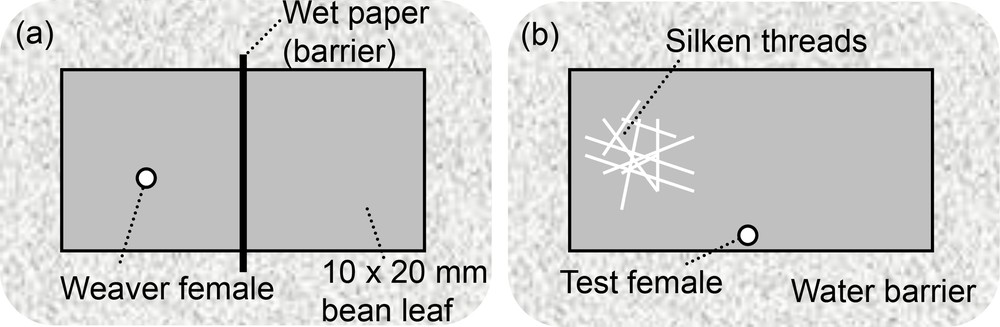
Experimental design for testing individual preferences for silken threads on a leaf surface.
For each replicate, ‘weaver’ and ‘test’ females were from the same strain. After 3 h, the leaf side (clean or silk-covered) on which the test female had settled was recorded (n = 57 for the strain LS-VL, n = 43 for the strain CAR, n = 30 for the strain CHA, n = 32 for the strain URT, n = 32 for the strain JPN2004 and n = 50 for JPN2009). Preliminary observations confirmed that females settled on a particular leaf position within that time (n = 30).
2.3 Influence of the quantity of silken threads on the spatial location of mites
The set-up described above was used (10 × 20 mm flat piece of bean leaf, Fig. 1) but we varied the amount of silken threads present on one side of the rectangular bean leaf. To obtain a larger quantity of silken threads on one side of the set-up, more ‘weaver’ females were introduced (one female on one side versus two, three, four, five and eight females on the other). After one hour on the bean leaf square, the ‘weaver’ females and the wet paper were removed. Then, a ‘test’ female was placed on the set-up and its choice was recorded after 3 h (n = 30 per silken thread quantity). Because the strain LS-VL was the least sensitive to silken threads in the first experiment (strain effect, point 2.2.), and hence seemed to have the largest potential for improving the preference for conspecific silk, the influence of the quantity of silk was only tested with individuals from this strain (‘weaver’ and ‘test’ females).
To obtain a more precise quantification of probabilities to observe that the settlement behaviour of mites depends on the number of congeners, the dynamics of choice of LS-VL mites during their settlement behaviour were analysed. Individuals were filmed during the three-hour experience (set-up with a silk-covered side versus a clean side, n = 19). Silk (from the silk-covered side) was spun by one ‘weaver’ female as described above (Section 2.2). A numeric camera (Panasonic WV-CP450/G) with a macroscopic lens (1:1.2/12.5–75) and a magnifying lens (1.2×) was focused on the bean leaf square and recorded female behaviour.
2.4 Influence of T. urticae stages on their response to silken threads
The response of larvae to silken threads was examined and compared with the response of females. In the same set-up (10 × 20 mm flat piece of bean leaf, Fig. 1) with one ‘weaver’ female versus one clean part, standardized larvae (i.e. < 24 h old) were tested. Because females from the strain LS-VL were the least sensitive to silk in the first experiment (strain effect, Section 2.2), only individuals from this specific strain (‘weaver’ females and ‘test’ larvae) were tested and their choices were recorded after 3 h (n = 32).
2.5 Data analysis
Experimental choices were analyzed using binomial tests, with the null hypothesis that an individual would choose each of the two sides with an equal probability of 0.5.
GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, California, USA; http://www.graphpad.com) was used to perform Chi-square and Fisher's exact probability tests. Curve fitting adjustments were performed with Matlab.
3 Results
3.1 Influence of the presence of silken threads on the spatial location of mites: strain effect
Fig. 2 shows the percentage of females that chose the ‘clean side’ (white box) or ‘the silk side’ (grey box) of the bean leaf set-up for the four strains tested. The spatial choices differed depending on the strain tested (χ2 = 9.996, DF = 4, P = 0.0405, Chi-square test comparing LS-VL, URT, CAR, JPN2004 and CHA strains). Indeed, individuals from the strain JPN2004 did not react similarly to individuals from the strains LS-VL and CAR (P < 0.0001 for both comparisons, Fisher's exact probability test). For LS-VL and CAR, females did not show a significant preference for a leaf side (P = 0.145 and P = 0.063 respectively; binomial test). For CHA and URT, females preferred to settle on the leaf side covered by silken threads (P = 0.008, and P = 0.004 respectively; binomial test). Likewise, for the Japanese strain, females preferred to settle on the leaf side covered by silken threads (P < 0.001 for JPN2004 and P = 0.032 for JPN2009). The results with the JPN strain also showed that more mite females settled in the silk part in 2004 (87.5%) compared with females tested in 2009 (64%). JPN2009 and JPN2004 did not react the same way to the presence of silken threads (P = 0.0225, Fisher's exact probability test comparing JPN2004 and JPN2009).
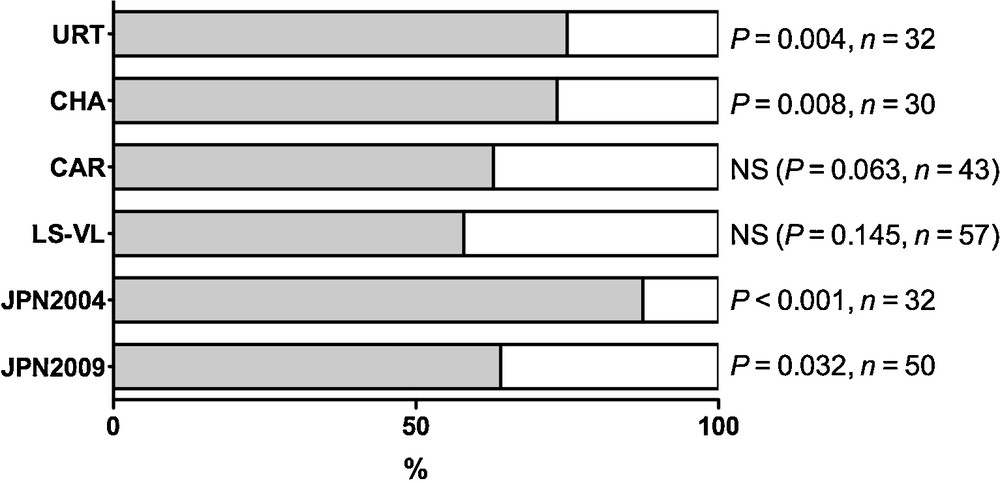
Choice of the silk part (grey box) of the bean leaf set-up. The choice of females differed according the strain tested (URT, CHA, CAR, LS-VL and JPN, binomial test).
3.2 Influence of the quantity of silken threads on the spatial location of mites
There was a trend of test females to settle on the bean leaf side covered with the larger quantity of silk spun by the higher number of weaver females. This preference was not statistically significant for small differences in silk quantities: one ‘weaver’ female versus two ‘weaver’ females, one versus three and one versus four (P = 0.123, P = 0.123 and P = 0.141 respectively; binomial tests; Fig. 3). However, for the situations comparing one versus five weavers and one versus eight weavers, test females significantly preferred to settle on the leaf side with more silken threads (P = 0.021 for both situations; binomial test; Fig. 3).

Influence of the quantity of silken threads on female choice (strain LS-VL). Females preferred to settle on a leaf side with silk (grey box) only significantly in the situations one versus five and one versus eight (binomial test).
Concerning the dynamics of choice during the settlement behaviour (data filmed), we observed that out of the 19 repetitions, 37% of mites (n = 7) had chosen directly (settled) one side of the set-up (i.e. no round trip between the two sides of the bean leaf). Within those 37%, 16% (n = 3) went directly to the silk side and 21% (n = 4) to the clean side. So mites went to a side randomly (they did not selected a specific side). The rest of the mites (63%, n = 12) did at least one round trip (the majority of them did one or two round trips) between the two sides.
This experimental distribution was rather well adjusted by the geometric distribution (Appendix A): P(i) = (1 − p)i−1p.
This theoretical distribution assumed that the probability of settlement (p) on one side after i – 1 round trips was constant. This preliminary estimation also suggests that the probability of settlement did not change over time.
The probability Oi of observing a mite on the side with i individuals at the end of the experiment (i.e. after 3 h) was well approximated by (for details of calculation, Appendix A):
| (1a) |
| (1b) |
In our experiments: Oi was the experimental proportion of mites on the i part (i.e. silk-covered part) of the set-up (i = 0, 2, 3, 4, 5, 8) at the end of the experience; M(i) was the probability of stopping on the i part and M(j) was the probability of stopping on the part j = 1. In our system, it became M(1).
Equation (1) was
Assuming that the individual tendency to stop on the part i increased with the amount of silk accumulated in that part [14]:
| (2a) |
| (2b) |
α expressed the proportionality between the number of weaver females (and therefore the quantity of silk on the substrate, i = 0, 2, 3, 4, 5, 8) and the probability of stopping in a side of the set-up. ɛ can be defined as the cooperativity between silken threads (= cumulated and cooperative effects of silken threads on the probability of stopping).
Therefore:
| (2c) |
| (2d) |
This equation (2c) fitted well with our experimental data (ɛ = 0.56, R2 = 0.94) and also with Oi (Fig. 4). Biologically, ɛ can be understood as the sensitivity of the response to change with the amount of silk. In our experiments, the probability of settling on the silk part i increased with the square root (ɛ = 0.56) of the quantity of silk present on the set-up.
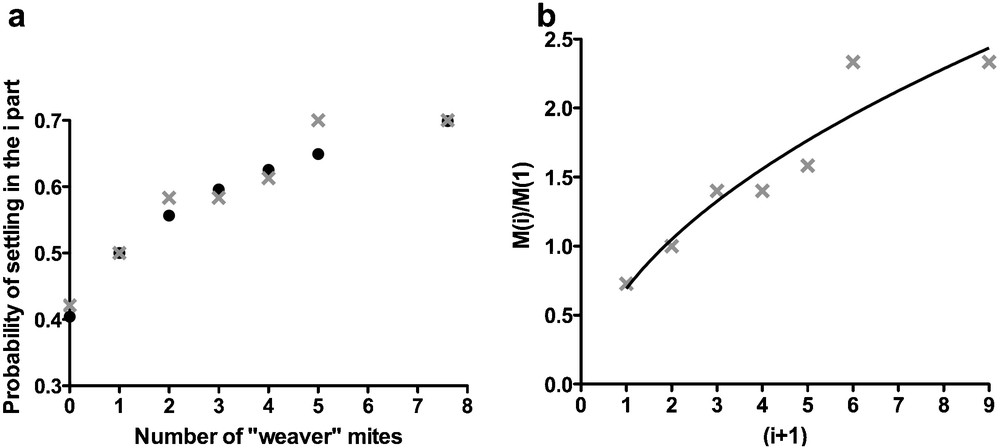
a: probability of settling (or proportion of mites) Oi on the silk part i of the set-up (LS-VL strain). Six different situations were tested with different silken thread quantities (i = 0, 2, 3, 4, 5, 8) (grey crosses = experimental data, black circles = theoretical data); b: our experimental data fitted to the equation (2,c): . The theoretical value of M(i)/M(1) for i = 1 was equal to 1 (i + 1 = 2). This theoretical reference was included in the fitting.
The probability of stopping after the first visit is (Appendix A and Fig. 5):
| (2e) |
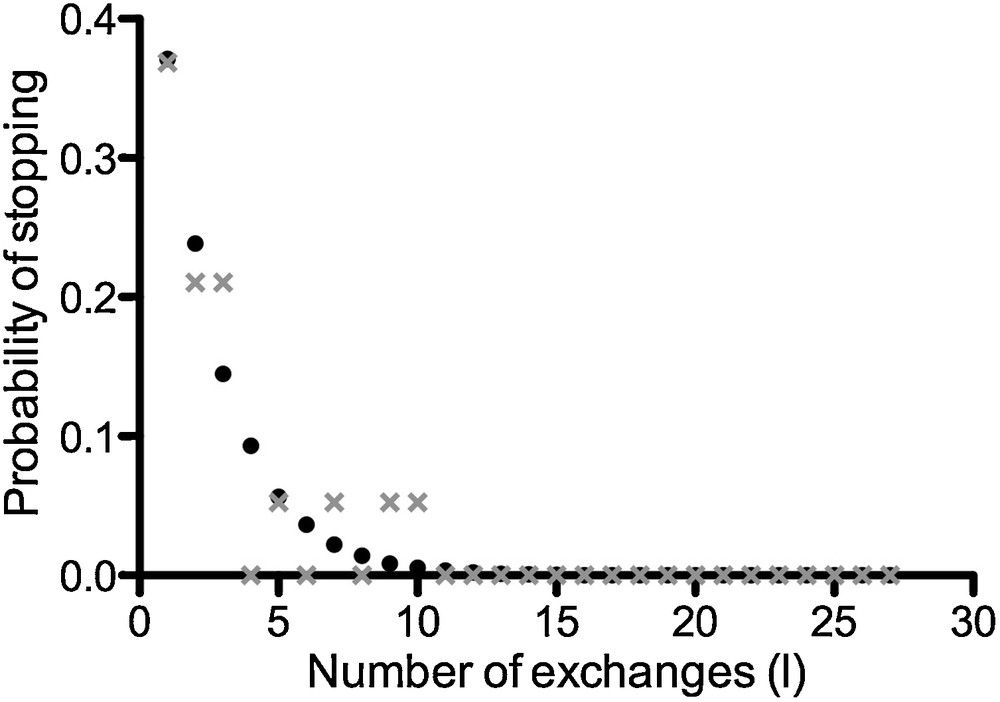
Distribution of the theoretical (black circles) and experimental (grey crosses) numbers of round trips (Equation (1,f); Appendix A). The theoretical and experimental mean numbers of exchanges were 1.67 (when i = 0 and j = 1 and without the outlier individual who has made 48 round trips).
If i = 0 and j = 1, the fraction of mites stopping after their first visit is 0.37, and therefore α ≈ 0.3.
3.3 Influence of the stage of T. urticae on the response to silken threads
Larvae from the strain LS-VL preferred to settle on the leaf side with silken threads (P = 0.025; binomial test). Indeed, 22 larvae chose ‘the silken side’, whereas only 10 chose the ‘clean side’. Adult females from the same strain (LS-VL) did not show a significant preference for a leaf side (P = 0.145; binomial test). There was no significant difference between responses of adults and larvae (P = 0.368; Fisher's exact test comparing the settlement decision between stages).
4 Discussion
Selecting a new place to live is crucial for individuals. Indeed, organisms may select a habitat with specific characteristics (e.g., physical habitat characteristics, biological constrains, presence of conspecifics) that enhance their own survival as well as that of the offspring [40–43].
Individuals of group-living species can benefit from the presence of conspecifics (i.e. Allee effect by gaining easier access to food, protection against predators, amelioration of the environment) [7,44–46]. However, being with conspecifics may also entail costs resulting from unavoidable competition among group members, deterioration of the habitat, and illness transmission [47]. There is therefore a trade-off when individuals choose to settle in a site already occupied by conspecifics. More importantly, the identification and use of reliable cues must have evolved to assess the quality of habitats. In this study, we did not quantify the optimal density resulting from this trade-off but we analysed the key factors used by individuals to evaluate the presence and number of conspecifics. In this context, we examined how an indirect indicator of the presence and number of conspecifics–the quantity of silk–affects the dynamics of settlement in a subsocial arthropod species, the two-spotted spider mite. Because settlers might benefit by living in aggregation [48,49], the by-product of the presence of conspecifics might serve as a social cue to assess the quality of environmental resources during the process of habitat selection.
Silken threads orient not only the dispersal [20,29], but also influence the spatial location of mites. Two steps are therefore initiated by silk: attraction and retention. This attraction and retention of individuals towards silk coupled to specific preference for some habitat features will lead to the formation of aggregations in areas suitable for this mite species. Moreover, the silk (especially the silk woven all around the host plant) provides protection against abiotic and biotic stress [16,23]. The more spider mites there are in one specific place, the more they are protected thanks to the size of both the group and the web. Silk retention would guarantee that the settling populations are large enough to overcome Allee effects. This may imply that colonies are not stabilized under a non-viable size. The use of silk becomes therefore a reliable cue, which has been selected to assess the occupation (presence of conspecifics), protection (presence of protective webs) and finally the quality of a habitat.
In natural conditions, the web of tetranychid mites protects them against adverse climate conditions such as wind or rain [50] and predators [51]. By contrast, in the laboratory, mites are raised in safe and controlled conditions with no exposure to rain or predators. One may expect that the average response of a ‘natural strain’ undergoing selection pressure to maintain their attraction towards silk is higher than the response of a strain reared for a long time in the laboratory. Indeed, in the field, individuals selecting an inappropriate habitat are rapidly eliminated. This is not the case in laboratory conditions where the pressure of selection is lower. The residence time in the laboratory could therefore lower the behavioural response of mites to the silk. Many examples in the literature highlight differences between lab and field strains of spider mites (e.g., acaricides resistance among field and lab strains) [52]. In our laboratory, LS-VL is the oldest strain: it was raised in the laboratory for 10 years compared to the three others kept in the laboratory between 1 and 5 years. Similarly, the response to silk of individuals from the same strain (JPN) but reared for longer in the laboratory (JPN2009, P = 0.032) is lower than individuals freshly harvested from the natural conditions (JPN2004, P < 0.001). A lower production of silken threads could be another cause for the lower response of the JPN2004 strain. The ‘recognition’ between a ‘silk-covered’ and a ‘clean’ side may be more difficult for a strain spinning less silken threads. Because silk production is costly in terms of fecundity [53], the mite population may have been selected to produce less silk under laboratory conditions where silk production is less necessary. In addition to the above possible directional selections during rearing periods, random genetic drifts may also be responsible for the inter-strain differences.
Settlement choices become more clear-cut as the amount of silk increases. From a selective point of view, this raises an interesting question: why some mites did not select the silk-covered part? Indeed, the choices are never uniform and seem to reach a plateau (around 75% of selection) when silk amount deposited by one female are compared to the amount laid by at least five females. The most plausible explanation is that the process of saturation (i.e. benefit of Allee effect) is offset by the cost of competition. To fully understand how silk is used as a social cue, further silk quantities (e.g. silk spun by 10 or 20 mites) should be tested. In such contexts, the silk as a social cue could vary as the group increases (i.e. silk quantity on the substrate). Second, this could be due to behavioural changes in larger groups (more than five females) where aggregated individuals reduce their movements and spin less than what resulted in a similar total amount of silken threads laid over the leaf. In group, mites could benefit from the silk spun by conspecifics [25,28,30]. This cooperative behaviour could lead to an individual reduction of silk production. The group size could therefore modulate the silk deposit. Third, even if the mite response increases with the amount of silk, individuals could show a maximal response to the chemical or/and physical properties of the silk for quantities deposited by more than five individuals. Finally, recorded data showed that 37% of mites went directly and randomly to one side, without any round trips. Within those 37%, 21% did settle on the clean side of the set-up. The plateau could thus be due partially to a part of the population, which went directly to the clean side in any conditions. Another interesting finding of our study is that test individuals seem unable to estimate the number of females who previously weaved on the substrate (i.e. individual cue left by weaver females). Indeed, in our experiments, different quantities of silk spun by an increased number of females (and therefore silk belonging to different origins) were tested. We did not obtain differences in situations 1 vs 2, 1 vs 3, 1 vs 4 (i.e. no preference for the side with more silk), suggesting that individuals seem to assess the quantity of silk (being attracted/retained to a certain silk threshold) rather than the number of weaver females.
The theoretical part of this study provides an insight into the growth and distribution patterns of T. urticae populations exhibiting this positive response to conspecific silk. Experimental and theoretical data from this study allow quantifying how the probability of settling on the silk-covered area increases with the presence of silk (and therefore indirectly with the presence of conspecifics) on a microhabitat. The agreement between the experiment and the model suggests that the response to silk by two-spotted spider mite females increases with the square root (ɛ = 0.56) of the quantity of silk. This response less than linear, probably due saturation effects discussed above, could prevent the emergence of overcrowded areas. Moreover, in such areas, competition could provoke a decrease in the retention effect of silk, and individuals would therefore have a lower probability of selecting a silk-covered area. A high value of ɛ could also lead to a strong amplification, which can induce suboptimal choices (as individuals will be trapped by a weak quantity of silk).
While we did not obtain a significant comparison between larvae and females, larvae seem to be more sensitive to the presence of silk. This is the first result suggesting differences in silk detection and response between stages of spider mites. This juvenile stage is often considered the most susceptible one within a mite population: larvae retain water less effectively than adults [54] and move more slowly than older mites, making them more vulnerable to predator attacks. Since the web preserves suitable conditions of relative humidity and temperature and provides a barrier against predators, efficient detection and response to silk seem essential for the adequate settlement/survival of larvae and finally for the growth of the colony. Further experiments should be done with ‘fresh strains’ to better understand how silk detection and response can differ between members of natural populations.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We thank Aina Astudillo Fernandez for her help and valuable advice on the data analysis. We are grateful to Lebdi Grissa Kaouthar, Thomas Van Leeuwen, Pierre Raulier, and Marc Migon, who supplied the different T. urticae strains used in our experiments. We also thank Fabrice Saffre for his useful comments on the manuscript. We are grateful to the team of UCL and the team of ULB. This work was supported by the ‘Fonds pour la formation à la recherche dans l’industrie et dans l’agriculture’ (FRIA) of Belgium. This paper is number BRC179 of the Biodiversity Research Centre (Université catholique de Louvain).
Appendix A
The model was based on the following assumptions:
- • at time t = 0 (time of introduction of the mite on the set-up), the mite chose randomly one side (0.5) of the bean leaf;
- • during each visit to one part, the mite had a probability M(i) to stop on the silk-covered part (i part) marked with silk of i mites (from 0 to 8 mites) and M(j) corresponded to the probability of stopping on the the silk-covered part with silk of j mites. M(i), M(j) supposed that the probability depended only on the local marking i or j (silk deposited by the ‘weaver’ females). M(0) was minimal and corresponded to an unmarked zone;
- • when the individual did not stop on one part then it moved on the other one.
The probability Oi to observe a mite at the end of the experiment (after 3 h) on the i part in competition with the j part was:
| (1,a) |
| (1,b) |
| (1,c) |
| (1,d) |
Therefore (1,a):
| (1,e) |
If M(i) and M(j) were < 1, M(i)M(j) was < M(i) + M(j) and (1,e) was well approximated by (2,a):
| (2,a) |
| (2,b) |
Our model predicted that the probability of stopping after l visits (the number of visits ‘l’ is equal to the number of round trips + 1):
| (1,f) |
If the difference between Mi and Mj was small, (1,g) was a good approximation of (1,f):
| (1,g) |
The theoretical mean number of exchanges was:
Fig. 5 shows the distribution of the theoretical and experimental number of round trips (Eq. (1,f)). When i = 0 and j = 1, the theoretical mean number of exchanges was 1.67. The experimental value was 1.67 (without the outlier) and 4.1 with the outlier individual. The outlier individual made 48 round trips during the observation time (i.e. 3 h). This value is much higher than those obtained with other tested individuals who have made in average of 1 or 2 round trips.


