1 Introduction
With the economic crisis in Benin, there has been an increase in the number of gardens as well as in the number of cultivated urban areas [1]. But the poor farming practice regulations pose also a threat to the health safety of garden products [2]. In fact, in Benin, market constraints, land and parasitic pressures force farmers to practice intensive phytosanitary treatments. It is well known that some farming practices are responsible for the introduction of traces of metal in gardening soils. Products intended to improve the physicochemical properties of soils are often richer in heavy metals than the soil itself [3]. Among them, we can cite fungicides [4], fertilizers, and composts [5]. Otherwise, water for market garden irrigation is a limiting factor for gardening, and many gardens are compelled to remain in the vicinity of swamps, areas that are used for dumping and are therefore sources of water as well as soil and vegetable contamination by xenobiotics [2]. This study attempts to establish that, among soils, use of phytosanitary products, location of the gardening site (rural or urban), irrigation water quality is the first factor responsible for the contamination of market garden products by heavy metals. In this study, we also make an assessment of health risks associated with the consumption of vegetables contaminated by toxic metals in the watering water.
2 Materials and methods
Two sites in Cotonou and another in Aplahoué, about 150 km far from Cotonou, have been our study framework (Fig. 1).
Map of the study area (Cotonou [Houéyiho + Godomey] and Aplahoué).
On two of the sites located in downtown Cotonou, Houéyiho (H) and Godomey (G), irrigation water comes from neighbouring swamps, and on the Aplahoue site (A), it comes from a small river streaming through cotton and crop fields.
2.1 Processing and analysis
Composite samples of eight different vegetables Amaranthus hybridus (amaranth), Daucus carota (carrot), Lactuca sativa (lettuce), Spinacia oleracea (spinach), Allium cepa (green onion), Brassica oleracea (cabbage), Corchorus olitorius (fiddle), Salanum macrocarpum (nightshade) were collected and treated with or without phytosanitary products, whereas water and soil samples were collected during the same periods on three major gardening sites in Benin.
The numbers of soils, water and vegetables samples are presented in Table 1.
Numbers of soils, water and vegetables samples.
| Sites | Godomey | Houéyiho | Aplahoué |
| Number of soils samples | 7 | 7 | 7 |
| Number of water samples | 7 | 7 | 7 |
| Number of vegetable samples | 8 | 8 | 8 |
The samples underwent the necessary treatment before they are tested for lead (Pb), cadmium (Cd) and arsenic (As) by atomic absorption spectrophotometry. First, all samples are cleaned and stored initially in the oven, first at 50 °C for 12 hours, then the temperature is increased to 120 °C and maintained for 24 hours. But water samples are left at 4 °C. After grinding in a mortar, 2 g of each lyophilized sample is soaked in a mixture of hydrogen peroxide (H2O2) and nitric acid for 24 hours, and then undergo an acid digestion in a digester (sand bath at 125 °C for 2 hours). The digest was recovered in a 100-ml flask and completed with distilled water to the mark. It is then filtered and stored at 4 °C until analysis for the extraction of heavy metals by atomic absorption spectrophotometry (Thermo Orion corrected Solaar S2), in the Laboratory of Management, Treatment and Recovery of Wastes of the University of Lomé in Togo according to the method described in [6] and [7] by electro-thermal atomic absorption spectrophotometry. The analysis was performed by interpolation on calibration curves obtained with standard solutions. The results, displayed by software Solaar S2 on the computer screen connected to the spectrophotometer, are expressed in mg/kg or mg/l or ppm. The corrected concentration is obtained by the formula:
The health risk assessment of Cd was performed using the standardized approach proposed by [8]. DED, the daily exposure dose, is expressed in μg/kg/day and calculated by crossing data of consumption of food contaminated with metal by the averaged poison content measured in the sample (Table 5). For the Cd-contaminated Amaranthus hybridus consumed by people, we have:
QD is the quotient of danger.
Statistical treatment is used for a pairwise and one-against-all comparison, using Student's t-test P (T > t) = 0.05.
3 Results
The results are given in ppm (mg/kg) and are compared in Tables 2–4 as mean ± standard deviation, and in Figs. 2–5 showed the presence of toxic metals in irrigation water, soils and vegetables on the three sites with some significant differences between the results. In addition, the risk assessment is summarized in Table 5 and a daily exposure dose of 8.05 μg/kg/day is shown, whereas the amount suggested is 1 μg/kg/day. The hazard quotient or quotient of danger (QD) therefore is shown to be 8.05.
Contamination of vegetables by toxic metals at the three sites.
| Lead (ppm) | Cadmium (ppm) | Arsenic (ppm) | |||||||
| Norms | 0.3 [9] | 0.05 [10] | 0.1 [10] | ||||||
| Sites | G | H | A | G | H | A | G | H | A |
| L. sativa | 4.84 | 3.10 | 6.03 | 0.89 | 0.63 | 0.07 | 237.62 | 260.48 | 315.13 |
| A. hybridus | 5.01 | 3.73 | 5.92 | 0.93 | 5.13 | 0.32 | 171.63 | 259.92 | 302.15 |
| S. macrocarpum | 2.52 | 3.38 | 5.99 | 0.82 | 0.64 | 0.81 | 282.73 | 316.28 | 219.19 |
| B. oleracea | 6.69 | 3.12 | 6.32 | 1.73 | 0.55 | 0.79 | 230.81 | 325.30 | 195.08 |
| D. carota | 1.06 | 1.14 | 2.37 | 1.22 | 0.72 | 0.53 | 300.51 | 251.45 | 270.18 |
| C. olitorius | 3.5 | 4.76 | 5.52 | 0.91 | 1.17 | 1.10 | 271.76 | 241.41 | 255.12 |
| A. cepa | 2.85 | 3.36 | 4.80 | 0.43 | 0.26 | 0.34 | 358.67 | 323.52 | 196.4 |
| S. oleracea | 4.08 | 3.46 | 4.95 | 0.34 | 0.52 | 0.75 | 231.84 | 204.01 | 232.02 |
| Average | 3.82 | 3.25 | 5.24 | 0.91 | 1.20 | 0.59 | 260.70 | 272.80 | 248.16 |
| ± SD | ± 1.73 | ± 1.00a | ± 0.88b | ± 0.43 | ± 1.60 | ± 0.33 | ± 56.04 | ± 44.25 | ± 45.56 |
| C. factors | 12.73 | 10.86 | 17.46 | 18.2 | 24 | 11.8 | 2607 | 2728 | 2481.6 |
Soil contamination by toxic metals at the three sites.
| Lead (ppm) | Cadmium (ppm) | Arsenic (ppm) | |||||||
| Norms [11] 85 | 0.80 | 29 | |||||||
| Sites | G | H | A | G | H | A | G | H | A |
| 9.527 | 49.75 | 4.62 | 0.74 | 0.09 | 0.63 | 151.2 | 138.79 | 112.34 | |
| 9.956 | 47.92 | 4.62 | 0.81 | 0.04 | 0.72 | 165.1 | 142.58 | 107.43 | |
| 11.24 | 50.72 | 5.02 | 0.74 | 0.03 | 0.56 | 158.8 | 151.33 | 115.55 | |
| Soils | 10.12 | 50.20 | 5.10 | 0.83 | 0.08 | 0.93 | 162.0 | 145.94 | 104.76 |
| 9.75 | 48.51 | 4.92 | 0.75 | 0.04 | 0.89 | 160.8 | 139.84 | 92.69 | |
| 10.81 | 51.67 | 4.72 | 0.77 | 0.03 | 1.10 | 153.3 | 137.91 | 92.22 | |
| 9.90 | 49.10 | 4.91 | 0.64 | 0.04 | 0.68 | 163.2 | 153.34 | 115.28 | |
| Average | 10.18 | 49.7 | 4.84 | 0.75 | 0.05 | 0.78 | 159.20 | 144.24 | 105.75 |
| ± S D | ± 0.61 a | ± 1.29b | ± 0.19a | ± 0.06a | ± 0.02b | ± 0.19a | ± 5.16a | ± 6.16 a | ± 9.89 a |
| C. factors | 0.11 | 0.58 | 0.05 | 0.98 | 0.06 | 0.98 | 5.48 | 4.97 | 3.64 |
Contamination of irrigation water by toxic metals at the three sites.
| Lead (ppm) | Cadmium (ppm) | Arsenic (ppm) | |||||||
| Norms [12] 0.4 | 0.21 | 0.1 | |||||||
| Sites | G | H | A | G | H | A | G | H | A |
| 4.81 | 2.85 | 2.24 | 1.65 | 0.79 | 0.92 | 583.11 | 335.41 | 281.02 | |
| 5.07 | 3.51 | 3.10 | 1.45 | 0.82 | 0.62 | 592.82 | 344.52 | 280.51 | |
| 3.97 | 3.52 | 2.09 | 1.43 | 0.79 | 0.74 | 571.81 | 353.12 | 269.61 | |
| Water | 4.96 | 3.45 | 2.51 | 1.16 | 0.92 | 0.90 | 542.51 | 343.94 | 282.41 |
| 5.41 | 3.52 | 3.50 | 1.80 | 0.72 | 0.83 | 502.41 | 342.14 | 265.71 | |
| 5.71 | 3.54 | 2.81 | 1.62 | 0.83 | 0.65 | 573.71 | 336.54 | 275.91 | |
| 5.54 | 3.35 | 2.41 | 1.63 | 0.99 | 0.85 | 572.93 | 356.13 | 285.90 | |
| Average | 5.06 | 3.39 | 2.66 | 1.53 | 0.83 | 0.78 | 562.75 | 344.54 | 277.29 |
| ± SD | ± 0.58b | ± 0.24a | ± 0.50a | ± 0.20b | ± 0.09a | ± 0.11a | ± 30.75b | ± 7.76a | ± 7.29a |
| C. factors | 12.67 | 8.47 | 6.67 | 7.33 | 4 | 37.6 | 5626.6 | 3445.5 | 2773.0 |
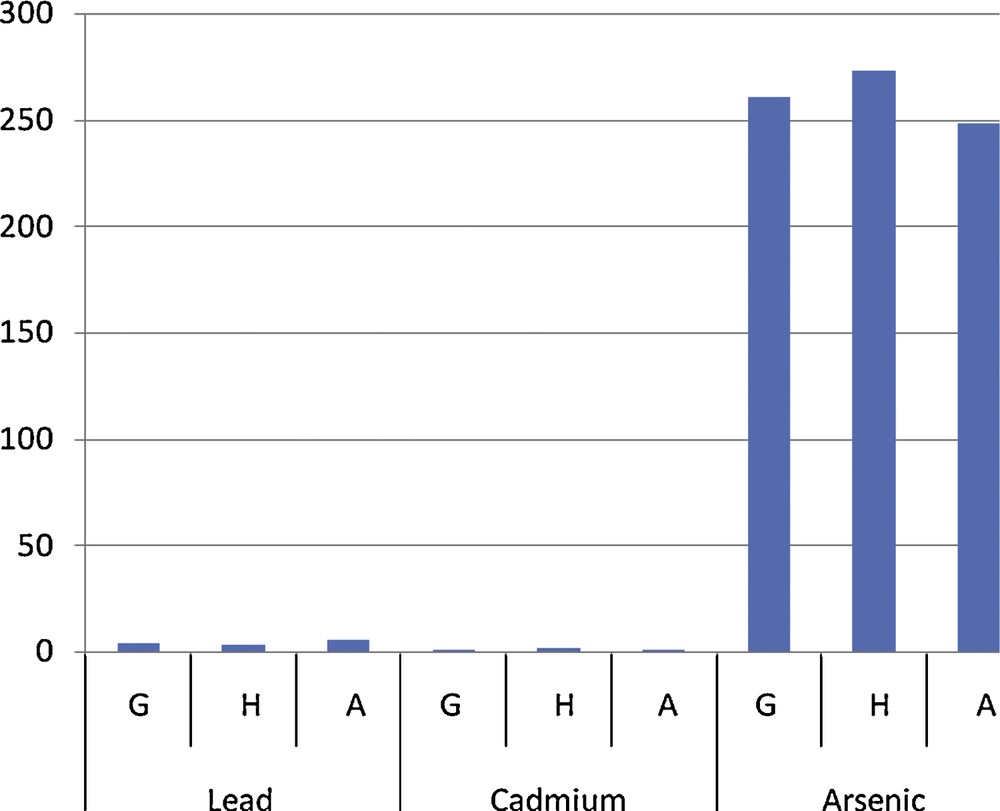
Vegetable contamination by site.
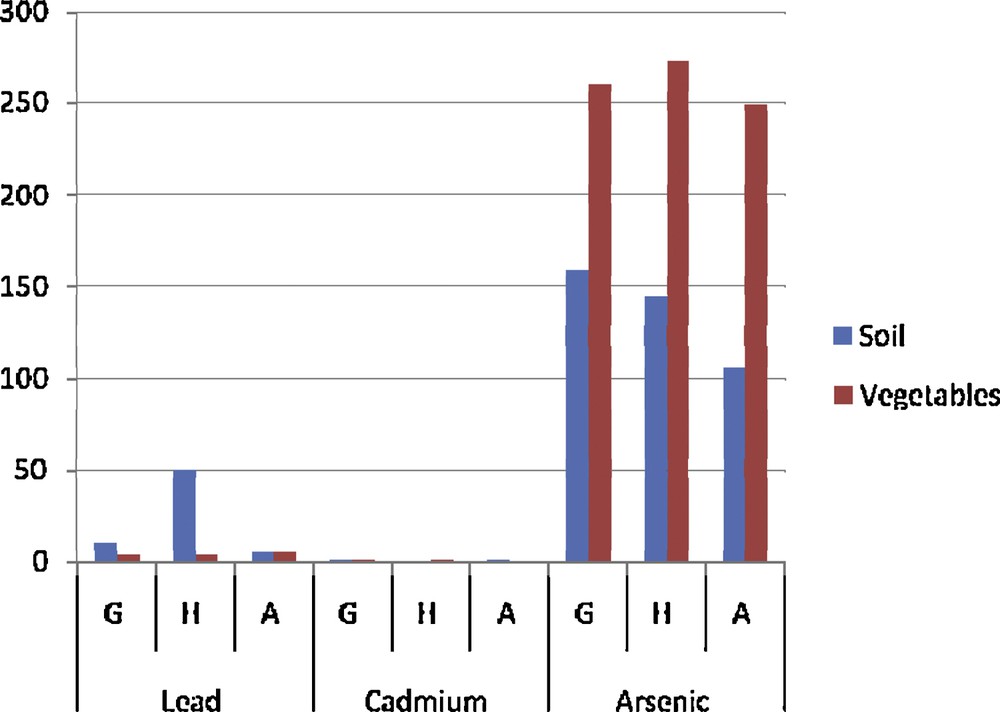
Comparison between vegetable and soil contamination.
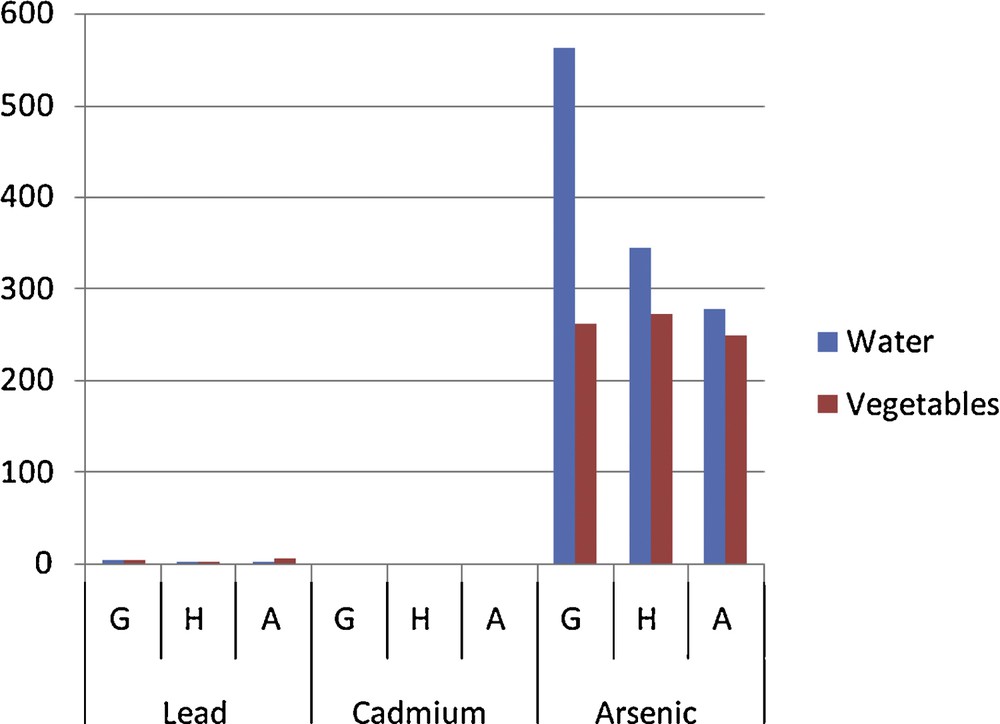
Comparison between vegetable (légumes) and water (eaux) contamination.
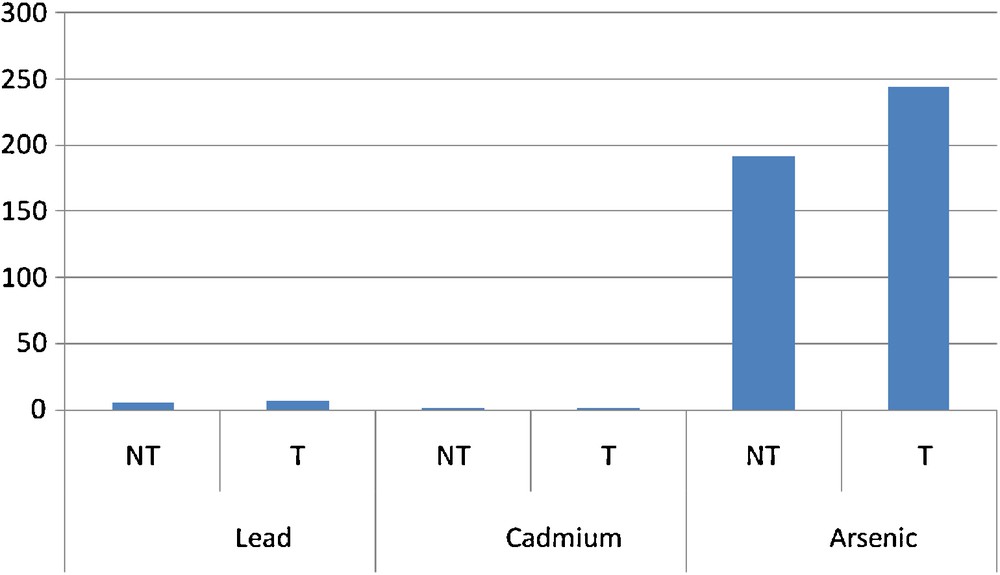
Contamination of pesticide-treated vegetables (T) compared to that of untreated ones (NT).
4 Discussion
An analysis of the results revealed that multiple sources of contamination by toxic pollutants of the gardening soil and water from swamps and the river crossing agricultural fields affect the sanitary quality of the grown vegetables (Table 2). Comparison of the contamination level of all vegetables on the gardening sites showed no significant difference (P > 0.05) from one site to the other (Fig. 2). But apart from lead, the lowest levels were recorded on the rural site of Aplahoué (Fig. 2). We might think immediately that the polluted atmosphere of the city contaminates the vegetables the most. The results also showed that the irrigation water for irrigation of the vegetables as well as the soil on which they are grown is contaminated by toxic metals (Tables 3 and 4). By comparing the levels of metals in vegetables and in the soil (Fig. 3), we notice that an arsenic and cadmium bioaccumulation phenomenon has taken place (their contents in vegetables exceeded their level in the soil). This is not the case with lead levels in soil, which exceed those in vegetables. In fact, Cd and As are highly soluble and thus are more bioavailable to plants [15]. In this case, their concentrations in the soil solution or irrigation water are not significantly different (P > 0.05) from those found in the vegetables [16] because these plants grow faster and are quickly harvested. Apart from the only case of As level on the Godomey site, this hypothesis is verified (Fig. 4). It is not the case with lead because it is less soluble [17]. If it is found abundantly in vegetables; it probably comes from different origins. Mench et al. [18] believe that the Pb found in vegetables is especially atmospheric; this is the reason why smaller amounts were found in samples from the rural site in Aplahoué and these samples are apparently less polluted. Leafy vegetables will be more exposed than non-leafy ones: lead levels in Daucus carota and in Allium cepa, non-leafy vegetables, are among the lowest; this also proves this hypothesis (Table 2). However, this result does not allow us to conclude about the origin of vegetable contamination. As for the soil, the concentrations of toxic metals appear to be like ancillary data for plants harvested early, because the comparison between vegetables and soils (Fig. 3) showed significant differences (P < 0.05). Therefore, the contamination of the vegetables does not come only from the ground. On the contrary, the levels of toxic metals in irrigation water seem to influence the contamination of the vegetables for two over three sites; when we compare vegetables and water (Fig. 4), there is no significant difference (P > 0.05) between the levels in water of Cd on the one hand and of as the other. This is justified because the ionic forms of these two metals are very soluble in the soil solution or the irrigation water that can be bioavailable for vegetables. An analysis of the soil has shown that its level of toxic metals is incidental. However, some results contradicted this argument, which lets us assume that the contamination of vegetables does not depend exclusively on the toxic levels in the soil or in the irrigation water. The contamination of S. macrocarpum whether pesticides are used or not (Fig. 4) showed a significant difference (p < 0.05) increase for lead and arsenic in treated vegetables (T) compared to untreated ones (NT). Therefore, the contamination of vegetables depends on the use of pesticides. Studies have highlighted other factors involved in the bioaccumulation of toxic metals by living organisms: metal speciation, the intrinsic nature of the organism bioaccumulative, biotic and physicochemical [19]. Finally, the content of Cd in vegetable (5.13 mg/kg) was used to calculate (Table 5) the daily exposure dose (EDI), taking into account the total exposure and the hazard quotient (QD). It appeared that the consumption of 100 g of this vegetable exposes an adult of 65 kg to 523 μg of Cd per day, when he only accepts a maximum of 65 μg. So, there is an annual accumulation of more than 167 g of Cd in the body of the consumer, hence the chronic risk whose magnitude is, for the moment, unknown. The QD greater than 1 confirms this observation.
5 Conclusion
This study showed that a contamination of vegetables by toxic metals depends on the soil, water, the environment of the gardening site and the use or not of pesticides. Many authors have highlighted that many factors – such as water – are primary responsible for the contamination of vegetables by pollutants. Our results prove this observation. In any case, due to the imminent risk of toxicity, the adoption of reasonable behaviour and the development of sustainable agriculture are needed to combine food security, economic development, environmental protection and public health.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


