1 Introduction
The introduction of exotic species is rightly considered as one of the most common causes of biodiversity losses worldwide [1–3], because they are a potential threat to native species through predation, grazing, competition, parasitism, disease, hybridization or habitat alteration [4–9]. However, this threat varies widely depending on both the impacted taxons (they only rank the 5th rank in birds threat worldwide [10]) and on sites. For instance, 88% of bird extinctions have occurred on islands (mainly endemic species that become extinct due to mammal predation [11–16]) even though most bird species (> 80%) live on continents. Data on the real impact of exotic species on continental fauna is more poorly documented, or minor compared to other causes of biodiversity losses [17]. On the other hand, most introduced species do not impact native species or ecosystems [18,19]; risk is generally related both to the alien species’ own characteristics [20–22] and the host environment (invasibility [23], lack of native predators [24]). Additionally, the success of introduced species is frequently related to previous perturbations in ecosystems [25–34]. Didham et al. [35] warned against the tendency of many studies to focus on single-factor explanations for extinction threat, without considering the full complexity of the synergies between hosts of other extrinsic drivers of the population decline of native species. Thus the contribution of aliens to the decline of native species is still controversial, particularly among birds, for whom it is generally being considered either as minor [36–41], or as major [13,42,43]. In addition, while native species can also be invasive [44], some authors consider only alien species as dangerous [45], particularly among birds, although they only represent 1.6% of the 10,771 alien species recorded in Europe [46]. Very few bird species have had any proven impact on continental species or ecosystems [39,41,47], contrary to mammals (81% of the invasive species that are a threat to birds around the world) or plants (26%) [10,48]. Despite all these facts, the progress of the real threat to native species by a few aliens is often generalised to all introduced species, particularly through the media. According to [49,50], alien species are more and more perceived as “public enemy number one” by many naturalists and managers, even without any data on their real impact on native species or habitats (only 16% of the 153 French alien vertebrate species have been partially studied [51]). Thus, following an often inappropriate application of the Precautionary Principle which needs a potential serious risk of threat [52,53], their immediate destruction is frequently demanded before it is too late. Moreover, the definition of biological invasion, and its two main criteria (geography and impact) are very confused and debatable [54–60], and differ widely between species or taxons. Above all, administrative boundary criteria (country, and even administrative subdivisions such as in [51]), are only or mainly taken into account to define species as native rather than using ecozones. Such reckless and debatable bioxenophobia (see review in [61]), based on the myth of stable and pure fauna or flora, has however been stigmatised by several authors [3,49,50,59,62–75], for whom the real impact must be studied and demonstrated on a case-by-case basis. This balanced point of view, which also recognises the benefits of some introduced species [3,76–78], has already been recommended by the 1992 Rio Convention on Biological Diversity. Moreover, listing a prey in a diet is not sufficient to classify an alien species as having an impact as a competitor or predator; a true demonstration of the impact (or at least a credible potential impact) on the dynamics of the native population is needed. However, up to now, few studies have shown that the available evidence about the impacts of invasive species is often poor or overestimated [40,41,79–81]. Some authors still consider that all introduced species should be considered as potential invaders, since many lie dormant for years or decades [82,83], but practically all of their examples concern plant invasion, never birds.
The case of the Sacred ibis (Threskiornis aethiopicus Latham) is particularly representative and symbolic of such recent debates. The bird originates from Africa and the southern part of the Western Palaearctic where it was locally threatened (in Iraq) or had even disappeared (in Egypt after 1850). Altogether its number gradually decreases worldwide [84–86] so that the species is listed in Annex II of the Bern Convention asking for the protection of its native populations, but as a “Little concern species” because of its very wide distribution. This nomadic species can move over distances up to 1,500 km in Africa depending on the seasons [87], and some birds were able to reach the Caspian and Black seas [88]. Since the 1970s, many observations of vagrant birds have been made in southern Europe (Spain, Portugal, France, Italy and Greece), and more rarely up to the Netherlands, Belgium, Germany and Poland [89]. However, the presence of escaped birds from zoos has made it impossible to distinguish them from the possible natural movements of ibises from their African range due to climatic warming and the increased protection of wading birds and wetlands, as seen previously for Cattle egret or Little egret [90]. In Brittany, a feral population escaped from the Branféré zoo and the numbers increased up to about 3000 birds between 1975 and 2004, without any threat to other bird species [91]. The first few isolated predations cases on tern eggs were reported under debatable conditions in 2004 [92], and predation on herons was suspected on the Mediterranean coast where some Sacred ibises had escaped from the Sijean zoo [93]. While admitting the knowledge gap about the impact of these feral populations, [89] and [94] depicted the species as potentially very dangerous for rare birds and possibly for health. They mainly based their opinion on the global threat to biodiversity represented by all kind of introduced opportunistic species around the world whatever the situation [95]. These papers, prolonged by the recent inclusion of the species in the list of the “One hundred Most Invasive Alien Species” in Europe by the same authors [46], resulted in an AEWA motion in 2008, asking France to eradicate the species, followed by the shooting of almost 5000 Sacred ibises in Brittany between 2007and 2010 (Office National Chasse Faune Sauvage pers. com.). This generated a large debate in France because the Sacred ibis’ beauty, its name, as well as its historic role in human civilisation, play a special role in positive public perception. The example of Brittany aroused suspicion about it, not only in new introduction areas such as the Everglades [96], but also in its native area where predation on birds is now stigmatised [97]. The species was even considered by Kumschick et al. [98,99] to have as severe an impact as the most effective alien mammals in Europe by referring to Clergeau and Yésou [95], but Strubbe et al. [41] rejected this conclusion by re-analysing the data.
The objective of the present paper is to answer the two following questions by means of a long-term study and by examining the worldwide literature: firstly, is the Sacred ibis a real threat for the population dynamics of rare birds, and secondly, is its population increase related to its own characteristics or to local anthropogenic ecosystem perturbations, such as water level changes, refuse dumps or invasive prey species? This example could be of relevance to many other cases of introduced bird species that are depicted as vermin in spite of the scarce local data on their real impact on native species [45,46,51,98–101], in contrast to numerous studies about other taxons [16,102,103].
2 Materials and methods
2.1 Studied population
The Sacred ibis has a large breeding range in Africa, from South Africa to Mauritania, but with scattered populations in periodically favourable wetlands, with a mean density of only one to two birds per kilometre square [86]. It generally settles in small colonies ranging between four and 40 nests, except on recent dams in intensive farmland in South Africa (105–170 nests [104]). Record numbers of 2000 nests along with other wading birds in large wetlands [105] seem to be exceptional. It is also present in Madagascar and the Aldabra islands, and is threatened in Iraq. In Brittany, the Branféré park imported about 20 Sacred ibises from Africa or zoos between 1975 and 1980. The original population inbred on site, and reached 280 individuals in 1991, and 350 in 1993 [91]. In 1993 local authorities compelled the zoo to catch these free birds, but the disturbance frightened them away. They then settled among two mixed colonies of Spoonbills and/or herons in the Grand-Lieu Lake (Loire Atlantique) and the Gulf of Morbihan [91]. The total breeding population increased relatively slowly between 2000 and 2004, fluctuating between 300 and 600 pairs, and it rapidly increased thereafter, but without any extension of the breeding range.
2.2 Data
We counted the numbers of occupied nests in most of the colonies every year (Grand-Lieu 1991–2009, Brière 1999–2004, St-Nicolas 2007, Bacchus 2006–2007, Govihan 2002–2004, Reno 2007–2009, and Bilho in 2006 and 2007), or used counts made by local observers: Bilho in 2004, 2005 and 2008 (Office National Chasse Faune Sauvage), and small and often sporadic colonies of gulf of Morbihan, Goulaine and Erdre.
The diet of Sacred ibises was mainly studied in the mother colony of Grand-Lieu Lake between 1994 and 2009, immediately after the colony settled in 1993 and before deterring management, and occasionally in four short-lived daughter colonies: Besné (2000–2001) situated in the Brière marshes, Bacchus sea island (2006), Bilho and St-Nicolas islands (2007) located in the Loire estuary (Fig. 1). Sacred ibises that bred at Grand-Lieu fed mainly in the wetlands surrounding the lake (4000 ha of variably flooded marsh grasslands, and secondarily in clearings in reed beds, channels or river banks), but about 30,000 ha of wetlands were also available within a radius of about 25 km extending to the Loire estuary and the Bourgneuf marshes. They also occasionally fed in slurry pits around the lake between 1998 and 2007, and intensively on a rubbish dump that was closed at the end of 2008, situated 6 km from the lake (La Marne). Sacred ibises breeding in the other three studied colonies mainly fed on the Brière peat bog marshes (19,000 ha), composed of a mosaic of reed beds, ponds, channels and marsh grasslands, but some birds from the Bacchus colony also exploited moist grasslands or farm lands. Sacred ibises that fed in Brière also irregularly used the rubbish dumps of St-Nazaire (Cuneix) before it was closed in December 2006; however this was mainly in winter, similarly to the rubbish dump of St-Michel-Chef-Chef which closed in 2008.
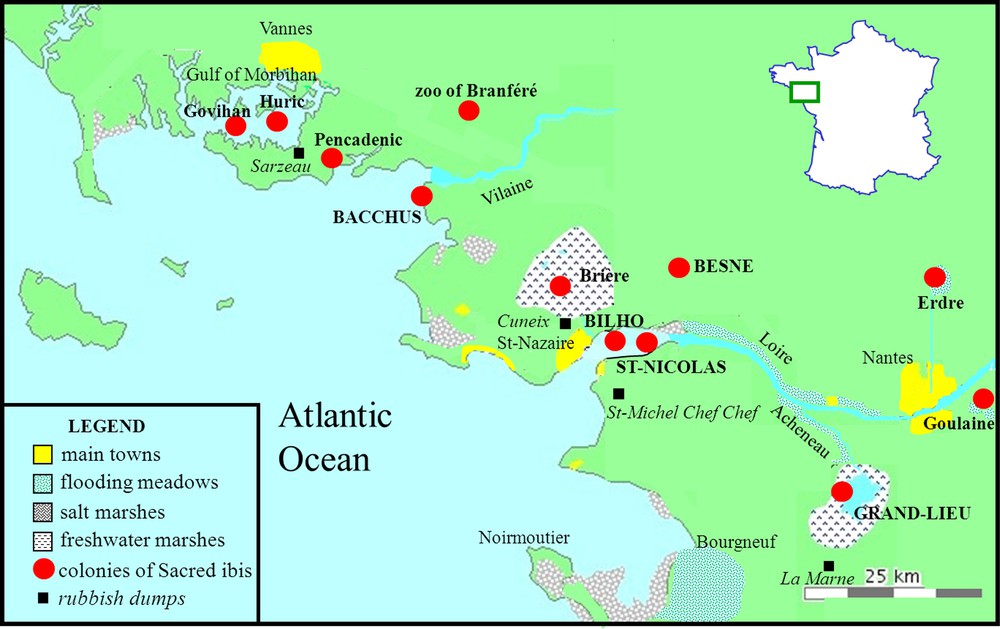
Distribution of the main Sacred ibis colonies and of refuse dumps in Brittany (1993–2009).
The diet of Sacred ibises was studied from spontaneous regurgitations in the nests (one per nestling recently fed by adults) during manipulation of nestlings for ringing or during colony censuses in May–June and more occasionally at the beginning of July (n = 380 at Grand-Lieu, 96 at Bilho, 50 at St-Nicolas, 20 at Bacchus). Each prey or each piece of meat (poultry, pork, beef, etc.) was counted as one food item. Vegetation (aquatic plants, algae, and some seeds including maize) was negligible in volume, so it was not taken into account due to the difficulty in separating it into items and because it was probably ingested partly accidentally along with prey. In spring 2002, 3520 cumulated Sacred ibises were directly observed foraging individually or in groups in the feeding areas within and around Grand-Lieu over a period of two months (160 hours from 22nd May to 22nd July), in 10 habitual feeding sites around the lake and four outside the site (Acheneau valley, Loire estuary, Bourgneuf marshes and La Marne dump). Foraging ibises were discovered by exploring all the potential feeding areas by road and by following Sacred ibis flights from the colony. All these marshes are natural flat meadows, generally without any trees, with a water level that decreases from April to the end of May (Grand-Lieu, Acheneau) or unflooded meadows in spring (Bourgneuf, Loire estuary). Most of the meadows are used by cattle.
At first, diet was analysed combining samples collected in all colonies over the whole 1994–2009 period, and then diet composition was compared among colonies. Annual fluctuations were only described for the permanent colony of Grand-Lieu. Data obtained from direct observations in the feeding area were only used for comparison. A Principal Component Analysis (PCA) and a Hierarchical Ascendant Classification with aggregation according to variance with Chi2 Ward distance were performed on diet composition between years. Relationships between Sacred ibises diet, population size (number of occupied nests counted each year in May–June), water level (mean value of average daily levels in flooded meadows in April and May) or Red swamp crayfish abundance at Grand-Lieu Lake (annual weight of bycatch captures in the fyke nets of professional fishermen in the lake) were analysed using Spearman's tests.
3 Results
3.1 Colony dynamics
All of the colonies fluctuated widely and most of them were temporary. The mother colony of Grand-Lieu fluctuated between only 45 and 150 pairs before 2000 and even a decrease to 34 pairs in 1996; then it fluctuated between 105 and 289 pairs between 2001 and 2006, with two intermediate decreases, followed by a sharp increase to 815 pairs from 2007 to 2009 (Fig. 2). In or near the Gulf of Morbihan, the breeding population only fluctuated between 10 and 190 pairs distributed into generally small colonies between 1994 and 2009, with several intermediate decreases (1998, 2001, 2004 and 2006). Between Grand-Lieu Lake and the Gulf of Morbihan, the colonies that fed mainly in the Brière marshes (Besné, Brière, Bilho, St-Nicolas, Bacchus) only existed for two or three years each. In the three latter sites, their short lifetime was mainly due to deterring management.
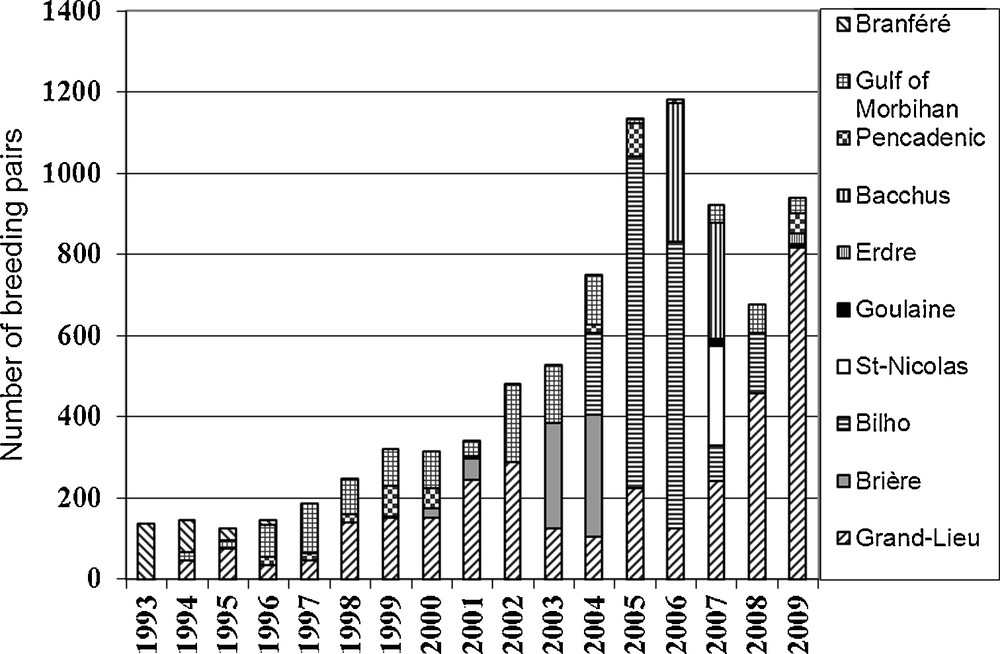
Sacred ibis colony sizes (numbers of breeding pairs) as related to years in Brittany (colonies of the gulf of Morbihan are grouped).
3.2 Global diet including all colonies
A total of 4744 food items were collected from the regurgitations of Sacred ibises from five colonies in Brittany (Grand-Lieu, Bilho, St-Nicolas, Bacchus and secondarily Besné). In numbers the most abundant prey were: Red swamp crayfish Procambarus clarkii (41%), then Syrphidae Eristalis sp. larvae (24%), Orthoptera (10%), aquatic insects including Dytiscidae, Heteroptera and Odonatidae (11%), scraps of meat (8%), Tipulidae (2%), shrimps Crangon crangon and Palaemonetes varians (2%), Opiliones and fish (about 1% each), and a few voles and molluscs. No birds were found.
3.3 Differences between colonies
There were large differences between colonies even within the same years 2006–2007, however the differences were smaller between Grand-Lieu and Bacchus (X2 = 28.91) than between Grand-Lieu and Bilho-St-Nicolas (X2 = 633.95) or between Bacchus and Bilho-St-Nicolas, X2 = 797.91 (P < 0.00001 for the three comparisons). At Grand-Lieu (n = 3382), throughout the study the diet was dominated by Red swamp crayfish and Eristalis, at Bilho and St-Nicolas (n = 898) by Red swamp crayfish, while at Bacchus (n = 433) it was more diversified with Orthoptera (mainly Chorthippus parallelus, and secondarily Platycleis sp. and a few Oedipodinae) and Red swamp crayfish (Fig. 3).
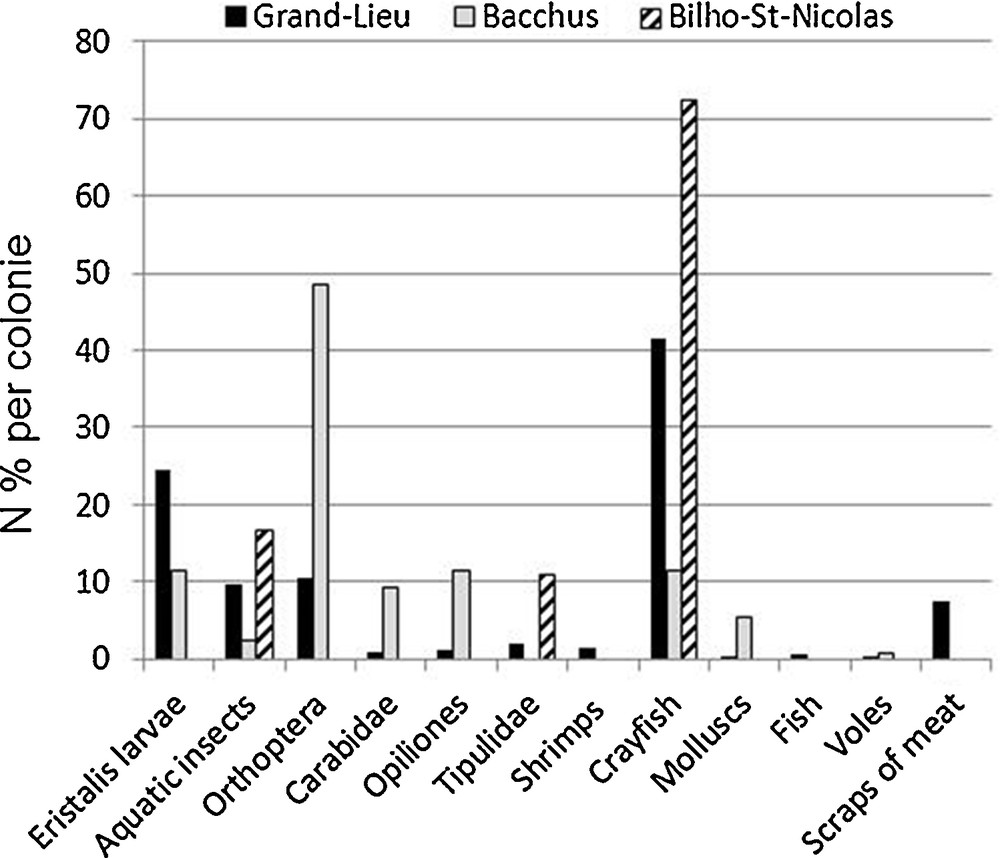
Compared Sacred ibis’ diets (% of the number of prey or of the items of scraps of meat from regurgitations) in food samples from colonies in Brittany (n = 3382 at Grand-Lieu, 898 at Bilho-St-Nicolas, 433 at Bacchus).
3.4 Temporal diet variations
Annual diet variations were also important, as evidenced by the permanent Grand-Lieu colony, which was studied over a long period (1994–2009, Fig. 4). There were three main distinct periods: (i) Eristalis larvae generally dominated between 1994 and 2005 (67% of the diet), with aquatic insects mainly between 1995 and 2001; (ii) Eristalis larvae and aquatic insects both totally disappeared from the diet after 2005, when Red swamp crayfish appeared, at first at low levels in 2005 and 2006, then as a main food item in 2007 and almost the exclusive food item in 2008 and 2009; (iii) in-between these two contrasting periods, scraps of meat were found between 1998 and 2007 (between 2% and 60% of the diet, with a peak in 2002). The Hierarchical Ascendant Classification (Fig. 4) separated 2008 + 2009 (diet essentially composed of crawfish) and the years 2006 (an exceptional year for Orthoptera), 2002 (important for scraps of meat) and 2007 (a transition with crawfish), from all the other years (dominated by Eristalis). A strong relationship between the proportion of Red swamp crayfish in the diet and its abundance in the lake was observed, starting in 2001 (r Spearman = 0.90, R2 = 80.8%, P < 0.01). Other preys were largely sporadic and generally rare, except Orthoptera in 1995 (20.80%) and 2006 (44.25%), and aquatic insects during the exceptional spring floods of 1998 (47.62%) and 2001 (19.10%). Their abundance in the diet throughout the total period studied was related to the water level in the surrounding marsh grasslands (r Spearman = 0.65, R2 = 42.8%, P < 0.015).
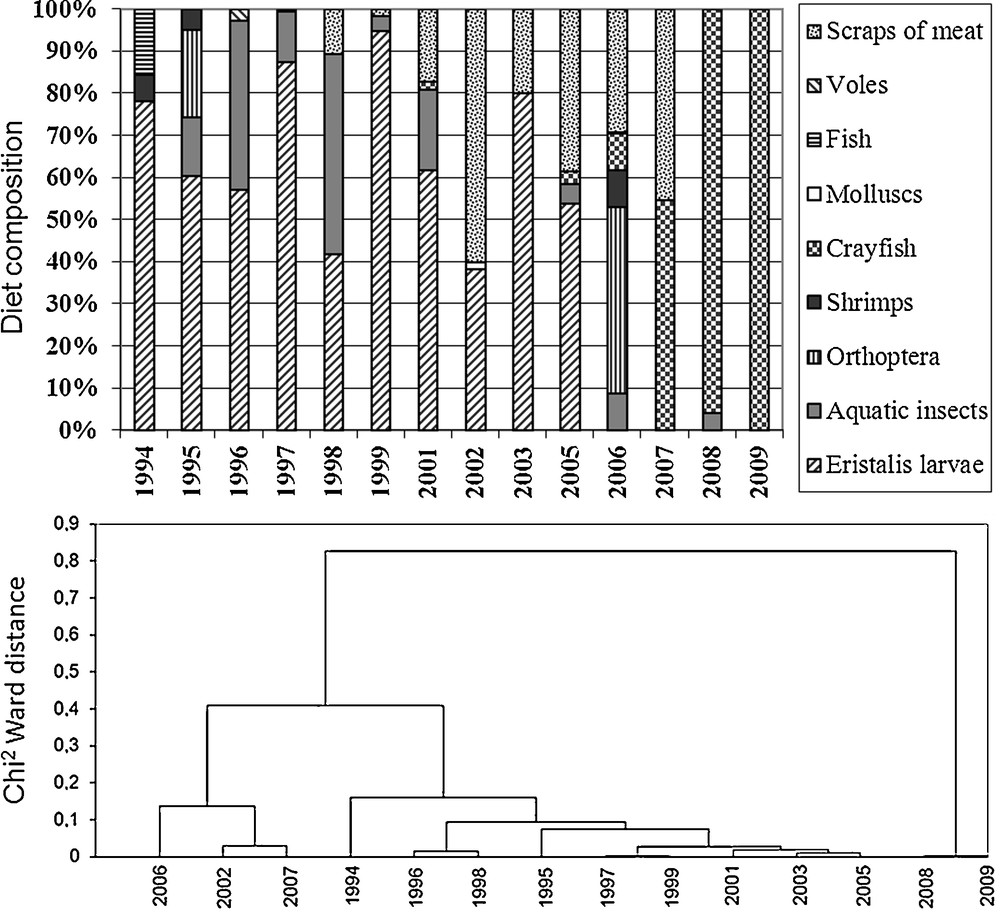
Annual variations in Sacred ibis’ diets (% of the number of prey or of the items of scraps of meat from regurgitations) in the Grand-Lieu colony between 1994 and 2009 (above), with Hierarchical Ascendant Classification of years (below).
3.5 Study of the diet from direct observations in feeding areas
Although we spent 160 hours directly sighting 3520 cumulated Sacred ibises in 10 feeding areas of Grand-Lieu Lake colony in 2002, we were only able to visually identify nine preys: two aquatic insects, two frogs, two voles and three fish; however few of these vertebrates were killed and none was swallowed or cut up and thus not eaten. All the other hundreds of prey, too small to be identified, were swallowed directly without being temporarily taken out of the water or the grass into the beak. On the rubbish dump of La Marne, Sacred ibises were unable to swallow bacon slices or entire steaks (in spite of having sometimes struggled for them), but fed essentially on small food items swallowed directly.
3.6 Factors influencing colony dynamics: diet and disturbances
3.6.1 The role of the diet
The PCA from regurgitations shows that the number of breeders in the colony of Grand-Lieu was clearly related to Red swamp crayfish (r Spearman = 0.69, P < 0.006, R2 = 0.48), in contrast with the presence of Eristalis (r = −0.49, R2 = 0.24), which is located on the opposite side of the graph (Fig. 5). Moreover, Orthoptera, which were closely associated with shrimps (caught far away from the colony in the Bourgneuf or Loire estuary marshes) and scraps of meat (taken during trips to Bourgneuf) in the diet (r = 0.96 and 0.71 respectively), are located opposite from colony size and Red swamp crayfish, which was mainly taken from Grand-Lieu lake. The role of invasive Red swamp crayfish as a key species in the surprising Sacred ibises population boom in South Brittany in the 2000s is confirmed by the relatively low population size when Red swamp crayfish was absent (Figs. 2 and 6): at Grand-Lieu before 2000 when Sacred ibises fed mainly on Eristalis, and in the Gulf of Morbihan, where Red swamp crayfish was not present and where Sacred ibises only fed on natural marine prey and from small rubbish dumps. Most of their periodic decreases were probably due to emigration to the Brière area related to the attractiveness of Red swamp crayfish. The phenomenon was mainly observed between 2003 and 2007, when the birds nested in Brière itself or in nearby refuge colonies of Bilho, St-Nicolas and Bacchus, with a corresponding low breeding population at Grand-Lieu (Fig. 2).
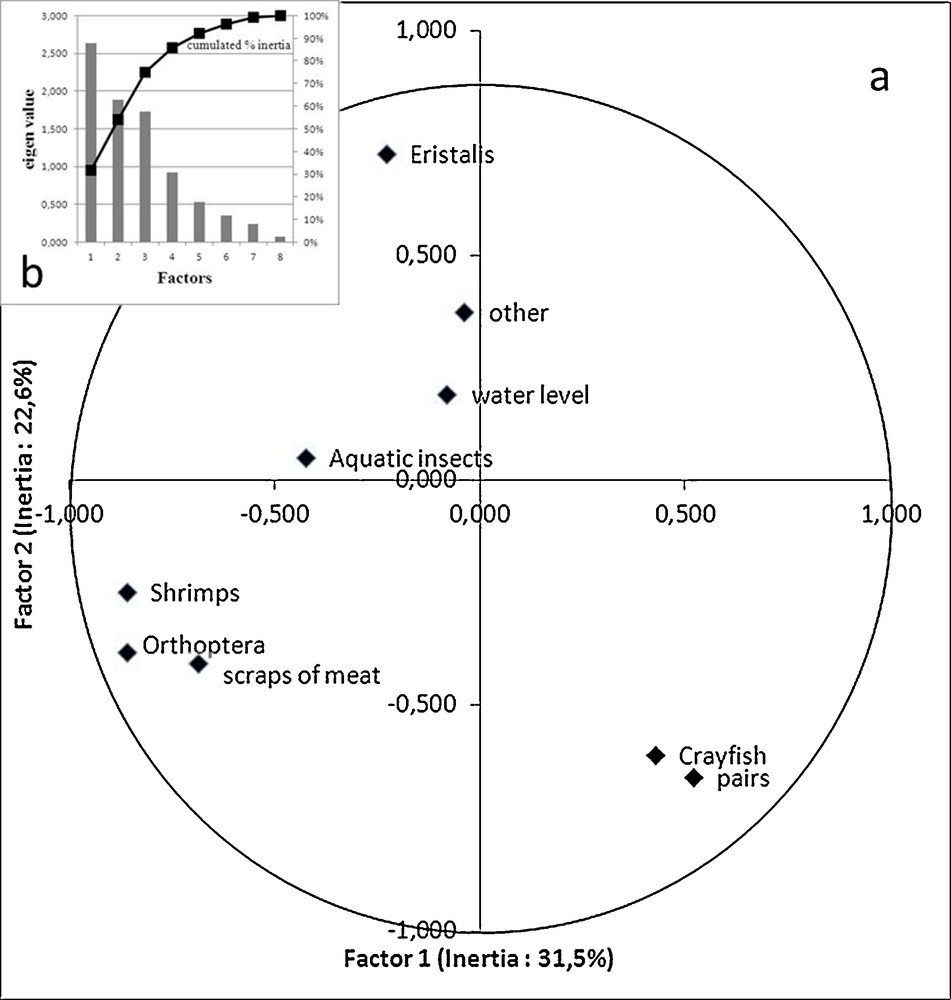
Principal Component Analysis showing the relationships between diet and numbers of Sacred ibis breeding pairs and water level at Grand-Lieu lake, according to years (1994–2009): a: factorial design of factors 1 and 2; b: histogram of the eigenvalues identifying the relative contribution of the factors that defined the average structure.
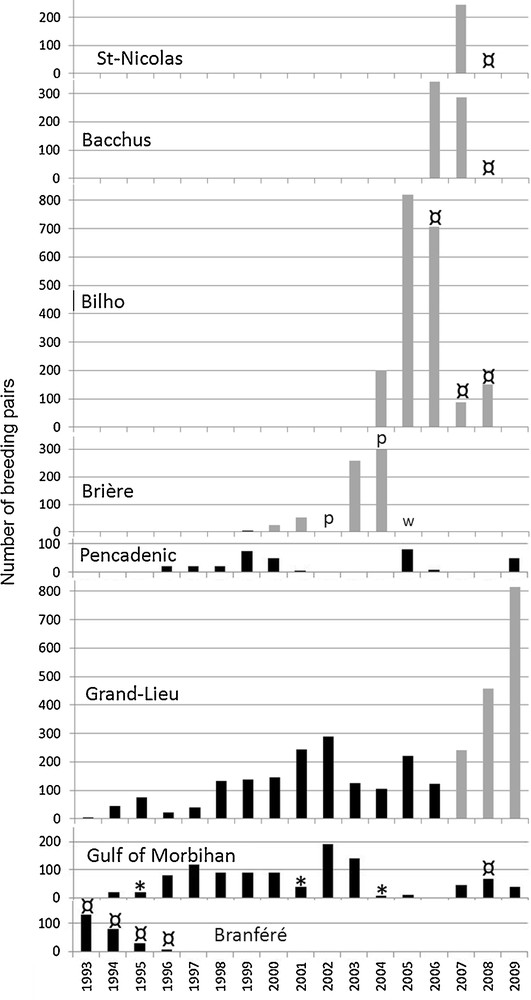
Annual trend of the size of the main Sacred ibis colonies as related to the dominance of Red swamp crawfish in the diet (grey columns), fox and/or wild boar predation (p), higher water level (w), cutting of trees (*) or other deterring management measures (¤).
3.6.2 The role of the disturbances
Fig. 6 also shows the role of the disturbances in colony dynamics. The cutting of trees in several Morbihan colonies (Huric in 1995, Île aux Oiseaux in 2001, Govihan and Le Ruault in 2004) also prompted Sacred ibises to take refuge in the Brière marshes in 2003 and 2004. There the first little colony of Besné (2000–2001) was abandoned in June 2002 due to decreasing water levels (−22 cm from April), which facilitated fox and wild boar predation (pers. obs.). The same phenomenon was observed at the end of June 2004 (−28 cm) with the newly-settled 2003–2004 ground colony in Brière, with a new decrease of water level (−28 cm). Conversely, a too high water level (+28 cm in March 2005 compared to 2004) forced this colony to finally escape to Bilho in the Loire estuary during the 2005 breeding season. Deterring management (egg-pricking and shooting of adults) requested by the French government at Bilho, caused some of the birds to escape to Bacchus island in 2006 and to St-Nicolas island in 2007, and finally a large shooting campaign in 2008 in the three latter colonies and in colonies in the Gulf of Morbihan (Runio, Île aux Souris and Pladic) forced most of the surviving birds to take refuge in the mother colony of Grand-Lieu.
4 Discussion
4.1 Factors affecting the diet
The present study confirms that the Sacred ibis essentially feed on invertebrates, even if it is an opportunistic feeder that adapts its diet to the available food. This explains the discrepancies between colonies at similar periods of study (2006–2007), and between years for the same long-term colony (Grand-Lieu). Bacchus showed a less aquatic feeding area with Orthoptera, only present in the diet of the Grand-Lieu colony in 1995 and above all in 2006. The occurrence of Orthoptera in both colonies in 2006 possibly indicates a particular abundance of this prey in the field at that time. Similarly, the abundance of aquatic insects in the diet was related to the years with exceptional spring floods, confirming the importance of water level for the Sacred ibis [106]. Eristalis larvae, which represent a vacant niche, were mainly caught in muddy areas in the eutrophic lake of Grand-Lieu and in slurry pits in cattle farms, when the colony first settle, but they totally disappeared after 2005. That year was the final legal time-limit for the French authorities to apply for grants to suppress such slurry pits (PMPOA II, Common European Agricultural Policy), but the decrease of Eristalis larvae in the diet was probably mainly exacerbated by the increasing abundance of more attractive Red swamp crayfish which invaded Grand-Lieu lake in 2007. At that time we estimated the stock to be 265 tons = 125 kg/Ha, well above the needs of Sacred ibises (Marion in prep.). This explains why Grand-Lieu was able to serve as refuge for more than 800 pairs of Sacred ibises from 2008 when shooting campaigns took place at Bacchus, Bilho and St-Nicolas. The existence of the latter colonies in the previous years was also clearly due to the availability of this prey in the Brière marshes, which were progressively invaded by this crayfish from the mid-1990s, with a density roughly estimated to be 100–300 kg/Ha and locally up to 2500–3300 kg/Ha in 2003–2004 [107,108]. However, crayfish availability for Sacred ibises increased in spring when the water level decreased (by an average 31 cm from February to May between 1996 and 2006) in relation to farming pressure. But in winter, the water was too deep for the length of Sacred ibises’ legs (such as at Grand-Lieu), and crayfish was also less active. Crayfish availability probably explained why Sacred ibises mainly used the rubbish dump of the large town of St-Nazaire (Cuneix) in January between 1997 and 2006 (53% of all sightings of ringed birds, n = 147) and not during the breeding season (except in April 2004). Similarly, crayfish availability also explained why no scraps of meat were observed in the diet at Bacchus in spring 2006 (the last year the rubbish dump was open), or in a previous little colony at Besné in 2000 and 2001, where only Red swamp crayfish was noted. At Grand-Lieu the presence of scraps of meat only appeared in the diet from 1998, probably because Sacred ibises had not yet discovered the rubbish dump of La Marne created in 1981 in a wood (200,000 tons of rubbish per year until 1997, but progressively less afterwards). Latter, Sacred ibises were scared away from it in 2008 by deterring shooting campaigns before it was closed in June 2009. Before the shooting campaigns, the rubbish dump was mainly used during the breeding season (64%), compared with only 4% in summer and 15% in winter (Fonteneau pers. com.). These results contradict Clergeau and Yésou [95] who supposed that refuse dumps became one of the most important food sources in western France, and allowed Sacred ibises to survive throughout harsh times (winter and summer).
4.2 Impact of the Sacred ibis on other birds
4.2.1 In the non-native area
The present study arouses doubts as to the impact of the Sacred ibis on rare birds described in recent literature (Introduction). Despite the Sacred ibis’ opportunism, the long-term data collected in the French core area showed that it largely feeds on invertebrates during the breeding season (91.66%), and secondarily on scraps of meat (7.59%), while vertebrates were insignificant in its diet (0.74%). Despite the different ways used to report diet composition (by occurrence, mass or number of prey), which do not facilitate comparisons among previous studies (Table 1), birds were absent or found in negligible portion in stomach contents and regurgitations in both the native and non-native areas. This high specialization for invertebrates is in accordance with the Sacred ibis’ feeding behaviour: it mainly searches for prey probing blindly in the mud (Eristalis), in the water or through vegetation (other preys), thanks to its innervated beak like Spoonbill. This flexible rounded beak does not seem fit for active vertebrates predation, contrary to Ardeids or storks, and this explains why Sacred ibises have difficulties in killing and swallowing vertebrates. For the same reason, refuse dumps played a far less important role in its diet than previously suspected; this was probably due to difficulties in swallowing large pieces of meat as carcasses did not seem to be cut up, as already reported by Marion and Marion [91] concerning Catfish dumps at Grand-Lieu lake and in the present study concerning direct observations in the feeding areas. Diet observation in the feeding areas, also reported by J. Pourreau (pers. com.), Locquard [109] and Clergeau and Yésou [95] from second-hand observations from birdwatchers, seems totally biased as it is impossible to observe the ingestion of invertebrates. Consequently, the method thus privileges the very rare catches of vertebrates, held for a long time in the beak, and finally rarely ingested, except for eggs. Of course, direct observations made only in other bird colonies (Table 1) were entirely focused on the predation of birds, and do not represent the complete diet of Sacred ibises.
Comparison of Sacred ibis’ diet composition according to studies carried out in native (Africa) and non-native areas.
| Non-native areas | Native area | ||||||||||
| Studies | [91] | [110] | [92]* | [93]* | [96] | [111]* | Present study | [112] | [97]* | ||
| Countries | France | France | France | France | USA | France | France | South Africa | South Africa | ||
| Locality | Grand-Lieu | Aude | Brittany | Brittany | Vendée | Aude | Everglades | Grand-Lieu | Brittany | Pretoria | Penguin island |
| Methods | Regurgitations | Stomach content (2) | Regurgitations (2) | Direct observations in other bird colonies | Direct observations in other bird colonies | Stomach content | Direct observations in other bird colonies | Regurgitations | Stomach content | Direct observation in marine bird colony | |
| n samples | 22 | 39 | 25 | 160 | 3 | 546 | 100 | ||||
| n years | 1 | 2 | 2 | 1 | 10 (¤) | 5 (#) | 7 | 14 | 2 | 3 | |
| Invertebrates | |||||||||||
| Insects | 20%M | ||||||||||
| Diptera | 78.13%N | 51.4%O | 24%O | 100%O | 26.50%N | 50%O | |||||
| Dermaptera | 4%O | ||||||||||
| Coleoptera | 33.3%O | 68%O | 100%O | 3.2%M | 9.32%N | 53%O | |||||
| Lepidoptera | 12.8%O | 4%O | 6.3%O | 17%O | |||||||
| Odonata | 6.3%O | 0.8%M | 0.7%N | ||||||||
| Orthoptera | 5.1%O | 10.41%N | 5%O | ||||||||
| Hymenoptera | 12.5%O | ||||||||||
| Hemiptera | 1 %O | ||||||||||
| Heteroptera | 0.37 %N | ||||||||||
| Arachnida | 5.1 %O | 8%O | 1.05%N | ||||||||
| Myriapods | 3%O | ||||||||||
| Isopoda | 5.1%O | ||||||||||
| Annelids | 17.9%O | 2%O | |||||||||
| Decapods | |||||||||||
| Crayfish | 20.5%O | 52%O | 93.8%O | 26.6%M | 41.38 %N | ||||||
| Shrimps | 6.25 %N | 4%O | 25%O | 1.52%N | |||||||
| Crabs | 4%O | 6.3%O | 7%O | ||||||||
| Molluscs | 43.6%O | 24%O | 81.3%O | 0.44%N | |||||||
| Vertebrates | |||||||||||
| Amphibia | 12.8%O | 2%O | |||||||||
| Reptilia | 1%O | ||||||||||
| Mammalia | 0.11%N | 2%O | |||||||||
| Fish | 15.63%N | 5.1%O | 75%O | 0.65 %N | |||||||
| Aves§ | 5.1 %O | 6.3%O | 5 to 30 | 5 to 50 | 79 | 1%O | 65 | ||||
| Plants | 17.2%M | ||||||||||
| Seeds | 23.1%O | 8%O | 87.5%O | 6.2 %M | 26 %O | ||||||
| Fibres | 12.8%O | 28%O | 87.5%O | ||||||||
| Waste | 43.3%O | 20%O | 100%O | 63.2% M | 7.55%N | 57%M |
Nevertheless, Clergeau and Yésou [95] highlighted several second-hand reported cases of predation on eggs and occasionally small young birds (terns Sterna sandvicensis, S. hirundo, Chlidonias niger, Chlidonias hybridus [92], Cattle egret Bubulcus ibis [93], waders Himantopus himantopus, Vanellus vanellus from S. Reeber [pers. com.] and Shag Phalacrocorax aristotelis from Le Névé pers. com.). Such occasional predations were largely exaggerated by Clergeau et al. [89] and Yésou and Clergeau [94] to justify the eradication of the Sacred ibis from western France. These occasional cases have to be put into perspective. In their recent paper about regurgitations and stomach contents, Clergeau et al. [110] themselves found only 6.3% occurrences of undetermined birds (including feathers from dumps?), compared with very large occurrences of invertebrates (Table 1). Similarly, Vaslin [92] reported from D. Montfort and A. Mauxion the “methodical” predation of about 20 broods of C. hybridus by 60–70 Sacred ibises in Brière in 2000, which caused the desertion of the colony. In fact, the group of Sacred ibises was only seen to knock over the flimsy floating nests by feeding on small aquatic prey and so all the eggs disappeared, but no active predation was really observed. Breeding success of the C. hybridus in Brière does not depend on the presence or absence of the Sacred ibis but on water level, because the water meadows that harbour these colonies are drained during the breeding season and the birds are thus left to the mercy of disturbance by cattle or predation by mammals or gulls (D. Montfort in [113]). A similar situation occurred in the cases of predation of three C. niger nestlings and one V. vanellus nestling, reported by Vaslin [92] from S. Reeber at Grand-Lieu lake in 2003–2004, which corresponded exactly to the draining of the water meadow at the beginning of June as part of the farming management. Between 2005 and 2009, predation (mainly of eggs) occurred in these drained wet meadows, totalling one case on Tringa totanus, four on V. vanellus, eight on Fulica atra, eight on C. niger, 13 on H. himantopus, and more than 23 on Larus ridibundus [111]. Such predations were not representative of the situation of the lake, despite of the number of Sacred ibises breeding and feeding there since 1993. The fact that birds’ eggs and some nestlings were not observed in the diet through regurgitations from Grand-Lieu in the present study during the same period could be explained by the enormous quantity of other food sources taken by Sacred ibises. For instance, the cumulative number of breeding Sacred ibises between 2003 and 2009 at Grand-Lieu lake was 4758 adults, corresponding to about three million 15-g items of prey such as Red swamp crayfish, compared to tens of birds’ eggs or young.
At Grand-Lieu, predation of bird species by the Sacred ibis had no proven consequence on their population dynamics (Table 2): the numbers of breeders increased for all the species in the lake since the first settlement of breeding Sacred ibises, despite the main nest failure caused by cattle, which was omitted by Clergeau et al. [89] and Reeber [111]. In spring 2010, a 5-Ha enclosure was created to prevent disturbance by cattle.
Estimated impact of predation on eggs (or small young) by the Sacred ibis on native bird species at Grand-Lieu lake between 2003 and 2009 (calculated from [111]), and status of these species in France (from [113] and [114]). The dates when native breeding pairs first settled are taken from [115] and [116], in comparison with 1993, the year when the Sacred ibis first settled at Grand-Lieu.
| Fulica atra | Larus ridibundus | Vanellus vanellus | Tringa totanus | Chlidonias niger | Himantopus himantopus | |
| n pairs at Grand-Lieu | 3200 | 985 | 52 | 25 | 62 | 41 |
| Breeding since | Centuries | 1961 | 1970 | 1994 | 1994 | 1997 |
| n laid eggs | 21,000 | 2500 | 200 | 75 | 185 | 170 |
| Mean n predation 2003–09 | 1.14 | 6.14 | 0.7 | 0.11 | 1.57 | 2.57 |
| Mean % predation per year | 0.005 | 0.25 | 0.35 | 0.19 | 0.85 | 1.51 |
| n pairs in France | 125,000 | 38,000 | 18,000 | 1400 | 230 | 2500 |
| Trend n pairs in France | +78% since 1970 | Stable | −20 to −50% since 1970 | Stable | −17% since 1963 | +35% since 1996 |
| Status | – | – | – | – | Vulnerable | – |
The third case reported by Vaslin [92] concerned a colony of S. sandvicensis at Noirmoutier, where two Sacred ibises were seen in the morning of 9th July 2004 feeding on eggs of all of the 30 nests present and one egg of S. hirundo. In fact, many eggs were already scattered on the ground at the beginning of the observation, and the author did not mention that the colony had been totally destroyed in one night, three weeks before, by a fox that killed 17 nestling terns and caused all the others (about 400 pre-fledging young belonging to 204 nests) to be drowned (M. Vaslin pers. com.). A return visit to this colony by a fox during the night of 9th July to predate late new broods was probable (it is the usual behaviour of such predators) and could explain the scattering of eggs on the ground before their predation by Sacred ibises, with defenseless by the stressed terns.
In these three cases (Brière, Grand-Lieu, Noirmoutier), only C. niger is considered to be “vulnerable” in France [117]. In Brittany, these predated species were not endangered by the Sacred ibis. Accidental predation of a few eggs and rarely of young, known to have had only a minor effect on the population dynamics of birds compared to adult mortality, has to be compared with the loss of all of the 1400 broods of C. hybridus at Grand-Lieu lake (the largest French colony) in 2006 due to a storm, which were rapidly replaced. Populations of breeding Ardeids, Spoonbill Platalea leucorodia or Cormorant Phalacrocorax carbo are also abundant and increasing at Grand-Lieu and in Brière in mixed colonies with the Sacred ibis. Conversely, the presence of the Sacred ibis is very attractive for the Spoonbill at Grand-Lieu lake (r = 0.87 [118]), the main and pioneer French colony, and this is true although a recent increase of Sacred ibis numbers in Grand-Lieu late in the breeding season due to shooting elsewhere disturbed the statistical relation between the respective breeding timings of breeding of the two species [119]. Destruction of Sacred ibis nests in mixed colonies could also induce indirect disturbing effects on Spoonbills which escaped from usual colonies [120]. In Mediterranean coastal marshes (the Camargue and other large wetlands with 16,000 breeding pairs of Ardeids [121]), Kayser et al. [93] suspected one Sacred ibis to be responsible for the failure of about 50 herons nests, but they only observed one case of one 15-day-old nestling Cattle egret (the most abundant heron species in France with 14,000 pairs compared to only 100 in 1974 [121]) being transported by a Sacred ibis. Causes for nest desertion are too numerous in Ardeids to attribute them to the presence of one Sacred ibis alone within a colony. However, two Sacred ibises were seen in another colony “robbing” Cattle egret nests. In Brittany, the case of predation of Shag's eggs mentioned by Clergeau and Yésou [95] was only hypothesized and solely based on the short presence of two Sacred ibises before the breeding season.
Such biased descriptions of Sacred ibis’ diet in France by Clergeau and Yésou [95] and this unrealistic impact on predated birds largely influenced Herring and Gawlik [96] in their opinion of the potentially negative impact of this species in the Everglades, although their own limited diet study (Table 1) showed no presence of vertebrates, even from direct field observations. Direct observations made specifically on other bird colonies rather than at the scale of the whole feeding area of Sacred ibises give a biased representation of their diet, that focuses on the occasional predation of birds’ nests, possibly by specialized individuals.
4.2.2 In the native area
In the Sacred ibis’ native area, predation on the eggs or small young of colonial birds by Sacred ibises has been reported occasionally, but only on marine islands in South Africa where other food resources seemed rare: Phalacrocorax capensis [122], Sterna bergii [123], particularly as a result of disturbance (however, predation was mainly made by Larus dominicanus and Larus hartlaubii), L. hartlaubii [124]. Apart from general citations of food in avifauna books [125–128], for which methods of reported predation were not detailed and seemed to include anecdotal cases, only one study focusing on the Sacred ibis’ diet quantified vertebrates predation (Table 1 [112]). More recently, Williams and Ward [97] observed more significant predation in the colony of Penguin Island in South Africa where 10,000 pairs of Morus capensis nested, together with 4800 pairs of P. capensis and other species such as gulls and Spheniscus demersus. For 3 years, a few specialized Sacred ibis individuals out of the 400 that roosted on the island fed on 152 eggs of P. capensis. However, the effects of this predation should be compared with the 14,500 adult P. capensis found dead due to avian cholera in the South Africa colonies in 1991, which represented only 8% of the population [129], and with the 13,000 individuals found dead in 2002 [130], without the species being endangered. Predation by the Sacred ibis has not been observed elsewhere in continental Africa, even in a six-year study of the behaviour of this bird in Ethiopia by Urban [131]. The study cumulated 200 hours of observations in mixed colonies of Sacred ibises, P. carbo, Pelecanus onocrotalus and Ciconia abdimii, and only showed Sacred ibises occasionally feeding on the contents of Pelican's eggs broken by Egyptian vultures. Modha [132] also mentioned Sacred ibises eating crocodile's eggs excavated by Varanus salvator at Lake Rudolf (Kenya-Ethiopia). These two latter cases of waste food ingestion, although incorrectly reported as active predation by BirdLife International [86] and Clergeau et al. [89], show the difficulties in interpreting the feeding behaviour of Sacred ibises in seabird or wading birds colonies where abandoned eggs or nestlings and food remains are abundant. Mortality during breeding, due mainly to a lack of food, usually affects half of the young in most colonial species due to starvation, even without any predators.
Even if regurgitations in nests underestimate some rare cases of killed but non-swallowed vertebrates, possibly by a few specialized individuals, a more balanced consideration of the real impact of the Sacred ibis is necessary. We should take into account its positive effect on the vulnerable Spoonbill [118], as well as the beneficial effect of predation of Red swamp crayfish on the ecosystem, rather than regarding it suspiciously, mainly based on the fact that the Sacred ibis is not native to Western Europe. In fact, Europe is included in the biogeographical West Palaearctic, a fauna the Sacred ibis belongs to [88], and all the bird species very occasionally predated in France cohabit with the Sacred ibis during wintering or breeding in South Mauritania and Senegal. So the situation of the Sacred ibis greatly differs from that of invasive species in small oceanic islands where they can endanger endemic fauna that previously had only low or no predation or competition (Introduction). The situation in France, only ranked 144th in the list of countries with threatened birds [10], is very different. Moreover, the other criticisms of the Sacred ibis by Clergeau et al. [89] are unproven. The decay of coniferous trees (in fact Cupressus, an allochthonous ornamental species introduced in the 1930s) observed in colonies in the Gulf of Morbihan, was not due to this species but to native mixed colonial species such as the Grey heron and above all the Cormorant, while the Sacred ibis and the Spoonbill do not have a serious impact on Salix trees in the Grand-Lieu or Brière colonies. Disease transmission suspected from the use of slurry pits or water treatment plants has not been demonstrated, whether by the Sacred ibis or by other bird species using rubbish dumps (storks, gulls, Corvus corone, Milvus migrans, Buteo buteo, etc.). Moreover, these slurry pits and water treatment plants spray their activated sludge on land under cultivation for human food, so the risk cannot be considered as serious. A recent veterinary thesis [133] showed that the Sacred ibis has a low infection level by various parasites, e.g. Salmonella and Chlamydiaceae, similar to other autochthonous species, and presents a low health risk for farm animals. This contradicts Clergeau and Yésou [46] who justified the presence of the Sacred ibis on the list of the 100 most invasive alien species in Europe by these few words: Predation on several threatened species (e.g. insects, amphibians) and especially on protected colonies of terns and herons. Vegetation rapidly affected at breeding sites. Epidemiological role suspected since foraging ibises frequently visit rubbish dumps and slurry pits. An economic impact has not been documented, but destruction of salt pan structure has been observed.
The present paper shows that all these impacts are as unjustified as they are inexact (threatened insects or amphibian species, health risks, etc.) or greatly exaggerated. Yet, Kumschick et al. [98,99] used these papers to classify the Sacred ibis as the third bird species with highest impact in Europe, using a method that greatly lacks precision and was rejected by Strubbe et al. [41]. In fact, all the wetlands used by the Sacred ibis in Brittany were mainly ecosystems perturbed by human activities (with general eutrophication or low water levels imposed by farming, slurry pits, rubbish dumps), and real invasive species such as the Coypu (Myocastor coypus), Red swamp crayfish and Ludwigia peploides that largely impact native aquatic plants and ecosystem functioning [134] as in other wetlands [135]). A similar situation occurred in South Africa, where the Sacred ibis did not breed before the beginning of the 20th century, and increased 2–3 folds in Free State between 1972 and 1995 due to the increased number of dams and agricultural practices such as rubbish dumps, dung heaps, sewage farms, carcasses, etc. [104]. Without the recent invasion of Red swamp crayfish, the Sacred ibis populations would probably have only fluctuated in Brittany at a relatively low saturated level (less than 600 pairs in only a few permanent colonies, five times less than herons in that area). So the Sacred ibis clearly appears as a “passenger of change” in degraded ecosystems rather than a driver, according to the theory of Didham et al. [28]. Consequently, a clear balanced demonstration of both the detrimental and positive effects of this allochthonous species on native species and ecosystems is needed to prove that it is harmful before the species is labeled as “invasive” and then managed as such, as Davis et al. [50] recently argued.
Disclosure of interest
The author declares that he has no conflicts of interest concerning this article.
Acknowledgements
We wish to thank Pierrick Marion for his help in collecting field data in the colonies, Guy Bourlès for breeding counts of Erdre colony, Frank Potiron for Goulaine, Jean Caziot for Pencadenic, Pierre Yésou for some other colonies, Jo Pourreau who provided direct diet observations in the field from birdwatchers before 2005 and sightings of Sacred ibises in Cuneix refuse dump, Benoît Faure for diet observation of Sacred ibises in the wetlands around Grand-Lieu lake, Alexandre François for his help in determining insects in regurgitations, the Parc Naturel Régional de Brière for water level data, and Aldyth Nys Traductions and Annie Buchwalter for linguistic improvements. We also thank Julie Lockwood and Stefano Volponi for valuable comments. This study was supported by a grant from SESLG.


