1 Introduction
Seed plants exhibit a wide variety of breeding systems that has fascinated biologists since Darwin [1]. Within variation in sex expression, trioecy, the co-occurrence of male, hermaphrodite and female individuals within the same population represents a small percentage of flowering plants (3.6% of the species; [2,3]). Understanding the evolution and maintenance of such mating system is a major challenge as it involves the stable coexistence of three sexual morphs, which compete for reproduction. Stable coexistence of two sexual morphs (gyno- and androdioecy, the coexistence of females, respectively, males, and hermaphrodites) has been extensively studied [4–13]. In contrast, trioecy has received little attention, mainly for three reasons. First, this mating system is rare in nature; second, trioecy has not been readily identified and defined. Finally, trioecy is a complex system to study.
Trioecy is usually defined as the co-occurrence of males, hermaphrodites and females in the same population, but under this definition, other mating systems may be confused with trioecy. To date, only a few species have been recognized as trioecious: Carica papaya (Caricaceae; [14]), Silene acaulis (Caryophyllaceae; [15]), Spinacea oleracea (Chenopodiaceae; [16]), Thymelaea hirsuta (Thymelaeaceae; [17,18]), Atriplex canescens (Chenopodiaceae; [19]), Pachycereus pringlei (Cactaceae; [20]), Juniperus seravschanica (Cupressaceae; [21]), Mercurialis annua (Euphorbiaceae; [22]), and Coccoloba cereifera (Polygonaceae; [23]). However, three out of these nine species, Atriplex canescens, Spinacea oleracea and Thymelaea hirsute were described by the authors as tetramorphic species, with dioecious individuals (males and females) and sequential monoecious hermaphrodites that are either protogynous or protandrous. This specific sexual polymorphism is considered more as a combination of true dioecy (males and females) and heterodichogamy or temporal dioecy (protogynous and protandrous individuals) by Dommé et al. [18] than as a case of true trioecy. Other authors found a significant degree of lability in sex expression of these three species under different environmental conditions [19,24]. In contrast, the Mexican columnar cactus Pachycereus pringlei is thought to be a true trioecious species consisting of male, female and hermaphrodite individuals, with a stable sex expression and only one type of flowers on a given individual [20]. However, both gynodioecious and trioecious populations of this species are found in the Sonoran Desert of Mexico [25], suggesting that trioecy is not stable. Field observations and a theoretical analysis suggest that pollinator abundance and pollen limitation play an important role in the maintenance of trioecy in this plant [25,26]. A true trioecious species should be defined as a species where three morphological and functional sex types co-exist: males producing effective pollen only, hermaphrodites producing simultaneously both effective pollen and viable seeds, and females producing seeds only. This definition of trioecy may include environmental variations in sex determination.
So far, no theoretical approach has specifically targeted the issue of the maintenance of trioecy. Nevertheless, three different general models of mating systems evolution have yielded conditions for the coexistence of three sexual morphs. First, Charnov et al. [27] proposed a phenotypic model of sexual strategies, assuming nuclear inheritance of sex in a large panmictic population and showing that trioecy is always an unstable equilibrium in the absence of selfing. Gouyon [28] extended the model to include selfing and did not find any stable conditions for trioecy. Maurice and Fleming [26] extended the model further and demonstrated that three sexual phenotypes can co-exist in a population under female frequency-dependent pollen limitation, moderate inbreeding depression and intermediate selfing rates. Finally, in a genetic model of the evolution of dioecy from hermaphroditism, Maurice et al. [13] found that trioecious reproductive systems can be stable under nucleocytoplasmic sex determination. Some of the conditions for maintenance of trioecy outlined in these approaches are highly model-specific and cannot be fruitfully used to infer the stability of a trioecious mating system in natural populations. However, in the two latter models, two conditions are necessary (although not sufficient) for stability of trioecy: the male fitness (pollen production) of the male individuals must be twice larger than that of hermaphrodites and, the female fitness (ovule production) of the female individuals must be larger than that of the hermaphrodites. All these models suppose that the sexual types are genetically determined.
The common ash, Fraxinus excelsior L. (Oleaceae), has a complex, continuous distribution of three sex types [29–32], leading to a polymorphic breeding system that has previously been described as trioecious [31]. In this paper, detailed information on the determination and expression of sexual types are provided. The reproductive success of males and females relative to hermaphrodites was followed by studying the number of flowers, the pollen grains per flower, the fruits, and the flowering phenology. This study was realized in a natural population of trees and, in a seed orchard where several genotypes were represented by a varying number of trees obtained by cloning.
2 Materials and methods
2.1 Study species and sites
Common ash (F. excelsior L., Oleaceae) is a deciduous forest tree distributed throughout Europe and Asia Minor [29]. This species is wind-pollinated and its fruits (samaras) are wind-dispersed. In France, flowering occurs in early spring (March–April), before foliage emergence (May), and flowering occurs during three to four weeks [33].
Two populations were studied; the first population is a natural population located in the national forest of Dourdan (latitude 48° 31′ 47′′, longitude 02° 00′ 42′′). This stand is of native origin and is managed by the French National Forest Service (ONF). F. excelsior is the dominant species in this area of about 12 ha. All trees with a diameter at breast height larger than 10 cm were located and marked. As the trees were very tall on average (about 30 m), we sampled branches to determine the sexual phenotype of each tree by shooting them down with a shotgun. The second population is a seed orchard (latitude 48° 29′ 56′′, longitude 00° 07′ 07′′). This seed orchard contains 414 trees. Sixty-six genotypes were identified: 41 clones (genets) represented by several trees (ramets) and, 25 single trees. Clones are represented by 2 to 25 ramets (mean = 9.49 ramets per genet, SD = 5.92).
Additional observations of sex expression were conducted in years 2000 and 2001 on a sub-sample of 32 clones from the seed orchard for which one ramet per clone was planted in the experimental field of the INRA (French National Institute of Agronomic Research) located in Orleans (France), 200 km from the seed orchard.
2.2 Sex expression
In each population, all the sampled individuals were observed for sex expression during two consecutive years (2000 and 2001), and in 2008 only in the seed orchard. The sexual phenotype was determined from only a few branches per tree as sampling was performed exclusively with a shotgun in the natural population. In contrast, in the seed orchard with shorter trees, sexual phenotype was assessed from the whole crown. Sex expression in F. excelsior appears to be a continuous trait, which could be best described using continuous measures, such as quantitative gender estimate [34]. Unfortunately, this estimate requires an accurate estimation of the total number of functionally female and male flowers for each individual, which was not achievable here given the height of the trees (especially, in the natural population). We, thus, chose to use discrete categories instead.
Perfect flowers consist of one pistil and two purple stamens attached to the base of the ovary [31]. Staminate flowers comprise only two stamens; pistillate flowers carry one pistil, with or without rudimentary stamens [32]. There is a continuum in sex expression from pure male individuals to pure female, with all kinds of hermaphrodites in between (pure hermaphrodites, andromonoecious, and gynomonoecious individuals)[29]. The sexual phenotype are: (1) pure male, bearing only staminate flowers (MM), (2) mostly male, with a few (less than 50% within an inflorescence) perfect flowers (MH), (3) andromonoecious bearing mainly perfect flowers, with a few (less than 50% within an inflorescence) staminate flowers (HM), (4) pure hermaphrodite, bearing only perfect flowers (HH), (5) gynomonoecious bearing mainly perfect flowers, with a few (less than 50% within an inflorescence) pistillate flowers (HF), (6) mostly female, with a few (less than 50% within an inflorescence) perfect flowers (FH), and (7) pure female, bearing only pistillate flowers (FF). To increase sample sizes per sex type, these seven sexual phenotypes were grouped into three gender categories. These gender classes were chosen on the basis of the pollen and fruit productions of the different sexual phenotypes: males (M) producing pollen and grouping MM and MH, hermaphrodites (H) producing pollen and seeds simultaneously and grouping HM and HH, and females (F) producing seeds and grouping HF, FH and FF.
2.3 Phenotypic trait measurements
In each population, all the sampled individuals were observed for flowering and fruiting in spring and autumn, respectively, during two consecutive years (2000 and 2001). For each sampled tree, we assessed flower and fruit densities, i.e., the total flower or fruit production relative to the crown volume, on a scale from 1 (sparse flower or fruit production) to 4 (dense flower or fruit production). A flower or fruit density of 0 refers to no flower or fruit production. To evaluate accumulated reproductive effort in time in this perennial plant and to account for temporal variation in flower or fruit production, accumulated flower and fruit densities over 2000 and 2001 were also estimated as the sum of the flower or fruit densities in each year, using all trees that were sexed either year, or with a same sexual phenotype scored both years.
The number of pollen grains per flower was estimated in 2000 by collecting several inflorescences per tree. Pollen was extracted following the method described in [35]. For individuals with only one floral type producing pollen, the pollen grains were extracted from two flowers. For trees carrying both staminate and perfect flowers, the pollen grains was extracted from two flowers of each floral type in the natural population, and from two flowers of the major floral type in the seed orchard. A sample of the total amount of pollen grains of the two flowers extracted was counted under microscope using a Malassez cell.
Flowering phenology was measured in the natural population and trees were classified in three phenological categories: early, mid and late. Flowering phenology in the natural population was studied only in 2001 because in 2000, when we started the observations on the field, some trees were already at the end of their bloom. Tree size was also measured for each sampled individual.
2.4 Statistical analyses
Analysis of flower density, pollen grains production, fruit density, and accumulated flower or fruit densities were performed by the Kruskal–Wallis test using the R software (The R Foundation for Statistical Computing ISBN 3-900051-07-0). In the seed orchard, one genotype per clone was chosen randomly within each clone, as the influence of clones could not be nested within sex when using Kruskal–Wallis test. A Chi-square test was used to test for independence between gender and flowering phenology.
3 Results
3.1 Sex expression and genetic determinism of sex
3.1.1 Natural population
The natural population contained a majority of male individuals [216 (56.1%) in 2000; 218 (62.1%) in 2001] with some hermaphrodites [104 (27.0%) in 2000; 86 (24.5%) in 2001] and a few females [65 (16.9%) in 2000; 47 (13.4%) in 2001] (Fig. 1).
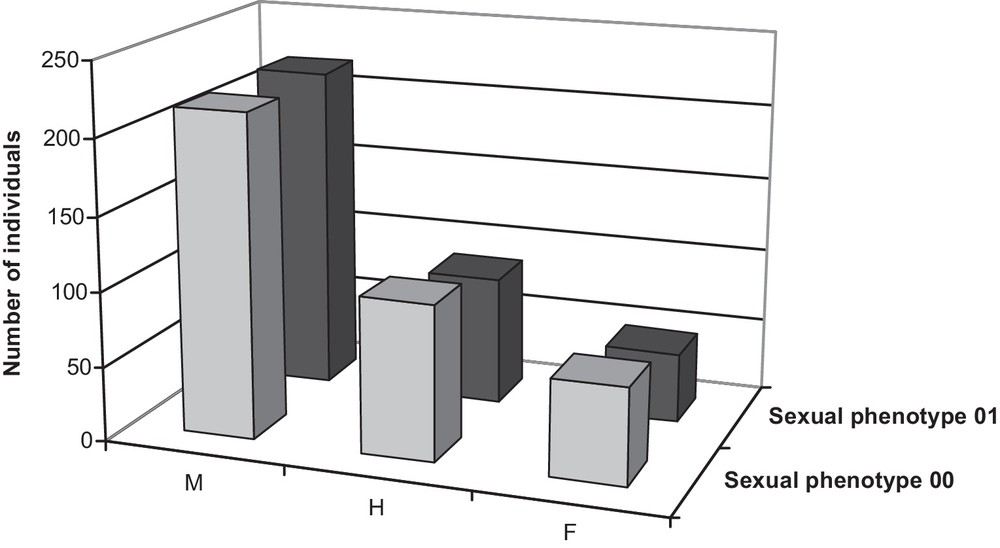
Total number of individuals observed in each class of sexual phenotypes in the natural population in 2000 and 2001.
In this natural population, 86% of the trees that flowered both years exhibited the same sexual phenotype in 2000 and 2001. Nonetheless, sex stability varied across genders (Table 1). Nearly all males in 2000 (171 out of 174) remained males in 2001, two became hermaphrodites, and one was recorded as female. Among the 55 hermaphrodites in 2000, two-thirds (36) were still hermaphrodites in 2001, 11 became males and eight became females. Female phenotype was far less constant, with only 12 of the 27 females in 2000 remaining females in 2001, 14 being scored as hermaphrodites in 2001 and the remaining one scored as a male in 2001.
Transition probabilities among genders from 2000 to 2001 both in the natural population (n = 256) and in the seed orchard (n = 241), and from 2000 to 2008 in the seed orchard (n = 183).
| Male 2001 | Hermaphrodite 2001 | Female 2001 | |
| Natural population | |||
| Male 2000 | 0.983 | 0.011 | 0.006 |
| Hermaphrodite 2000 | 0.200 | 0.655 | 0.145 |
| Female 2000 | 0.037 | 0.519 | 0.444 |
| Seed orchard | |||
| Male 2000 | 0.966 | 0.034 | 0 |
| Hermaphrodite 2000 | 0.020 | 0.700 | 0.280 |
| Female 2000 | 0 | 0.227 | 0.773 |
| Male 2008 | Hermaphrodite 2008 | Female 2008 | |
| Seed orchard | |||
| Male 2000 | 0.97 | 0.03 | 0 |
| Hermaphrodite 2000 | 0.02 | 0.93 | 0.05 |
| Female 2000 | 0 | 0.58 | 0.42 |
3.1.2 Seed orchard
In the seed orchard, similar levels of sex stability were observed, with 88% of the ramets that flowered in 2000 and 2001 years (n = 241) displaying the same gender. Again, sex stability depended on the gender (Table 1). As in the natural population, the male gender was the most stable, with 142 of the 147 males exhibiting the same gender in 2000 and 2001. In the seed orchard, hermaphrodites and females exhibited comparable levels of gender stability, with 35 (34) individuals out of 50 (44) exhibiting a hermaphroditic (female, respectively) gender both years. Only one hermaphrodite became male in 2001, and 14 became females. The ten females that changed sex were all scored as hermaphrodites in 2001. The same results are observed over a longer period (2000–2008, Table 1).
Sex expression appeared to be strongly genetically determined. The repetitions of the same genotype present in the seed orchard and in Orleans are very stable in sex expression. In 2000, 25 clones were homogeneous in sexual phenotype representing 210 individuals; 10 clones were heterogeneous representing 77 individuals with only 22 individuals differing from the others individuals of the clone. In 2001, 27 clones were homogeneous in sexual phenotype representing 191 individuals; 10 clones were heterogeneous representing 96 individuals with only 24 individuals differing from the others individuals of the clone.
3.2 Flower and fruit densities
In the natural population, the sexual phenotypes differed in their flower density (Table 2). Males produce significantly more flowers than hermaphrodites. In the seed orchard, the flower densities do not differ between sexual phenotypes. The different sexual phenotypes also differed in their accumulated flower density over the two years in the natural population and in the seed orchard (Ac flowers, Table 2). In both populations, males produce more accumulated flower than the others sexual phenotypes.
Statistical results.
| 2000 | 2001 | |||||
| Mean | SD | N | Mean | SD | N | |
| Natural population | ||||||
| Flower density | K = 6.6 df = 2 P = 0.037 | K = 24 df = 2 P = 6e – 6 | ||||
| F | 1.6 | 0.84 | 62 | 1.7 | 0.97 | 44 |
| H | 1.55 | 0.87 | 103 | 1.61 | 1.04 | 82 |
| M | 1.88 | 1.07 | 213 | 2.33 | 1.26 | 209 |
| Fruit density | K = 0.2 df = 1 P = 0.66 | K = 6.3 df = 1 P = 0.012 | ||||
| F | 0.93 | 1.41 | 59 | 1.27 | 1.45 | 40 |
| H | 1.02 | 1.43 | 99 | 0.6 | 0.98 | 77 |
| Pollen production | K = 38 df = 1 P = 6e – 10 | |||||
| H | 100.2 | 98.6 | 103 | |||
| M | 165.3 | 91.6 | 210 | |||
| Ac flowers/Ac fruits | K = 76 df = 2 P < 2e – 16 | K = 0.65 df = 1 P = 0.42 | ||||
| F | 1.96 | 1 | 66 | 1.41 | 1.7 | 63 |
| H | 2.1 | 1.3 | 103 | 1.2 | 1.6 | 103 |
| M | 3.6 | 1.8 | 229 | |||
| Seed orchard | ||||||
| Flower density | K = 4.9, df = 2, P = 0.08 | K = 3.6, df = 2, P = 0.17 | ||||
| F | 1.5 | 1.02 | 11 | 1.43 | 1.06 | 7 |
| H | 1.59 | 1.22 | 11 | 2 | 1.3 | 9 |
| M | 2.21 | 1.07 | 30 | 2.34 | 1.09 | 31 |
| Fruit density | K = 0.03, df = 1, P = 0.86 | K = 0.2, df = 1, P = 0.67 | ||||
| F | 2 | 1.49 | 11 | 1.21 | 1.11 | 7 |
| H | 1.9 | 1.35 | 10 | 1.61 | 1.56 | 9 |
| Pollen production | K = 2.1, df = 1, P = 0.14 | |||||
| H | 148 | 71 | 11 | |||
| M | 196 | 96 | 30 | |||
| Ac flowers/Ac fruits | K = 2.3, df = 2, P = 0.002 | K = 0.1, df = 1, P = 0.75 | ||||
| F | 2.7 | 0.97 | 9 | 2.8 | 1.75 | 6 |
| H | 2.8 | 2.12 | 9 | 3.5 | 2.5 | 6 |
| M | 4.6 | 1.65 | 29 |
No effect of sex on fruit density was revealed in either population, except in 2001 in the natural population where females produce more fruits than hermaphrodite (Table 2). Similarly, the sexual phenotypes did not show any difference in their accumulated fruit density over the two years in the natural population and in the seed orchard (Table 2).
3.3 Pollen production
The number of pollen grains per flower varied across sexual phenotypes in the natural population in 2000 (Table 2). Males produce significantly more pollen grains per flower than hermaphrodites. In the seed orchard, the same year, pollen production per flower is similar across genders (Table 2).
3.4 Phenology
In the natural population in 2001, flowering phenology depended on gender (χ2 = 55.4, df = 4, p < 0.001). Males tended to flower earlier, whereas there was an excess of females and hermaphrodites flowering mid and late in the blooming season, respectively.
4 Discussion
4.1 F. excelsior exhibits a trioecious breeding system
We found three phenotypic genders in both populations, as expected in a true trioecious breeding system: trees producing pollen but no seeds, trees producing both pollen and seeds simultaneously (hermaphrodites) and, trees producing seeds (females). The three genders defined here are justified on a functional basis and also from the point of view of the stability of the phenotype. Indeed, the studies of phenotypic stability described below showed that both in the orchard and in the population, the same individual, or different trees of the same genotype exhibit few cases of change from one gender to another.
4.2 Stability of sex expression and genetic determinism of sex
The present work is the first investigation of sex ratios in a large natural population of F. excelsior. The natural population consisted of a majority of male and hermaphrodite individuals, females being the rarest gender (Fig. 1). The stability of sexual phenotypes varied among gender. Females were notably far less stable than males and hermaphrodites in the natural population (Table 1). This result can be explained partly by the fact that female types are more difficult to observe and to determine accurately than male ones. Effectively, F. excelsior pistillate flowers exhibit a greater variability in their morphology than staminate and perfect flowers.
In the seed orchard, we found a strong genetic determinism for sexual phenotype in the species, as shown by the partition of nearly all variation in sex expression among clones for a given year, leaving very little variation within clones. This lack of within clone variation could definitively be attributed to a strong genetic component (broad sense heritability) to sex expression. The stability of sex expression between the two years of the study, associated with the genetic control of sex expression allow us to argue that F. excelsior reproductive system is truly trioecious: F, H and M really co-exist and are not a mere effect of environmental variation in sex expression.
4.3 Reproductive successes and the maintenance of trioecy
4.3.1 Pollen production of males vs hermaphrodites
In Maurice et al. [13]’s theoretical approaches, trioecy can be stable only if males exhibit a larger male fertility than hermaphrodites. Male advantage can be assessed either indirectly at a pre-zygotic stage by measuring, for example, flower or pollen production, or directly at a post-zygotic stage by quantifying male seed siring success with paternity analysis. Our results showed a male advantage in pollen production in F. excelsior in the natural population. Moreover, males produce a higher flower density than hermaphrodites in the natural population and also a higher accumulated flower production. Combining pollen and flower production, males have a higher pre-zygotic advantage over hermaphrodites. Our results agree with those of Wallander [32] who also found that F. excelsior males produced significantly more pollen grains per anther than hermaphrodite individuals (n = 4 trees). Male fertility advantage at the post-zygotic level has also been detected for F. excelsior in controlled crosses [36]. Furthermore, male reproductive success in F. excelsior is likely linked to flowering phenology. We observed that, in the natural population, males flowered earlier than hermaphrodites. Moreover, F. excelsior perfect flowers are protogynous: their stigmata become receptive about a week before the anthers start to disperse pollen [32]. The difference in blooming period between males and hermaphrodites, along with the protogyny of the latter, might favour pollen grains from male vs hermaphrodite plants. This mechanism can account for a larger male advantage of male vs hermaphrodite trees in F. excelsior populations.
4.3.2 Female fertility of females vs hermaphrodites
In addition to the male advantage, the maintenance of trioecy also requires a larger fertility of females vs hermaphrodites [26]. However, our results showed no significant difference in fruit densities between females and hermaphrodites, except in 2001 in the natural population. No difference between females and hermaphrodites in accumulated flower and fruit densities over the two studied years was detected in the natural population and in the seed orchard. Female fertility advantage in trioecious species has already been suggested for F. excelsior [32] and observed for Pachycereus pringlei, where females produced 1.62 times more fruits and seeds per season than hermaphrodites [20,37]. Our study failed to reveal a female advantage in F. excelsior. Nevertheless, female fitness was measured only by fruit production observed for two years. Other mechanisms, such as inbreeding depression when hermaphrodites can self, allow females to outperform hermaphrodites via the production of a higher quantity of seeds or, of higher offspring quality [5,8,38]. In F. excelsior, some complementary studies on other life history traits, such as quantity and quality of offspring are needed to find a possible female advantage. Further investigations of fruiting patterns of females and hermaphrodites during several years are also required.
4.4 Evolution of F. excelsior breeding system
In this study, we have shown both morphological (occurrence of three sexual morphs) and functional (males producing pollen, hermaphrodites producing pollen and seeds, females producing seeds) trioecy in F. excelsior. Theoretically, the maintenance of trioecy requires fitness advantages of males and females over hermaphrodites. We found a male advantage of males over hermaphrodites, but failed to show a female advantage of females over hermaphrodites. In the more general context of the evolution of plant breeding systems, trioecy in F. excelsior might be seen either as a stable reproductive system or as a breakdown of dioecy evolving towards androdioecy.
In F. excelsior, given the lower male fitness of hermaphrodites, the equivalent female fitness of females and hermaphrodites and the rarity of female individuals, hermaphrodites might be replacing females and, present trioecy might be seen as a transitory step towards androdioecy. However, both controlled crosses [36,39] and paternity analyses in natural conditions [40] showed that selfing rates in this species are very low. Thus, the necessary condition of self-compatibility for androdioecy to evolve from dioecy [41] does not seem to be fulfilled in F. excelsior. Moreover, the phylogenetic study of Wallander [32] on Fraxinus species suggested that dioecy in this genera was derived from androdioecy which had evolved from hermaphroditism. These findings are more in favour of a stable case of trioecy in F. excelsior rather than a transitory step in the breakdown of dioecy towards androdioecy.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors thank P. Bertolino, O. Cudelou, O. Jonot, L. Nowak, S. Triki-Teurtroy, J. Fernandez, E. Klein, B. Jung-Muller, J. Dufour, D. Desseaux and M. Lemonnier for their precious help. Financial support: FCPR grant, DERF, and the European Union.


