1 Introduction
Stevia (S.) rebaudiana (Bert.), belonging to the family Asteraceae, is cultivated throughout the world for its Stev. and Reb-A contents (SGs), which are 300 times sweeter than commercial sugar [1–3]. Stev. and Reb-A are low-calorie, non-toxic, non-mutagenic compounds; they have been recently approved by FDA in different countries, including Canada, China, Indonesia, Japan, Korea, Mexico, South America, UK, and the United States as a food supplement, especially for obese and diabetic patients [4,5]. S. rebaudiana species originated from Paraguay and Brazil, and is currently considered as an alternate substitute for cane and beet sugar [1]. According to the literature cited, Brazilian and Paraguay tribes used these valuable species for the treatment of heartburn in medicinal teas and Yerba mate [6]. Beside its sweet taste, antioxidant activities [7], anti-pathogenic [8], anti-cancerous, anti-hyperglycaemic [4], anti-hypersensitive properties [9] have been reported for this compound, as well as an effect in the prevention of dental caries [4]. The major part of SGs in the leaves is under Stev. and Reb-A forms. Other components are present, but in lower quantities such as steviolbioside 2, Reb C-F and dulcoside-A. Some evidence exists that steviolbioside and Reb-B are not genuine constituents of S. rebaudiana, but rather are formed by partial hydrolysis during the extraction process [5,10].
S. rebaudiana plants are conventionally propagated through cuttings, but this traditional method cannot produce a large number of plants. The seeds of S. rebaudiana are smaller in size and the germination percentage is very low [11]. Therefore, modern techniques of propagation such as in vitro regeneration or tissue culture are needed to enhance the production of this important specie. For these reasons, tissue culture techniques are widely used to produce a maximum mass from a single plant in a short span of time and also to provide opportunities for germplasm conservation of important plants [1].
The aim of the present study was to develop a feasible and cost-effective system for regeneration with enhanced SGs production. Different PGRs and agar concentrations were used to check its effect on micropropagation and major SGs biosynthesis. There are enough reports on the composition of SGs in the leaves of stevia, but the effect of different PGRs and agar concentrations on SGs have not yet being reported. Different studies showed that seed germination and stem cutting are not suitable approaches for healthy biomass and SGs production. Because Stevia seeds loose viability during storage, while stem cutting need higher input stock and also laborious. This promising approach will provide an opportunity for the selection of suitable PGRs and agar concentration for micropropagation and higher SGs production for commercial applications.
2 Material and methods
2.1 Micropropagation
2.1.1 Collection and sterilization of leaf explants
Fresh leaf explants were taken from laboratory cultures of S. rebaudiana at the Nuclear Institute for Food and Agriculture (NIFA), Peshawar, Pakistan. The collected leaf explants were immersed in autoclaved double-distilled water to maintain viability and to remove agar-medium particles. For surface sterilization, the procedure reported by Ahmad et al. [1,12] was applied. Ethanol (70%, 1 min) and mercuric chloride (0.2%, 120 s) were used for explant sterilization. The leaf explants were rinsed with double-distilled water and dried with the help of filter paper (autoclaved).
2.1.2 Media and growth conditions
For efficient in vitro regeneration of S. rebaudiana, Murashige and Skoog (MS) [13] medium was used as the basic medium, augmented with sucrose (3%) and solidified with two different agar concentrations (3.5 and 7.0 gL−1, Merck). Plant growth regulators (PGRs) from stock solution were added to the medium, and the pH (5.6–5.8) was adjusted with the help of a pH meter (330i/SET, Germany) by using a weak acid or a weak base. After pH adjustment, the media were boiled in a microwave oven and then autoclaved (Hirayama, Japan) at 121 °C for 20 min. All prepared media were then placed in a growth room (25 ± 1 °C) following a 16 h/8 h photoperiod [12].
2.1.3 Plant growth regulators (PGRs) for callus induction
Different concentrations and combinations of auxin were used for callus induction. Appropriate leaf portions were placed on MS-medium augmented with 2,4-dichlorophenoxyacetic acid (2,4-D; 1–3 mgL−1; T1–T3) alone or in combination with either α-naphthalene acetic acid (NAA; 1–3 mgL−1; T4–T6) or indole acetic acid (IAA; 1–3 mgL−1; T7–T9) with two different agar concentrations (3.5 and 7.0 gL−1). An MS-medium without any plant growth regulator (MS0; T10) was used as a control medium. Approximately 3 to 5 leaf portions were inoculated into the single food jar containing 30 to 40 mL of medium. Data on callogenesis were documented after four to five weeks of explant inoculation. Fresh and green callus was further transferred to a new medium for organogenesis.
2.1.4 PGRs concentrations and combination for shoots organogenesis
Eight treatments (T) of PGRs (cytokinin) were used for shoot regeneration from fresh callus cultures. The T1–T4 media contained different 6-benzyladenine (BA) concentrations (1–4 mgL−1) for shoot organogenesis. The T5–T8 medium was augmented with BA in combination with kinetin (Kin; 1–4 mgL−1). T9 was used as a control medium without the addition of PGRs. For all these treatments, two agar concentrations (3.5 and 7.0 gL−1) were used. Data regarding different parameters of shoot organogenesis (percentage shooting, mean shoot length, number of shoots per explant and number of leaves per explant) were documented after four weeks of callus subculture.
2.1.5 PGRs for roots organogenesis
Eight suitable treatments containing different combinations and concentrations of auxin were designed for root organogenesis. T1–T2 contained indole butyric acid (IBA; 1–2 mgL−1). T3–T4 contained indole acetic acid (IAA; 1–2 mgL−1). T5–T6 contained NAA (1–2 mgL−1). T7–T8 contained a combination of IBA (1–2 mgL−1) with IAA and NAA (1–2 mgL−1). For root induction, MS media without PGRs, with two agar concentrations (3.5 and 7.0 gL−1), were used as controls. Mean rooting percent, mean root length and number of roots per plantlet was recorded after three to five weeks of shoot transferring.
2.1.6 Acclimatization
Well-rooted plantlets were taken out from the medium and washed several times with sterile distilled water to remove agar particles. A suitable combination of soil, sand and manure was prepared and autoclaved before being poured into pots. The plantlets were shifted to these pots and completely covered with plastic bags to retain humidity. These pots were placed under growth room conditions. Plastic bags were periodically removed for a period of 21 days. After their successful establishment in pots, these plantlets were shifted to a lath house and finally to the field for further studies.
2.2 Extract preparation for HPLC analysis
In vitro regenerated shoots of S. rebaudiana were collected from cultured jars. These shoots were washed with sterile distilled water to remove agar particles and were dried with the help of filter paper. Finally, in vitro grown shoots from sixteen treatments (T1–T8; agar 3.5: T9–T16; agar 7.0: T17 control) were dried in an electric oven at 50 °C for 24 h. The dried tissues were ground to get well-powdered materials [14]. Powder from each treatment was extracted with HPLC-grade ethanol (Merck) and finally filtered through Whatman filter paper No. 1 (Whatman Ltd., England). The filtrate was dried by using a freeze dryer and stored at 4 °C in airtight bottles, and the extractive values in pure ethanol were recorded. The extracts obtained from each part were dissolved independently in HPLC-grade ethanol and filtered through 45 μm membranes for analysis.
2.2.1 Analytical method
Extraction of SGs (stevioside, rebaudioside-A and dulcoside-A) from dried powdered samples was carried out according to the recent method of Dey et al. [4] with little modification. The dried extract (20 mg) was independently dissolved in HPLC-grade ethanol (10 mL; 2 mg/mL) for analysis and stock solution preparation. HPLC analysis was performed at ambient temperature using a LC-8A, Shimadzu system (Japan) set with a binary pump, a solvent vacuum degasser, a 150 × 4.6 mm C-18 column (ODS), 5 μm particle size, a variable wavelength detector, an auto sampler with a 10-μl injection loop; the absorption was made at 210 nm.
The mobile phase contained HPLC-grade methanol (70%; A) and HPLC-grade water (30%; B). The flow rate was 1.5 mL/min and the injection volume was 10 μL. The SGs standard was purchased from Sigma (USA) and dissolved in HPLC-grade water (200 μg/mL). The standard was run on an HPLC system and the retention time for stevioside, rebaudioside-A and dulcoside-A was determined. Identification of stevioside, rebaudioside-A and dulcoside-A in different in vitro-grown shoots was achieved by comparing the retention time of samples with standard data and chromatogram (Supplementary data, Fig. S1). The results obtained for each sample were expressed in microgram/gram (μg/g) of dry weight (DW).
2.3 Statistical analysis
All the analysis was repeated three times, and the design of the experiment was completely randomized. Statistical analysis of mean values, standard deviation (±), and least significant difference (LSD) were carried out by using Statistix software (8.1 versions). For graphical presentation, Origin Lab (8.1) software was used. All the results are statistically significant when P < 0.01.
3 Results and discussion
3.1 Micropropagation
3.1.1 Callus induction from leaf portions
In this study, a feasible regeneration system was established from leaf explants of Stevia rebaudiana Bertoni (Fig. 1). The first step in indirect regeneration is the induction of callus culture from suitable explants. In this study, maximum callus induction (91.67%) was observed when suitable leaf portions were placed on MS-medium augmented with 2.0 mgL−1 of 2,4-D with 3.5 gL−1 agar concentration (Figs. 1–2). Significantly, a similar callus induction response was observed on a medium containing a combination of 2,4-D and NAA (2.0 mgL−1) along with 7.0 gL−1 agar concentration. In the literature cited, few reports are available on callus induction from leaf explants of S. rebaudiana. Ahmad et al. [1] reported similar callus induction response, but the explants were floral parts rather than leaf portions. Swanson et al. [15] reported that the elimination of agar from the medium or its modulation greatly affects callus texture and production from leaf explants. Hwang et al. [16] observed that the combination of 2,4-D and NAA influences callus induction from mature explants. In this study, good-quality callus (< 80%) was observed when the MS-medium was augmented with 2,4-D and NAA (1.0 mgL−1 or 2.0 mgL−1) with 3.5 gL−1 agar concentration. No callus was observed on the MS0 medium. The 2,4-D alone or in combination with NAA was more effective in callus induction than 2,4-D and IAA (Fig. 2). Furthermore, these results suggested that a lower quantity of agar accelerates the formation of a good friable callus more than the addition of a higher amount of agar to the medium.
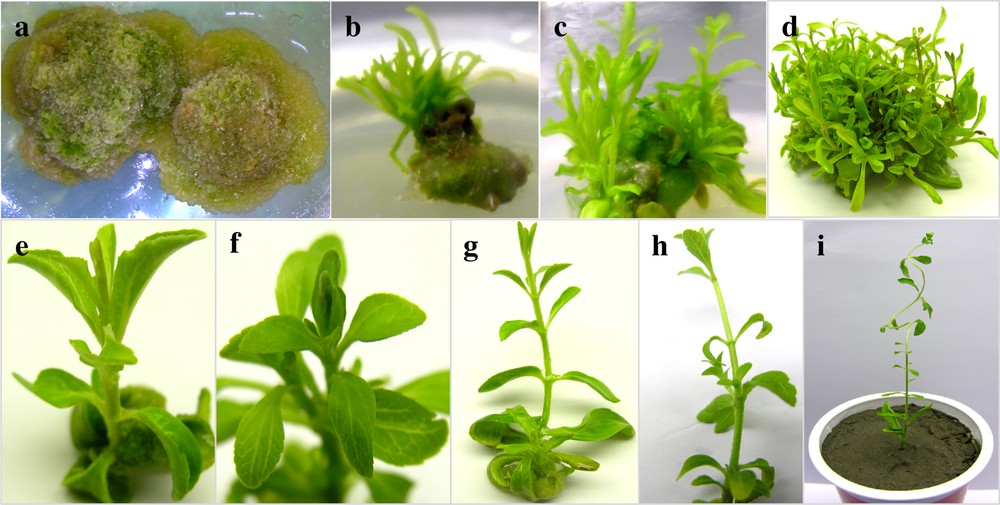
Callus induction from leaf explants of Stevia rebaudiana (Bert.). (a) Callus induced on a medium containing 2.0 mgL−1 of 2,4-D with 3.5 gL−1 agar, (b) Shoot regeneration on a medium containing BA (T1: 1.0 mgL−1 + 3.5 gL−1), (cd) Shoots multiplication, (ef) shoots maturation, (gh) shoots elongation, (i) acclimatization of an in vitro-grown plantlet.

Percent callus induction on media containing 2,4-D alone (T1: 1.0 mgL−1 + 3.5 + 7.0 gL−1 agar, T2: 2.0 mgL−1 + 3.5 + 7.0 gL−1 agar, T3: 3.0 mgL−1 + 3.5 + 7.0 gL−1 agar) either in combination with NAA (T4: 1.0 mgL−1 + 3.5 + 7.0 gL−1 agar, T5: 2.0 mgL−1 + 3.5 + 7.0 gL−1 agar, T6: 3.0 mgL−1 + 3.5 + 7.0 gL−1 agar) or in combination with IAA (T7: 1.0 mgL−1 + 3.5 + 7.0 gL−1 agar, T8: 2.0 mgL−1 + 3.5 + 7.0 gL−1 agar, T9: 3.0 mgL−1 + 3.5 + 7.0 gL−1 agar) and control (MS0: +3.5 + 7.0 gL−1 agar). Data were collected from three independent experiments. Mean values (mean ± SD + LSD) with the common alphabet are not significantly different when P < 0.01.
3.1.2 Effect of different PGRs and agar on shoot organogenesis
A fresh and good-quality callus was shifted to a new medium augmented with different concentrations and combinations of PGRs and agar for shoot organogenesis (Table 1). The highest (100%) shoot regeneration potential was observed when the agar concentration was kept at 7.0 gL−1 and the MS-medium was augmented with different BA concentrations (1.0, 2.0, and 4.0 mgL−1), as shown in Table 1. However, the addition of similar PGRs to a 3.5 gL−1 medium induced a shooting response of more than 80% after four weeks of callus culture. Similar results were also reported by Ahmad et al. (2011), showing that BA alone can influence the shooting response in S. rebaudiana. The combination of BA with Kin (2.0 mgL−1) also showed a better regeneration potential of 93.33% when 3.5 gL−1 agar was added to the MS-medium (Table 1). Ahmad et al. [12] also observed that addition of other cytokinin to an MS-medium containing BA influenced shoot organogenesis. Similar results were also documented by Wan et al. [17] and Huii et al. [18] in different plant species.
Shoot organogenesis (% shooting, mean shoot length, no. of shoots and leaves per explant) in Stevia rebaudiana on MS-medium containing various concentrations of BA alone (T1: 1.0 mgL−1, T2: 2.0 mgL−1, T3: 3.0 mgL−1, T4: 4.0 mgL−1, with 3.5 and 7.0 agar gL−1) or in combination with Kin (T5: 1.0 mgL−1, T6: 2.0 mgL−1, T7: 3.0 mgL−1, T8: 4.0 mgL−1, with 3.5 and 7.0 agar gL−1) and control (MS0 + 3.5 and 7.0 agar gL−1).
| Treatments + agar | Shoot induction (%) | Mean shoot length (cm) | No. of shoots per explant | No. of leaves per explant |
| T1 (3.5 gL−1) | 85 ± 7.6abc* | 2.12 ± 0.33b* | 8.36 ± 2.36b* | 28.16 ± 9.56bcd* |
| T2 (7.0 gL−1) | 100 ± 0.0a* | 13.03 ± 0.57a* | 28.0 ± 1.62a* | 109.67 ± 3.48ab* |
| T3 (3.5 gL−1) | 85 ± 7.6abc* | 1.44 ± 0.27d* | 4.68 ± 1.82bcd* | 20.13 ± 5.27d* |
| T4 (7.0 gL−1) | 100 ± 0.0a* | 9.47 ± 0.19c* | 15.0 ± 0.23bc* | 131.0 ± 2.15a* |
| T5 (3.5 gL−1) | 60 ± 10.0d* | 1.87 ± 0.19bc* | 2.33 ± 0.84d* | 21.55 ± 3.86cd* |
| T6 (7.0 gL−1) | 85 ± 7.6abc* | 6.03 ± 0.12de* | 7.0 ± 0.22c* | 49.67 ± 0.77c* |
| T7 (3.5 gL−1) | 70 ± 5.0cd* | 1.64 ± 0.15cd* | 2.88 ± 0.78cd* | 23.55 ± 3.12cd* |
| T8 (7.0 gL−1) | 100 ± 0.0a* | 8.23 ± 0.19cd* | 15.66 ± 1.15bc* | 99.67 ± 2.20ab* |
| T9 (3.5 gL−1) | 76.7 ± 1.7bcd* | 3.90 ± 0.04a* | 6.19 ± 1.61ab* | 34.27 ± 5.53ab* |
| T10 (7.0 gL−1) | 93.3 ± 6.7ab* | 12.30 ± 0.25ab* | 16.33 ± 0.96b* | 132.67 ± 3.17a* |
| T11 (3.5 gL−1) | 93.3 ± 6.7ab* | 2.01 ± 0.05bc* | 5.50 ± 0.76bc* | 37.92 ± 3.22a* |
| T12 (7.0 gL−1) | 93.3 ± 6.7ab* | 8.93 ± 0.27c* | 11.33 ± 0.22bc* | 83.33 ± 1.09bc* |
| T13 (3.5 gL–1) | 91.7 ± 8.3ab* | 2.06 ± 0.33bc* | 4.83 ± 1.33bcd* | 22.75 ± 7.13cd* |
| T14 (7.0 gL−1) | 70 ± 5.0cd* | 4.60 ± 0.17e* | 7.67 ± 0.45bc* | 44.0 ± 0.51c* |
| T15 (3.5 gL−1) | 61.7 ± 7.3d* | 1.87 ± 0.33bc* | 5.94 ± 1.36b* | 29.28 ± 3.29bc* |
| T16 (7.0 gL−1) | 91.7 ± 8.3ab* | 10.17 ± 0.58bc* | 12.67 ± 1.06bc* | 96.67± 4.61ab* |
| MS0 | 25.0 ± 4.6e* | 1.11 ± 0.27d* | 1.00 ± 0.00e* | 3.30 ± 0.53e* |
It has been observed that lower concentrations of agar significantly reduced the length of micro-shoots, but that higher concentrations promoted shoot elongation (Table 1). In this experiment, a lower concentration of BA (1.0 mgL−1) and a higher concentration of agar showed a maximum shoot length of 13.03 cm. Under similar PGRs condition, lower agar concentration reduced shoot length to 2.12 cm. Moreover, addition of BA in combination with Kin (1.0 mgL−1) also showed higher mean shoot length (12.3 cm). Shoot elongation in S. rebaudiana under in vitro conditions is a major problem, because elongated shoots begin curling after transferring to soil environment. Therefore, in this experiment, we get a reduced shoot length by the addition of a lower agar concentration. More recently, Dey et al. [4] reduced the length of micro-shoots by the addition of the IBA and chlorcholine chloride into the MS-medium.
Presently, the highest (28) number of shoots per explant was documented when the medium was supplemented with 1.0 mgL−1 BA along with a higher agar concentration (Fig. 1d; Table 1). Thiyagarajan and Venkatachalam [19] recently reported a total of 123 shoots per explant. The difference in the data is due to the explant type and the method of regeneration. We used an indirect regeneration system in which multiple shoots were obtained from callus culture and Thiyagarajan and Venkatachalam [19] followed direct regeneration from nodal segments. The current results are in agreement with the observation of Ahmad et al. [12]. Furthermore, BA alone (2.0 and 4.0 mgL−1) or in combination with Kin (1.0 mgL−1) produced > 15 number shoots per explant (Table 1). Ahmad et al. [1] also reported that the addition of BA induced a maximum number of shoots per explant in S. rebaudiana. However, 8.35 shoots per explant were obtained when the medium was augmented with 3.5 gL−1 agar and 1.0 mgL−1 of BA. Lower concentration of agar in this condition is less effective in shoot multiplication.
In this experiment, the number of leaves per explant under the influence of different PGRs and agar was also investigated. The addition of BA and Kin (1.0 mgL−1) to the MS-medium containing a higher agar concentration produced a significantly higher (132.67) number of leaves per explant (Table 1). Lower BA concentrations (1.0 and 2.0 mgL−1) also produced 109.67 and 131 leaves per explant. However, MS-medium containing lower agar concentrations with different PGRs produced a lower number of leaves as compared to the higher agar concentration. But we already mentioned that a lower agar concentration was effective for shoot length reduction. A reduction in shoot length is directly proportional to a reduction in the number of leaves, but the plantlets obtained were comparatively vigorous and strong enough for acclimatization.
3.1.3 Effect of different PGRs and agar on root organogenesis
Vigorous shoots with suitable length were transferred to a fresh MS-medium containing two agar concentration for rhizogenesis (Table 2). Nine treatments (T1–T9) have been applied for root organogenesis. 100% root induction was observed when the MS-medium was augmented with 2.0 mgL−1 of IAA along with 3.5 and 7.0 gL−1 agar concentrations after four weeks of culture.
Root organogenesis (% rooting, mean root length and No. of roots per shoot) in Stevia rebaudiana on an MS-medium containing IBA alone (T1: 1.0 mgL−1, T2: 2.0 mgL−1), or IAA alone (T3: 1.0 mgL−1, T4: 2.0 mgL−1, with 3.5 and 7.0 agar gL−1) or NAA alone (T5: 1.0 mgL−1, T6: 2.0 mgL−1) or IBA in combination with IAA and NAA (T7-T8: with 3.5 and 7.0 agar gL−1).
| Treatments + agar | Root induction (%) | Mean root length (cm) | No. of roots per explant |
| T1 (3.5 gL−1) | 70.00 ± 5.00cd* | 1.85 ± 0.25ab* | 9.78 ± 0.97bc* |
| T2 (7.0 gL−1) | 85.00 ± 7.64bc* | 2.98 ± 0.49a* | 11.64 ± 0.83bc* |
| T3 (3.5 gL−1) | 93.33 ± 6.67ab* | 0.61 ± 0.04bc* | 8.42 ± 1.88bc* |
| T4 (7.0 gL−1) | 61.67 ± 7.26f* | 0.72 ± 0.22c* | 10.11 ± 2.67bcd* |
| T5 (3.5 gL−1) | 83.33 ± 8.33abc* | 1.34 ± 0.47b* | 12.77 ± 3.93b* |
| T6 (7.0 gL−1) | 83.33 ± 8.33bcd* | 0.91 ± 0.44c* | 5.45 ± 0.99d* |
| T7 (3.5 gL−1) | 100 ± 3.33a* | 1.56 ± 0.58b* | 15.67 ± 3.86ab* |
| T8 (7.0 gL−1) | 76.67 ± 1.67cde* | 0.80 ± 0.05c* | 9.41 ± 2.21bcd* |
| T9 (3.5 gL−1) | 55.55 ± 5.55de* | 0.13 ± 0.09c* | 0.67 ± 0.18c* |
| T10 (7.0 gL−1) | 69.44 ± 2.78def* | 1.44 ± 0.12bc* | 13.05 ± 1.90ab* |
| T11 (3.5 gL−1) | 46.67 ± 3.33e* | 2.90 ± 0.60a* | 26.33 ± 6.64a* |
| T12 (7.0 gL−1) | 91.67 ± 8.33ab* | 1.30 ± 0.39bc* | 5.58 ± 2.58cd* |
| T13 (3.5 gL−1) | 83.33 ± 8.33abc* | 1.16 ± 0.47bc* | 7.80 ± 3.52bc* |
| T14 (7.0 gL−1) | 66.66 ± 3.78ef* | 1.90 ± 0.25b* | 9.83 ± 1.30bcd* |
| T15 (3.5 gL−1) | 77.77 ± 11.11bc* | 1.81 ± 0.46ab* | 5.17 ± 1.42bc* |
| T16 (7.0 gL−1) | 100 ± 2.33a* | 1.60 ± 0.09bc* | 19.02 ± 2.28a* |
| MS0 | 0 | 0 | 0 |
* Values are mean of three replicates with ± standard error. Data were collected after four weeks of culture and are not significantly different when P < 0.01.
The IBA alone (2.0 mgL−1) also showed better rooting (93.3%) as compared to control and NAA augmented media (Table 2). Ahmad et al. [12] observed that the MS-medium containing IBA (2.0 mgL−1) also influence the rooting percent response in Piper nigrum L. However, the combination of IBA (1.0 and 2.0 mgL−1), IAA (1.0 and 2.0 mgL−1) and NAA (1.0 and 2.0 mgL−1) was found less effective in percent root induction. But maximum shoot length (2.9 cm) with 26.33 roots per plantlet was observed when the MS-medium was augmented with 2.0 mgL−1 of NAA along with 3.5 gL−1 agar concentration (Table 2). Similar root length with same number of roots per shoot was also observed with the addition of 1.0 mgL−1 IBA, but the agar concentration was higher. It has been observed that application of similar PGRs and different agar concentrations in the medium greatly affects root organogenesis.
3.1.4 Acclimatization of regenerated plantlets
Regenerated plantlets were successfully transferred to pots containing a combination of soil, sand, and manure in a ratio of 2:1:1 (Fig. 1i). The establishment of plants in pots was confirmed after the emergence of new leaves on the shoot tips. The leaves on the lower part of the plants underwent welting due to lower humidity. But new leaves emerge from after four weeks of establishment. The pots containing the plantlets were kept in a growth chamber for five weeks and then transferred to the greenhouse. Healthy plantlets from pots were then transferred to field conditions for raising MV1 generation. Plants with vigorous growth, higher number of leaves will be selected for raising MV2 generation.
3.2 Major steviol glycoside production
Major steviol glycosides (Dulcoside-A, Steviosides and Rebaudioside-A) were determined in in vitro shoots obtained from media augmented with different PGRs and agar treatments. It has been observed that the combination of BA and Kin (3.0 mgL−1) along with 3.5 gL−1 agar significantly increased the dulcoside-A content (71.8 μg/g-DW) compared to control (50.81 μg/g-DW) (Fig. 3). As the agar concentration increases with similar PGRs, the dulcoside-A content decreases from 71.8 μg/g-DW to 54.0 μg/g-DW. The addition of a lower concentration of BA (1.0 mgL−1) into the medium with 7.0 gL−1 agar showed 53.6 μg/g-DW dulcoside-A content (Fig. 3). However, a significant reduction has been observed in the content (12.70 μg/g-DW) with the addition of a 3.5 gL−1 agar concentration. It has been clearly observed from these results that different agar concentrations in the medium make the dulcoside-A content fluctuate. A higher dulcoside-A content (60.24 μg/g-DW) was observed in a medium containing 3.5 gL−1 agar and 3.0 mgL−1 BA, but when the agar concentration was increased, the dulcoside-A content significantly decreases (13.24 μg/g-DW).

Dulcoside-A content in different in vitro-grown tissues of Stevia rebaudiana. T1: 1.0 mgL−1 BA, T2: 2.0 mgL−1 BA, T3: 3.0 mgL−1 BA, T4: 4.0 mgL−1 BA, T5: 1.0 mgL−1 BA + Kin, T6: 2.0 mgL−1 BA + Kin, T7: 3.0 mgL−1 BA + Kin, T8: 4.0 mgL−1 BA + Kin, with 3.5 and 7.0 agar gL−1. Values are mean of three replicates. Bars from mean values (±SD + LSD) with the common alphabet are not significantly different when P < 0.01.
Furthermore, a higher stevioside (82.48 μg/g-DW) content was observed in shoots on a medium containing a combination of BA and Kin (3.0 mgL−1) with a 7.0 gL−1 agar concentration (Fig. 4). However, a lower steviosides content (22.2 μg/g-DW) was noted with lower agar concentration with similar PGRs. It means that the agar concentration significantly influenced stevioside production under in vitro conditions. Similar stevioside contents in different in vitro cultures of S. rebaudiana were reported by many researchers, but the effect of agar concentration on stevioside production are not reported yet [4,15,16,20]. The addition of BA (3.0 mgL−1) alone into the MS-medium with 3.5 gL−1 also influences the steviosides content (63.77 μg/g-DW) as compared to the higher agar concentration (30.89 μg/g-DW) (Fig. 4). From these results, it was observed that the combination of BA and Kin is very effective for major SGs production than BA alone with different agar concentrations.
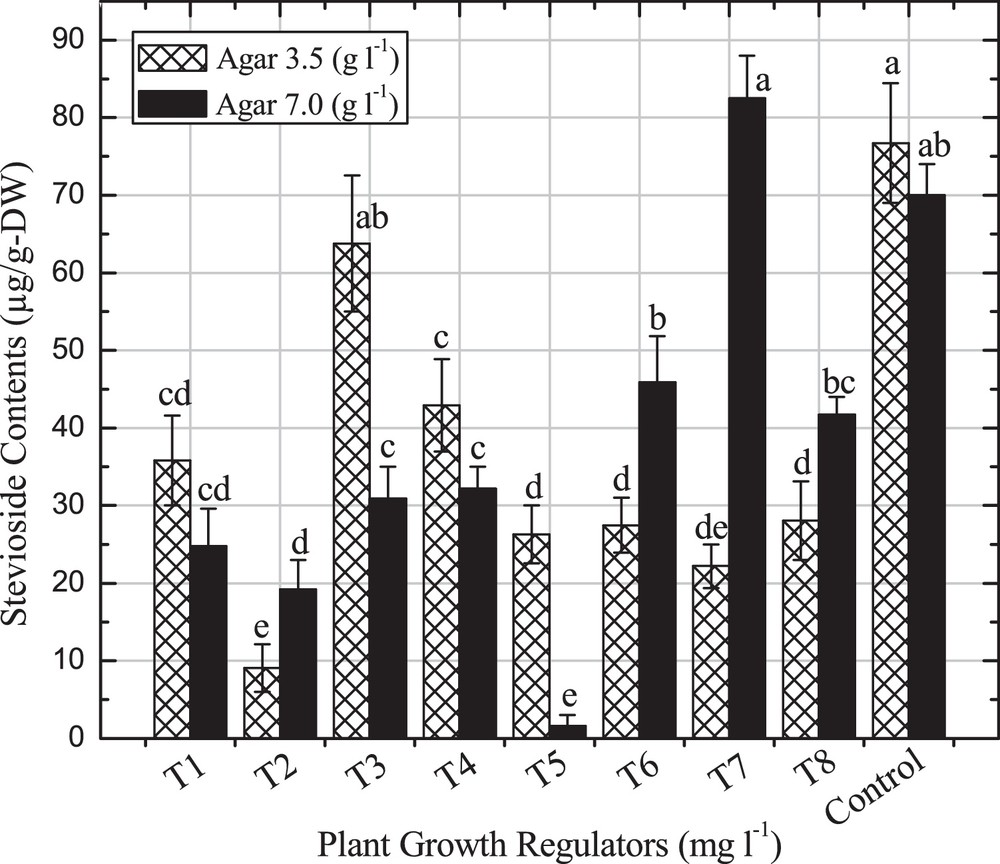
Steviosides content in different in vitro-regenerated shoots. Values are mean of three replicates. Bars from mean values (±SD + LSD) with the common alphabet are not significantly different when P < 0.01.
A lower amount of rebaudioside-A content was observed in all treatments as compared to dulcoside-A and steviosides content. A maximum rebaudioside-A content of 12.35 μg/g-DW was obtained with shoots grown on a medium containing BA (1.0 mgL−1) with 7.0 gL−1 agar compared to control (07.39 μg/g-DW) (Fig. 5). As the BA concentration increases, the rebaudioside-A content decreases with varying agar concentrations. The current data are in agreement with the reports of Swanson et al. [15], Ladygin et al. [20] and Bondarev et al. [5] in different in vitro-grown tissues of S. rebaudiana.
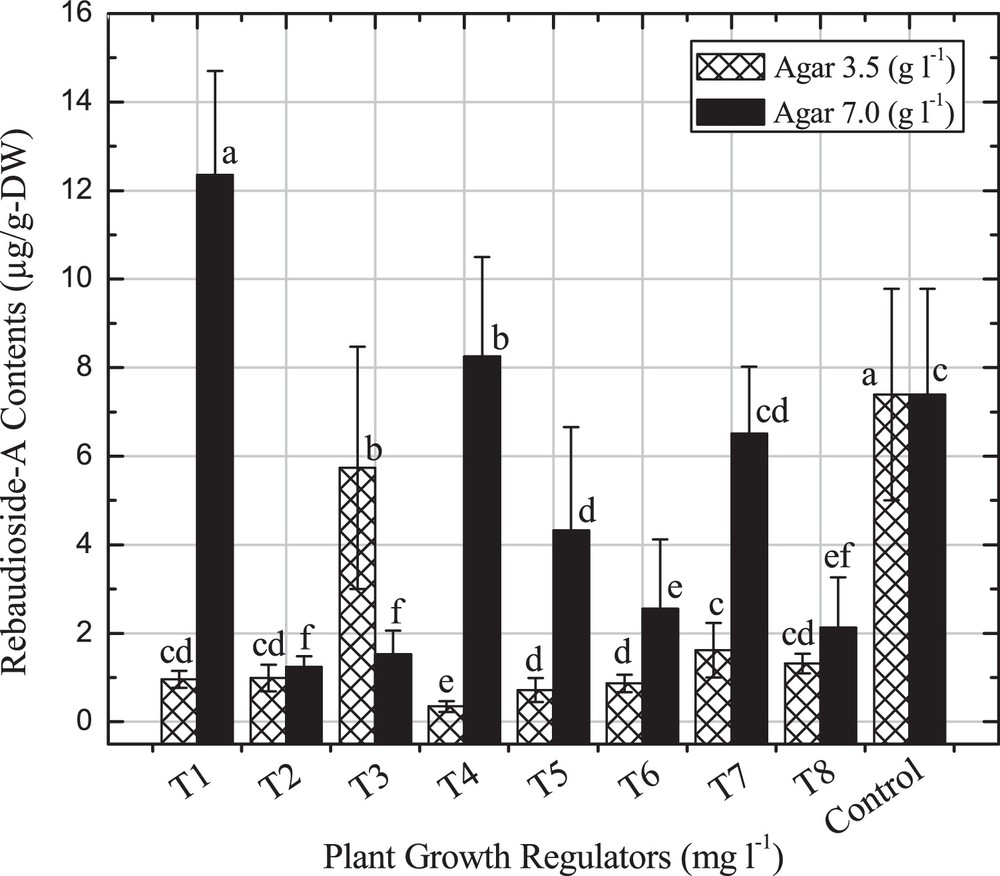
Rebaudioside-A content in different in vitro shoots. Values are mean of three replicates. Bars from mean values (±SD + LSD) with the common alphabet are not significantly different when P < 0.01.
S. rebaudiana has recently been considered as the most effective plant for diabetes and obesity limitation all over the world. In this experiment, the effect of selected PGRs and two agar concentrations have been used for feasible regeneration and consistent production of major steviol glycosides contents. These results showed that lower agar concentrations are more effective for callus cultures and root induction, while higher agar concentration influence shoot organogenesis. Selected PGRs and agar concentrations increase the dulcoside-A (71.8 μg/g-DW), steviosides (82.48 μg/g-DW) and rebaudioside-A (12.35 μg/g-DW) content compared to control. In conclusion, this study will be highly useful for further work on S. rebaudiana for the selection of appropriate PGRs for reliable regeneration and enhancement of SGs production for commercial purposes.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We acknowledge the support of Pakistan Science Foundation (PSF), for completion of this research work.


