1 Introduction
Temperature is the main factor driving marine species distributions [1–3]. Over the last century, the average global temperature has increased by 0.6 °C and is projected to increase by a further 1–6 °C by 2100 [4]. In the Mediterranean Sea, an increase in sea-surface temperature (SST) has already been documented [5–8], but no clear warming projection is currently available, due to the lack of data in the eastern basin [9]. In the Northern hemisphere, species distributions are expected to shift northward [10,11], or, when northward migration is not possible, e.g. for some marine species restricted to the Mediterranean north coast, to acclimate/adapt to new thermal conditions, or to become extinct [12]. Thermal tolerance differs between species and even between populations of the same species due to local adaptation [13,14]. Thus, when comparing species and populations, it is important to test for phenotypic plasticity and/or local adaptation in order to infer a species’ potential response to future climate changes [15].
The brittle star Ophioderma longicauda (Bruzelius, 1805) is a common Atlanto-Mediterranean species [16] reproducing via the production of a vitellaria larva [17]. However, it was recently shown to be a species complex [18,19] comprising at least six divergent mitochondrial lineages with contrasting life-history strategies. Three lineages produce dispersing larvae (lineages L1–L5–L6), while the three others brood their offspring (lineages L2–L3–L4) [18,19]. A recent study (Weber et al., submitted) confirmed that L1 and L3 are separate species since they occur in sympatry and in syntopy, but do not display the same reproductive mode (larvae vs. brooder), do not reproduce at the same period of the year (April vs. July, prezygotic isolation), and are genetically differentiated at a nuclear locus, independent of the mitochondrial genome used to define lineages [20]. L1 is the dominant species in the western Mediterranean and the Atlantic basins, but seems less abundant in the eastern Mediterranean, where the dominant lineages are brooders L2–L3–L4 [19]. For example, L1 and L3 are the only lineages present in Crete, L3 being more abundant than L1. A differential bathymetric distribution was also found, L1 being predominantly found from five meters depth or more, and L3 being dominant at the shallowest depths (2–3 m). Brooding lineages L2–L3–L4 are absent from the western Mediterranean basin [19].
Abiotic conditions in the eastern Mediterranean basin differ from those of the western basin, and include oligotrophic waters [21], higher salinity [22], and higher mean water temperatures [9]. At the study site in Marseilles, monthly average temperatures at 5 m depth range between 12 and 24 °C during the year, with maximum temperatures around 26 °C in summer, when excluding an exceptional thermal anomaly at 28 °C [23]. At the Crete study site, temperatures range between 15 and 27 °C during the year, with a maximum of around 28 °C in summer [9]. Short-term temperature variations can be very high and differences of 10 °C can occur in 24 h in Marseilles [23]. In contrast, summer seawater temperatures in Crete are much more stable (Thanos Dailianis, unpublished data).
To study the thermotolerance of O. longicauda, we must consider the occurrence of several lineages. Therefore, we used the different lineages L1 and L3, without geographical replicates, for this preliminary study. Based on its geographical and bathymetric distributions, the lineage L3 may be more adapted to elevated temperatures than L1. Thermal tolerances of L1 and L3 were measured using three parameters: survival, spontaneous autotomy as a proxy for thermal stress [24] and arm regeneration rate. We assumed that a temperature environment that is favorable to regeneration is also favorable to somatic growth of the not-injured brittle stars. Thus, experimental investigation of regeneration would help us making inference on the thermal tolerances of the lineages. Three different temperatures were tested, covering today's (17–26 °C) and future (30 °C) natural variability. Individuals from the species L1 and L3 were collected from two locations: Marseilles (France; L1) and Crete (Greece; L1 and L3).
2 Material and methods
2.1 Specimen collection and genetic analyses
O. longicauda specimens were collected by scuba diving during May 2012, in Crete (35° 16’ N; 25° 49’ E), Greece (n = 112) and in June 2012 from Marseilles (43° 16’ N; 5° 17’ E), France (n = 80). Specimens from Crete were shipped alive to Marseilles at ambient temperature in 2-L non-insulated plastic containers (10 individuals per container) filled with seawater saturated with O2. The transportation lasted 10 h in total. All individuals survived and did not display any autotomy or other signs of stress. To reduce possible stress due to transportation, all individuals were kept prior to experiment in an open seawater circuit aquarium for two weeks for acclimation (temperature ranging between 17 °C and 19 °C) and were fed once a week ad libitum with commercial fish pellet food.
All individuals used were genotyped to determine their lineage. A small piece of arm was cut from each individual and DNA extracted following a Chelex protocol [25]. A 689-pb fragment of Cytochrome Oxidase I (COI) was PCR amplified with the primers COIa (5’ AGT ATA AGC GTC TGG GTA GTC 3’) and COIf (5’ CTT GCA GGA GGA GGA GAY CC 3’) using the following parameters. An initial denaturation step of 5 min at 95 °C, followed by 40 cycles of 30 s of denaturation at 95 °C, 30 s of annealing at 52 °C and 45 s of extension at 72 °C, with a final extension step of 5 min at 72 °C. A Restriction Fragment Length Polymorphism (RFLP) protocol was then developed to rapidly recognize the lineage of each individual. BccI restriction enzyme (cutting site: CCATC(N)4/) (New England Biolabs) generates different fragments lengths specific to each lineage after digestion. Restriction simulations were done with the online program NEBcutter V2.0 and verified with PCR products of sequenced L1 and L3 individuals. For each individual, 10 μl of PCR product were added to 1U of Bcc I, 1X of buffer NEB 1, 1X of BSA and digestion was performed for 90 min at 37 °C. Restriction fragments were migrated on a 2% agarose gel stained with ethidium bromide. The RFLP analysis indicated that individuals collected from Crete belonged to lineages L3 (L3C; n = 80) and L1 (L1C; n = 32). All individuals collected in Marseilles belonged to lineage L1 (L1M; n = 78). Beside the missing piece of arm used for lineage determination, individuals did not show any sign of a recent wound.
2.2 Experimental design
Three different temperatures were chosen to test the thermal tolerance of O. longicauda, based on the natural temperature variation in Crete and Marseilles during spring and summer. A temperature of 17 °C corresponds to the average one during the studied period, whereas 26 °C is close to the maximal temperature observed at present (slight stress), and 30 °C would correspond to extreme conditions projected under a global warming scenario (strong stress). This temperature was chosen to ensure that the stress would be sufficient to cause mortality.
Two replicate tanks in semi-open circuit were used per temperature (17, 26, 30 °C) and per group (L1M, L1C, L3C) each containing five (L1C) or 13 (L1M and L3C) specimens, for a total of 18 tanks. After 2 weeks of acclimation, temperature was raised of ∼1 °C per day until the desired temperature was reached. Brittle stars were fed once a week ad libitum with commercial fish pellet food. At the beginning of the experiment, four tanks (one of L3C at 17 °C; L3C, L1C and L1M at 30 °C) displayed bacterial contaminations that rapidly killed all individuals; those tanks were excluded from further analyses.
2.3 Animal preparation and measured parameters
Dupont and Thorndyke [26] showed that length lost (LL) is the key intrinsic parameter regulating regeneration rate (RR). As the specimens of O. longicauda displayed heterogeneous sizes, arms were amputated at a fixed LL of 5 cm to standardize the RR. Experimentally induced amputations were performed on the arm already amputated for DNA extraction. The position of amputation was calculated for a LL of 5 cm from the linear relationship between disc diameters (DD) and longest arm lengths (AL), where AL = 0.48 DD + 0.52 (R2 = 0.88). There were no significant differences between regressions constructed with L1 and L3 data (n: L1 = 40; n: L3 = 42), thus L1 and L3 data were pooled to construct the linear regression (n = 82). For a few small individuals with arm lengths < 5 cm, the whole arm was cut from the base of the disc. Their regeneration trajectories are displayed in Fig. 1, but they were excluded from further statistical analyses testing the impact of temperature, lineage, and populations. All amputations were performed after anesthesia by immersion in 3.5% w/v MgCl2.6H2O in seawater. Experimental arm amputation was achieved by applying a scalpel blade across a natural inter-vertebral autotomy plane. The disc of each individual was photographed to measure disc diameter and to allow individual identification.

A–C. Individual regeneration trajectories in each group (L1M, L1C and L3C) and temperature (17, 26, 30 °C). Dashed lines trajectories correspond to individuals with DD < 10 mm, excluded from analyses as LL < 5 cm. 1D: mean Gomperz curves, calculated from the mean of estimated parameters ML, MR and tip for each group and temperature. L1M: individuals of lineage L1 collected in Marseilles. L1C: individuals of lineage L1 collected in Crete. L3C: individuals of lineage L3 collected in Crete. DD: disc diameter. LL: length lost. ML: maximal length of regenerate. MR: maximal rate of regeneration. tip: time to the inflexion point.
Aquaria were monitored daily during 14 weeks and dead individuals were immediately removed. The length of the regenerate was measured after anesthesia by immersion in 3.5% w/v MgCl2.6H2O for each individual at weeks 3, 6, 9 and 14. At the end of the 14th week, the remaining individuals were scored as intact or with autotomized arms.
2.4 Statistical analyses
At the end of the experiment, individuals were classified into three categories: intact, showing sign of autotomy, or dead. The effects of temperatures (17, 26, 30 °C) and brittle star group of origin (L1C, L1M, L3C) were tested by applying Fisher's exact test on the contingency tables displaying the number of individuals in each category for different temperatures or different groups. Data from replicate tanks were pooled to increase the robustness of the analyses and average the different responses per tank.
The kinetics of regeneration for each individual was modeled as a Gomperz growth between regenerate length (RL) and time. Specimens for which regenerate length was not available for the four time points (e.g., as a consequence of autotomy) were not considered for this analysis. Each individual trajectory was characterized by three parameters calculated as constants of the Gompertz equation of an asymmetrical growth curve estimated by the least-square method from the original data [27]. The calculated parameters were ML, the maximal length of the regenerate (mm); MR, maximum rate of regeneration (the maximum growth rate of the curve at inflection point, ip); tip, time to the inflexion point in weeks (Eq. (1)):
| (1) |
The effects of brittle star groups and temperature on these parameters (ML, MR, tip) were investigated using the Wilcoxon test as the data were non-normally distributed (unpaired data, unilateral test L1 > L3 and L1C > L1M). All statistical analyses were performed with the R software (www.r-project.org).
3 Results
3.1 Survival and autotomy
Autotomies and deaths occurred throughout the experiment but remained low in numerous cases, therefore we did not build LT50 curves and we displayed the final results only. The proportion of individuals in each category (intact, autotomy, dead) at the end of the 14th week period is presented in Fig. 2. For L1M, there was no significant difference between 17 °C and 26 °C. However, a higher mortality was observed at 30 °C compared to 17 °C and 26 °C (statistics in Table 1). For L1C, there was no significant difference between 17 °C and 26 °C, but 100% mortality was observed at 30 °C, all individuals being dead after nine weeks of experiment (Table 1). Temperature had no significant effect for L3, each temperature condition displaying around 10–20% of mortality, 40% of autotomy and 40–50% of intact individuals (Table 1).
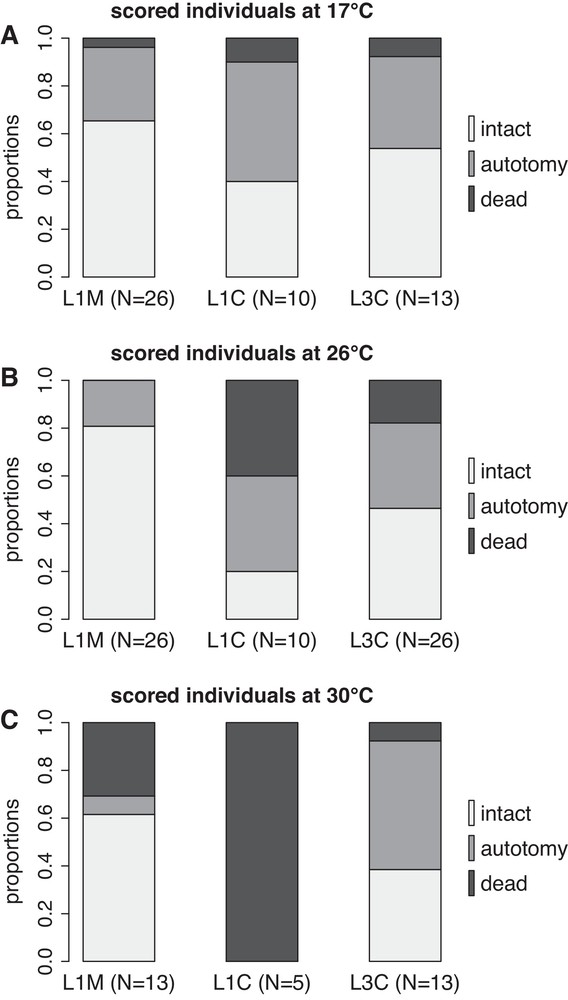
Proportions of intact, showing evidence of autotomy and dead individuals in each group (L1M, L1C, L3C) and temperature (17, 26, 30 °C) after 14 weeks of experiment. L1M: individuals of lineage L1 collected in Marseilles. L1C: individuals of lineage L1 collected in Crete. L3C: individuals of lineage L3 collected in Crete.
Comparison of survival, autotomy and death between three different temperatures (17, 26, 30 °C) for each group. Proportions of each category were compared by applying a Fisher's exact test.
| L1M | L1C | L3C | |
| 17 °C vs. 26 °C | NS (0.35) | NS (0.39) | NS (0.81) |
| 17 °C vs. 30 °C | * (0.048) | ** (0.006) | NS (0.84) |
| 26 °C vs. 30 °C | * (0.015) | NS (0.12) | NS (0.54) |
At 17 °C (Fig. 2A), there were no significant differences between groups (statistics in Table 2), each group displaying high survival and few autotomy. At 26 °C (Fig. 2B) there was no difference between L1C and L3C, but L1M was significantly different from L1C and L3C (Table 2). L1M did not display any mortality and only few specimens presented signs of autotomy in comparison to the higher proportion of autotomy observed in L1C and L3C. At 30 °C (Fig. 2C), all comparisons between groups were significant (Table 2). L3C had low mortality and high autotomy, whereas L1M displayed higher mortality but lower autotomy. For both L1M and L3 C, at least 40% of the individuals remained alive and intact after 14 weeks at 30 °C. Yet, mortality was lower in L3C (10%) compared to L1M (30%) and L1C (100%).
Comparison of survival, autotomy and death between three different groups (L1M, L1C, L3C) for each temperature. Proportions of each category were compared by applying a Fisher's exact test.
| 17 °C | 26 °C | 30 °C | |
| Between sites (L1 M vs. L1 C) | NS (0.24) | *** (2.0 × 10−4) | * (0.044) |
| Between lineages (L1 C vs. L3 C) | NS (0.83) | NS (0.232) | *** (8.17 × 10−4) |
| Between sites & lineages (L1 M vs. L3 C) | NS (0.64) | * (0.013) | * (0.049) |
3.2 Regeneration
The regeneration rate was estimated from the relationship between regenerate length and time following a Gomperz growth model (Fig. 1, Table 3). For L1C, all specimens died at 30 °C by the end of the experiment (14 weeks) and it was then not possible to measure any regeneration rate. Temperature had a significant impact on regeneration rate parameters (Table 4). For the three tested groups, the maximal rate of regeneration (MR) and maximal length of regenerate (ML) significantly increased when temperature increased from 17 to 26 °C and the time to the inflexion point (tip) decreased. The only exception was the absence of significant effect between 17 and 26 °C for ML in L3C. No significant difference between any of the regeneration parameters was observed between 26 °C and 30 °C. At the different temperatures tested, there was no significant effect of the group on any of the tested regeneration parameters (Table 5) with the exception of ML, which was significantly different between L1M and L3C at 26 °C. Gompertz curves were only available for individuals that remained intact until week 14. Thus we also compared regenerate lengths at the different sampling points to take advantage of higher sample sizes (Fig. 3). These results indicated that L1C and L1M regenerated significantly faster than L3C at 26 °C (p < 0.05, Wilcoxon test). e.g. at week 14, means of regenerate lengths were 21.8 mm, 19.7 mm and 16.8 mm for L1C, L1M and L3 C, respectively. This tendency was also confirmed by the mean regeneration Gompertz curves for each group (Fig. 1D). In addition, we observed that standard deviation of ML at 26 °C and 30 °C was higher for L1M compared with L3C. Inferences about L1C at 30 °C could not be done after week 6 because all L1C individuals were dead at week 9. The influence of the arm length lost is illustrated by the few individuals for which LL was lower than 5 cm (Fig. 1C, dashed lines). As expected, the plateau was reached sooner and corresponded to a lower length for these individuals.
Regeneration rate parameters estimated from a Gomperz model.
| 17 °C | 26 °C | 30 °C | ||||||
| L1M | L1C | L3C | L1M | L1C | L3C | L1M | L3C | |
| n | 14 | 6 | 9 | 22 | 3 | 16 | 6 | 6 |
| MR | 0.21 | 0.15 | 0.15 | 0.27 | 0.31 | 0.29 | 0.30 | 0.36 |
| 0.14 | 0.03 | 0.07 | 0.06 | 0.09 | 0.06 | 0.09 | 0.06 | |
| t ip | 13.28 | 12.97 | 16.89 | 6.74 | 6.0 | 6.94 | 6.04 | 5.51 |
| 4.68 | 2.63 | 7.06 | 0.99 | 0.8 | 1.38 | 1.03 | 1.01 | |
| ML | 11.3 | 12.06 | 20.13 | 23.71 | 24.54 | 19.77 | 23.32 | 20.25 |
| 6.55 | 3.79 | 14.93 | 7.83 | 6.20 | 4.66 | 8.54 | 4.91 |
Temperature effects on the regeneration rate parameters estimated from a Gomperz model.
| L1M 17–26 | L1C 17–26 | L3C 17–26 | L1M 17–30 | L3C 17–30 | L1M 26–30 | L3C 26–30 | |
| MR | ** (0.0028) | * (0.012) | *** (6.78 × 10−05) | * (0.0203) | *** (1.99 × 10−4) | NS (0.31) | NS (0.18) |
| t ip | *** (1.34 × 10−07) | * (0.012) | *** (5.87 × 10−06) | *** (2.58 × 10−05) | *** (1.99 × 10−4) | NS (0.16) | NS (0.16) |
| ML | *** (2.2 × 10−05) | * (0.012) | NS (0.26) | ** (0.00232) | NS (0.30) | NS (0.89) | NS (0.67) |
Group effects on the regeneration rate parameters estimated from a Gomperz model.
| L1M–L3C 17 °C | L1C–L3C 17 °C | L1M–L1C 17 °C | L1M–L3C 26 °C | L1C–L3C 26 °C | L1M–L1C 26 °C | L1M–L3C 30 °C | |
| MR | NS (0.153) | NS (0.304) | NS (0.516) | NS (0.830) | NS (0.396) | NS (0.303) | NS (0.845) |
| t ip | NS (0.138) | NS (0.264) | NS (0.548) | NS (0.358) | NS (0.180) | NS (0.119) | NS (0.650) |
| ML | NS (0.920) | NS (0.612) | NS (0.273) | * (0.0476) | NS (0.086) | NS (0.516) | NS (0.350) |

Boxplots of regeneration lengths at each measurement date (3, 6, 9 and 14 weeks) for each group L1M, L1C and L3C at each temperature 17, 26 and 30̊C. Each comparison was performed two by two with a Wilcoxon test (unpaired data, unilateral test L1 < L3; L1C < L1M) since the data were non-normally distributed. Shared letters mean non-significant comparison at 0.05 level of p-value. L1M: individuals of lineage L1 collected in Marseilles. L1C: individuals of lineage L1 collected in Crete. L3C: individuals of lineage L3 collected in Crete.
4 Discussion
4.1 Survival and autotomy
Our results showed that globally, the species complex O. longicauda displayed good resistance to elevated temperatures. Indeed, both O. longicauda L1 and L3 lineages could resist for three months a chronic exposure at 26 °C with high survival (100%, 60% and 80% for L1M, L1C and L3C, respectively) and more interestingly, there was 70% and 90% survival for L1M and L3C, respectively, after three months at 30 °C. Those results contrast with the idea that intertidal marine invertebrates already live at the edge of their thermal limits [28,29]. Christensen et al. [24] studied the brittle star Ophionereis schayeri and showed that O. schayeri could not resist more than a few days a temperature rise of 3 °C compared to the maximum SST experienced by this species. In addition, the Antarctic brittle star Ophionotus victoriae could not acclimate to a temperature rise of 2 °C compared to 0 °C [30]. Thus, we can speculate that adult O. longicauda, particularly the L3 lineage, would be little affected by future water temperature rise. However, it is important to consider that larval or juvenile survival may be more impacted by ocean warming (e.g., [31–33]).
When comparing at the intra-complex level, we showed that the L1C sample was more impacted by elevated temperature, since a higher mortality was observed between 17 °C and 26 °C, and at the end of the experiment, all individuals were dead at 30 °C. For L1M, there was a significant higher mortality at 30 °C compared to 26 °C, indicating that the specimens from Marseilles were also affected by elevated temperature, but to a lesser extent. In contrast, the L3C sample did not display any difference among 17, 26, and 30 °C experiments, indicating that temperature had no effect on mortality and autotomy. Furthermore, the L3C sample displayed the lowest mortality at 30 °C compared to L1M and L1C. Thus, when estimating thermotolerance by a response difference among temperatures, we showed that our L1 samples were less thermotolerant than our L3C sample. However, to confirm that this is actually an effect of the lineage (L1 versus L3) and neither an effect of the local populations considered nor a random effect, further experimentation should be performed including additional populations of L3 (e.g., from North and South coasts of Crete) and additional replicate tanks.
Interestingly, we observed that L1 individuals from Marseilles were more resistant to elevated temperatures (26 and 30 °C) than L1 individuals from Crete, despite higher average and maximal seawater temperatures in Crete. One explanation may be local adaptation to the rapid thermal variations typically observed in Marseilles [23], yet an experiment including short-term temperature variations would better test this hypothesis. There might also be a difference in reproductive times between L1C and L1M that could have placed some additional stress on L1C individuals, as the exact reproduction time of L1C is not known yet. Another possibility is that individuals from Marseilles were in better condition than individuals from Crete, because they were not transported to the experimental laboratory and did not undergo a change in seawater. However, such transportation effects are unlikely, since the proportions of intact, autotomized, and dead individuals did not differ among the three groups (L1C, L1M and L3C) at the control temperature of 17 °C, and in addition, the experiment started after an acclimation period of two weeks and lasted for 14 additional weeks. The difference between L1 individuals from Crete and Marseilles may not reflect a true difference among localities. We cannot exclude random effects affecting whole tanks and biasing the results, since we observed a contamination at the very beginning of the experiment, leading to complete mortality in four tanks. When analyzing the L3C response to thermal stress, our data support the hypothesis that L3 may be more resilient to elevated temperatures, as temperature had no significant effect on survival for this population, the samples at 17, 26 °C and 30 °C displaying similar relative proportions of intact, autotomized, and dead individuals. This result is in agreement with the hypothesis that temperature may contribute to the geographical distribution of L1 and L3, as the eastern Mediterranean displays higher temperatures.
An interesting aspect to develop in future studies would be to test the importance of local adaptation in thermotolerance, as it is expected to differ between populations of the same species [13,14]. The O. longicauda species complex comprising brooding and broadcasting lineages may then represent an ideal model to conduct research on local adaptation. Indeed, life-history differences among lineages have direct consequences on gene flow and connectivity, as confirmed by population genetic analyses [19]. Dispersal involves a cost to local adaptation, since maladapted genotypes are likely to be introduced into a non-native environment; thus, species reproducing via larvae might be less prone to local adaptation [29,34]. Such species however are able to explore more diverse environments and can produce more offspring, thus they may cope with a rapid change in environmental conditions better than non-dispersing species such as brooders [15]. Thus, conducting a thermotolerance study with several L3 and L1 populations sampled in eastern Mediterranean, and L1 populations from western Mediterranean (ideally after the reproduction period), would allow us to make inferences about the link between local adaptation and dispersal abilities, by discriminating the effects of species, populations and life-history trait variation.
4.2 Regeneration
To our knowledge, this is the first study comparing regeneration kinetics from different individuals (Fig. 1). Regeneration follows a growth curve with first a lag phase, followed by an exponential growth phase and ending with a plateau. Biressi et al. [35] have described the cellular processes during regeneration in O. longicauda (most probably belonging to lineage L1 given the sampling location) and Amphiura filiformis. They identified four phases: a repair phase, an early regenerative phase, an intermediate regenerative phase, and an advanced regenerative phase. During the repair and early regenerative phases, the complete healing of the epithelial layer is accompanied by extensive migration and proliferation of cells, leading to the formation of a blastema of undifferentiated cells. This phase is not associated with significant growth and corresponds to our lag phase. Intensive cell proliferation during the intermediate regenerative phase leads to rapid growth and corresponds to our exponential growth phase. The advanced regenerative phase is the time of slow differentiation and leads to a strong reduction in growth rates, as indicated by the plateau.
Furthermore, we showed that regeneration increased with temperature, as the time to inflexion point (tip) was divided by two between 17 and 26 °C. This is consistent with previous studies on the effect of temperature on brittle star regeneration (e.g., [36,37]). Our data also showed a threshold effect at 30 °C, as regeneration did not significantly increase between 26 and 30 °C, this result being congruent with the higher mortality observed at 30 °C for the lineage L1 and confirms the cost of survival at elevated temperatures [38]. Overall, our results demonstrate the importance to better define regeneration rate. Often, the regeneration rate is only considered as the linear relationship between regenerate length and time, but we clearly show that this rate is not constant during the regeneration process.
Our results did not display any difference in regeneration between the three groups (L1M, L1C, and L3C), except for the L1M vs. L3C comparison of ML at 26 °C, where L1M displayed a significantly higher ML (maximal length of regenerate) than L3C. The fact that only some of the surviving individuals were considered, because the stressed individuals autotomized their regenerate or died, thus reducing the number of analyzed individuals, may have reduced the differences among groups and their statistical significance.
We observed a high inter-individual variation, especially for the plateau parameter ML. These variations were not correlated with the size of the individuals, as DD and ML were compared and no correlation was found (data not shown). In addition, we showed that individual variation was higher for L1 compared to L3, as shown by the standard deviation comparisons. This may be a consequence of the higher genetic diversity of L1 compared to L3 [19]. Species with high genetic diversity may also display high phenotypic diversity (e.g., [38]). To confirm the correlation between genetic and response diversities, further regeneration studies should be performed on more numerous populations of L3 and L1. Yet, our results highlighted a physiological difference between L1 and L3, supporting the fact that they are truly separated species. The fact that the increase in regeneration rate with temperature is greatly reduced between 26 and 30 °C confirms that the temperature of 30 °C induces a significant stress and supports the idea that future experiments with geographic replicates may use the same temperatures tested in this study.
Although preliminary, our results are interesting because we compared the thermotolerance of two still undescribed sibling species and found potential differences. This is in agreement with the hypothesis that L1 may be less thermotolerant than L3 given their distribution across the Mediterranean, L1 being less abundant in eastern Mediterranean, where water temperature is more elevated, in particular at shallower depths. If future research confirms our results, the distribution of lineage L3 may not change considering future warming scenarios. In contrast, the distribution of L1 may shift both geographically and in bathymetry. Cryptic species are common in the sea and often undetected [39,40]. Although generally closely related, they may respond differently to environmental changes. Thus, researchers currently studying potential responses to climate change should be aware that different sibling species may occur within a nominal species before predicting global warming impacts on a given species.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are very grateful to Christos Arvanitidis from the HCMR (Crete) who hosted us in his laboratory, brought pertinent advice for general organization and organized the sampling sessions. We would like to thank him for his availability and his constant willingness to help; without him this study would not have been possible. We are also grateful to Thanos Dailianis, Elena Sarropoulou and Magdalini Christodoulou who helped for the sampling sessions and laboratory work. Many thanks to Panos Grigoriou for giving us the possibility to use the aquarium facilities at the Crete aquarium and for his advice. We also would like to thank Katerina Vasileiadou, Christina Pavloudi and Wanda Plaiti for their help in the lab and availability, and Frédéric Zuberer and Laurent Vanbostal for collecting the O. longicauda from Marseilles. We are very grateful to Marjorie Selva who helped to monitor the aquariums and settled the experiment, and to Pascal Mirleau and Marc Bally for their advices about the experiment. Finally, we would like to thank Michael Thorndyke for his useful comments to improve the manuscript.


