1 Introduction
A major evolutionary advance of the angiosperms is double fertilisation, with simultaneous formation of the embryo and endosperm. The main function of the endosperm is the nutrition of the embryo during development, both in the early and later stages of ontogeny [1,2]. In albuminous seeds, the endosperm remains relevant in germination [3], promoting seedling establishment.
Despite its relevance in angiosperms, the endosperm may be absent in some families, such as the Podostemaceae, Trapaceae, and Orchidaceae [4,5]. In Podostemaceae (Malpighiales), Baroux et al. [6] highlighted the ambiguity surrounding the occurrence of double fertilisation.
While the nutritional role of the endosperm is concentrated in the embryo and seedling, the nucellus has a critical role during the development of the megagametophyte, as it forms archesporial cells [7]. There is a great diversity of nucellus characters, ranging from the reduced nucellus in tenuinucellate ovules to extremely well-developed nucellus, as found in the Malpighiaceae and Euphorbiaceae families, where, in addition to being abundant, the nucellus projects through the micropyle [8].
Malpighiaceae is a complicated family belonging to Malpighiales [9]. The family has tropical and subtropical distribution, with approximately 60 genera and 1200 species [10]. In this family, the seeds remain poorly studied and typically exhibit reasonably homogeneous structure. Because of that, the seeds apparently have less utility for phylogenetic analyses. According to Corner [8], Malpighiaceae seeds are small, compressed obconic or pyriform, bitegmic, more or less exalbuminous. The integuments are not multiplicative, and in the mature seed, the testa and tegmen are reduced [8,11–12]. In Byrsonima intermedia [11], there are only the uniseriate phenolic testa and the sclerotic endotegmen. In species of Banisteriopsis and in Diplopterys pubipetala [12], the seeds have a pachychalaza, and the seed coat is collapsed. The nucellus expands and fills the seed, being compressed by the embryo and endosperm, which is mostly absorbed. There are no reports in the literature regarding the formation of perisperm in Malpighiaceae seeds, even though a broad nucellus is commonly noted [8,11–12]. A perisperm has been observed for the seeds of basal angiosperm groups, such as Nymphaeales [3,13], but also occurs in many derived families, mainly from the Caryophyllales [3]. According to Corner [8], the presence of perispermic seeds is an ancestral condition. Based on the new phylogenetic positions arising from the APG III [9], Friedman et al. [13] discussed the possible plesiomorphic state of this character for all angiosperms but considered that this trait could be an apomorphy for Nymphaeales.
Studying Byrsonima, Banisteriopsis and Diplopterys species, Souto and Oliveira [11,12] reported the occurrence of seeds with a well-developed nucellus, which is amplified during seed development, both by cell divisions and elongation. In parallel, the authors highlighted that the endosperm formed in these seeds is scarce and ephemeral.
Given these considerations, the aim of this study was to evaluate, via the study of the ontogeny of Malpighiaceae seeds, the formation of the endosperm and the role of the nucellus. We also elucidate other peculiarities of their seed development and their relationship with the embryogeny of Janusia, Mascagnia and Tetrapterys species. We included two species of Janusia, because this genus belongs to Gaudichaudieae, a single tribe recognised as monophyletic in Malpighiaceae, which is considered as derived in the family [14].
2 Materials and methods
The material was collected in the São Paulo State, Brazil, and consists of the ovules and seeds of the selected species. The vouchers are deposited in herbaria under the following indicated registers: J. mediterranea (Vell.) W.R. Anderson (R. Sebastiani et al. 306), J. occhionii W.R. Anderson (BOTU 25,788), M. cordifolia (A. Juss.) Griseb (BOTU 25,787) and T. chamaecerasifolia A. Juss. (BOTU 25,789).
For structural analysis, samples of 50 units of flowers and fruits in various stages of development were collected for removal of the ovules and seeds; we sampled approximately five individuals per species. The terminology adopted to describe the seeds was based on works by Martin [15], Corner [8], and Werker [3].
The ovule and seed samples were fixed in FAA 50 [16] and subsequently stored in 70% ethanol. Then, the samples were dehydrated in an ethanol series and infiltrated with 2-hydroxyethyl-methacrylate according to the protocol proposed by Paiva et al. [17]. The samples were sectioned with a rotary microtome, stained with 0.05% toluidine blue O, pH 4.7 acetate buffer [18,modified], and mounted in synthetic resin.
Histochemical tests were conducted in hand-sectioning fresh or fixed materials that were submitted to the following dyes or reagents:
- • ruthenium red to identify various polysaccharides and pectins [19];
- • phloroglucinol with hydrochloric acid to identify lignified cell walls [20];
- • Sudan IV to highlight the location of lipidic substances;
- • Lugol's solution for detecting starch;
- • ferric chloride with sodium carbonate to check for the occurrence of phenolic substances [16];
- • mercuric bromophenol blue to identify proteins [21].
The cross-sections were analysed with a light microscope, and the results were recorded in photomicrographs prepared in an Olympus photomicroscope with a digital camera attached.
3 Results
3.1 Ovule
The ovules of all the studied species are inserted unitarily in axial placentation in the apical portion of each carpel, so that they remain suspended (Fig. 1A). The ovules are subcampylotropous and crassinucellate (Fig. 1B–E). The ovules are unitegmic in J. mediterranea and J. occhionii (Fig. 1B–C and F) and bitegmic in M. cordifolia and T. chamaecerasifolia (Fig. 1D–E and G). A clear equatorial constriction occurs in the ovule of M. cordifolia (Fig. 1D), and the micropylar and chalazal portions are wider than the central region. In T. chamaecerasifolia, the constriction also occurs, but it is less evident.

Ovules of Tetrapterys chamaecerasifolia (A, E, K), Janusia mediterranea (B, F, H–J, L), J. occhionii (C) and Mascagnia cordifolia (D, G, M). Longitudinal sections. A. General view of a carpel, showing a single ovule with axial placentation. B–E. Subcampylotropous ovule of the studied species; note the median constriction in M. cordifolia (D, arrow). F. Detail of the single integument of Janusia species. G. Detail of the outer and inner integument of bitegmic species. H. Mycropilar region of the ovule, with nucellar cells protruding through the micropyle. I. Median region of nucellus; note the nucellar cells in reabsorption around the megagametophyte (arrowhead). J–K. Chalazal region of nucellus, with many cell divisions; the hypostase can be identified (arrow). L–M. Detail of the obturator, cells in direct contact with the nucellar projected cells. (*): nucellus protruding through micropyle; ch: chalaza; fu: funiculus; ie: inner epidermis of the integument; im: mesophyll of the integument; it: inner integument; mg: megagametophyte; ne: nucellar epidermis; nu: nucellus; ob: obturator; oe: outher epidermis of the integument; oi: outer integument; ow: ovary wall. Scale bars = 150 μm (A–B, D–E), 100 μm (C) and 50 μm (F–M).
The outer integument (or the single one) consists of up to six cell layers in the chalaza and three layers near the micropyle. At the fullest extension of the ovule, this integument has four cell layers in Janusia (Fig. 1F), three in M. cordifolia (Fig. 1G) and three to four in T. chamaecerasifolia. The outer epidermis is composed of rectangular cells in longitudinal sections that are thin-walled, voluminous, and have phenolic content (Fig. 1F), except in T. chamaecerasifolia. The mesophyll consists of cells shaped like the epidermis, and is smaller but also has thin walls, hyaline cytoplasm, and evident nucleus (Fig. 1F–G). The inner epidermis is uniseriate with even smaller cells (Fig. 1F) that are cubical in J. occhionii and slightly taller than wider in J. mediterranea. In M. cordifolia and T. chamaecerasifolia, the inner epidermis is not distinguishable from the mesophyll and, in some regions, it is also difficult to distinguish it from the inner integument (Fig. 1G). In the bitegmic species, the outer integument is longer than the inner, and therefore, it is the only feature that defines the micropylar pore. Several idioblasts with druses occur indiscriminately in the integuments.
The inner integument has one to five cell layers (Fig. 1G), and it is more common to find three layers in T. chamaecerasifolia and four in M. cordifolia. The outer epidermal and the mesophyll cells are similar; they are quadrangular longitudinal sections, more or less elongated, voluminous, with hyaline cytoplasm and nucleus clearly evident. The inner epidermis has smaller cells that are more cubical (Fig. 1G), also with evident nucleus.
The nucellus is very abundant and protrudes through the micropyle (Fig. 1B–D), being the longer projection in J. mediterranea (Fig. 1B and H). The nucellar cells are diverse depending on the region where they are located. We can recognise three regions in the nucellus (Fig. 1H–K). In the area that protrudes through the micropyle, including the near portion, the cells are voluminous in J. mediterranea (Fig. 1H) and M. cordifolia and smaller in J. occhionii and T. chamaecerasifolia. The cells have thin cell walls, hyaline cytoplasm and evident nuclei (Fig. 1H). In the middle area, the cells are smaller in J. mediterranea (Fig. 1I) and M. cordifolia and are voluminous in J. occhionii and T. chamaecerasifolia; the cell wall is pecto-cellulosic, and the cytoplasm is limited to the periphery of the cell, which presents a single central vacuole with an evident nucleus. Inserted in this region, we find the developing megagametophyte, and the nucellar adjacent cells appear to be apoptotic, with a degenerated nucleus and often ruptured cell walls, in addition to cellular residues, which confirm lysis (Fig. 1I). The third region near the chalaza presents meristematic-like cells juxtaposed to each other; numerous cell divisions occur in this region (Fig. 1J–K). Near anthesis, the central cells in the nucellus at the micropylar pole present irregularly thickened pectic walls.
The structure of the hypostase varies in all the species studied (Fig. 1J–K), presenting impregnation with phenolics of the cell walls between the chalaza and nucellus, which is more inconspicuous in T. chamaecerasifolia.
The funiculus is long, thick and coated by uniseriate epidermis (Fig. 1A–E) that eventually can be phenolic. The epidermis surrounds the parenchyma with phenolic and druse idioblasts, with only one vascular bundle (Fig. 1A–E). Near the insertion of the funiculus on the ovary wall, there are a set of elongated and narrow cells, irregularly shaped, with thick pectic walls and dense cytoplasm, which accumulates pectic substances (Fig. 1B–D and L–M). This tissue of the secretory aspect is directly in contact with nucellus projected cells (Fig. 1L–M) and is of variable size in the studied species; it is higher in J. mediterranea (Fig. 1L), broad in J. occhionii, and more restricted in M. cordifolia (Fig. 1M) and T. chamaecerasifolia.
3.2 Developing seed
The seed remains subcampylotropous with extensive chalaza and indistinct raphe already in early development, as there is no fusion between the outer integument and funiculus (Fig. 2A–D).

Developing seeds of Janusia mediterranea (A, J), J. occhionii (B, G, I, K), Mascagnia cordifolia (C, H, L) and Tetrapterys chamaecerasifolia (D–F, M). Longitudinal sections. A–D. General view showing the ample perisperm and the embryo in the early ontogeny (*); note the pachychalazal region (between the arrowheads). E–F. Detail of the pachychalazy. G. Seed coat of unitegmic species. H. Seed coat of bitegmic species; observe the integuments coalescing (arrow). I. Seed coat in a more advanced stage of development, with phenolic exotesta, mesotesta with ample vacuolated cells and endotesta with “U” thickened cell wall. J–K. Detail of the region near the embryo, indicated in the figures (A) and (B), showing perisperm cells and the absence of endosperm. L–M. Detail of the region near the embryo, indicated in figures (C) and (D), showing the contact region between the cells of endosperm and perisperm. ed: endotesta; em: embryo; en: endosperm; et: endotegmen; ex: exotesta; fu: funiculus; mt: mesotesta; pa: pachychalazy; pe: perisperm; ts: testa; vb: vascular bundle. Scale bars = 2 mm (A, C–D), 500 μm (B), 100 μm (E) and 50 μm (F–M).
The chalazal region expands, forming a pachychalaza (Fig. 2A–F). During this, the number of layers is enlarged and a vascular bundle is observed, which is thick and irregular (Fig. 2E–F).
The seed coat is not multiplicative and does not have periclinal cell divisions; thus, the number of layers remains the same as in the ovule (Fig. 2G–H). In the bitegmic species, testa and tegmen coalesce together during seed development, and it is not easy to individualise them (Fig. 2H). Thus, the seeds of all the species were originally or eventually become unitegmic. The exotesta remains phenolic (Fig. 2I). The cells of the developing mesotesta enlarge and form small schizogenous intercellular spaces. These cells have a thin cell wall, a little dense cytoplasm and an evident nucleus (Fig. 2I). The endotesta is still composed of small cells, but presents a U-like thickening (Fig. 2I). Near maturation, these thick cell walls become lignified.
The seed enlarges its size due to an increase of the volume of the nucellus (Fig. 2A–D), which occurs via cell divisions in the chalazal pole and differentiation of all nucellar cells, constituting the perisperm. Their cells have an irregular shape, a thin wall, a little cytoplasm and an evident nucleus (Figs. 2I–M and 3A). The cells from the periphery of the perisperm are smaller and have a denser cytoplasm (Fig. 3A). In the Janusia species, there is no endosperm (Figs. 2A–B, J–K, and 3B). In M. cordifolia and T. chamaecerasifolia, a nuclear endosperm is formed (Fig. 2C–D), which does not multiply much (Fig. 3C–D) and the cell walls are formed soon (Figs. 2L–M, 3C–F). Thus, the reserves for seed growth are supplied mainly by the perisperm in all species. The cells of the perisperm around the embryo show signals of lysis and the reabsorption of the cytoplasm.
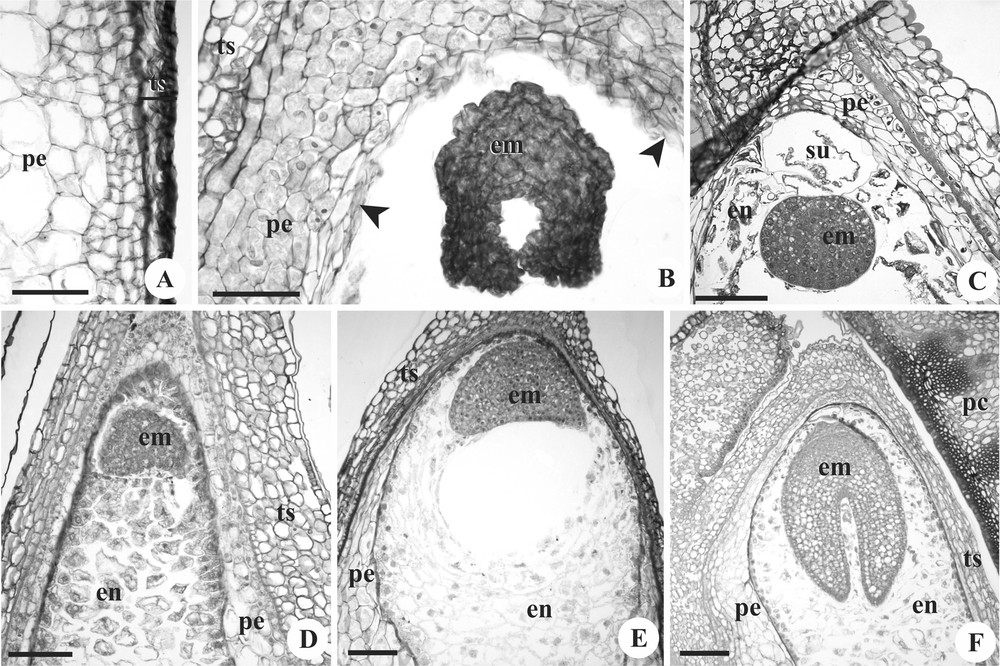
Developing seeds of Mascagnia cordifolia (A, C, E), Janusia mediterranea (B), and Tetrapterys chamaecerasifolia (D, F). Longitudinal sections. A. Perisperm detail, with smaller cells in the periphery and larger in the centre. B. Seed presented embryo post heart-shaped and perisperm in reabsorption near the embryo (arrowhead). C. Detail of the globular embryo, with unicellular suspensor; note the scarce endosperm. D–F. Embryos during differentiation, surrounded by endosperm. em: embryo; en: endosperm; pc: pericarp; pe: perisperm; su: suspensor; ts: testa. Scale bars = 150 μm (E–F) and 100 μm (A–D).
During seed development, the embryonic zygote divides, producing the embryo, which clearly goes through the globular (Fig. 3C) and heart-shaped stages (Fig. 3D–E), reaching the elongation of cotyledons and of the embryo axis (Fig. 3F). Until this stage, the suspensor can be seen; it consists of a voluminous highly vacuolated single cell (Fig. 3C).
3.3 Mature seeds
The mature seed is small and dark-yellow coloured, is sickle-shaped in J. mediterranea (Fig. 4A), pyriform in J. occhionii (Fig. 4B), elongated and slightly flattened in M. cordifolia (Fig. 4C) and globose in T. chamaecerasifolia (Fig. 4D).
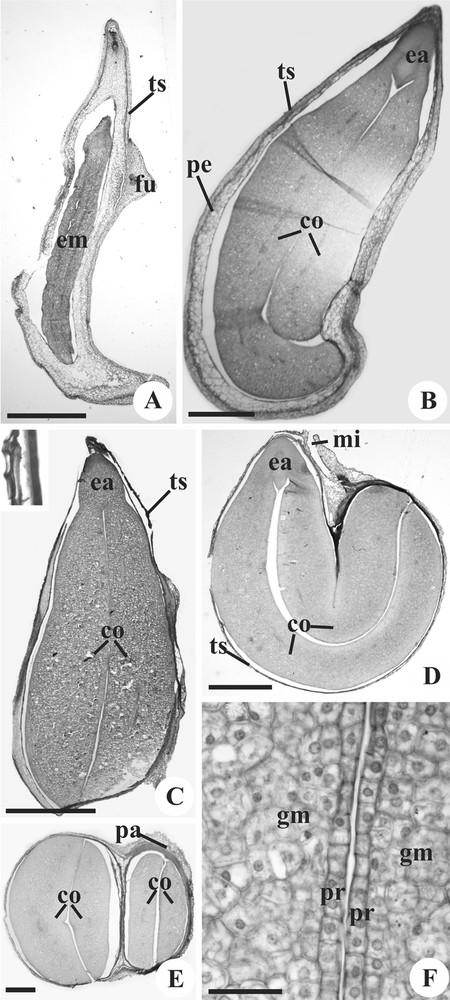
Mature seeds of Janusia mediterranea (A), Janusia occhionii (B, F), Mascagnia cordifolia (C) and Tetrapterys chamaecerasifolia (D–E). Longitudinal sections (A–D); Transverse sections (E–F). A–C. General view; observe the testa highlighted in (C). D–E. Seed general view, showing the cotyledons bent on themselves. F. Detail of the cotyledons, showing the protoderm and ground meristem. co: cotyledon; ea: embryo axis; em: embryo; fu: funiculus; gm: ground meristem; mi: micropyle; pa: pachychalazy; pe: perisperm; pr: protoderm; ts: testa. Scale bars: 2 mm (A, C), 1 mm (D), 500 μm (B, E) and 50 μm (F).
At maturity, the mesotesta is collapsed, and it is possible to observe only the phenolic exotesta and the U-thickened endotesta (Fig. 4C). In pachychalazal areas, the vasculature is also visible (Fig. 4E). The seed is exalbuminous and the perisperm is totally consumed during the embryo growth (Fig. 4C–E); it is possible to find residues of not consumed perisperm cells at some points.
The embryo is total and fills the seed in all species, straight in M. cordifolia (Fig. 4C), curved in Janusia (Fig. 4B), and curved and folded in T. chamaecerasifolia (Fig. 4D–E). The hypocotyl radicle axis is short (Fig. 4B–D) and both cotyledons are big and fleshy (Fig. 4E); in Janusia, one cotyledon is bigger and folds over the other (Fig. 4B). The embryo has uniseriate protoderm, small and cubic cells, with thin wall, dense cytoplasm and evident nucleus (Fig. 4F). The ground meristem is composed of cells that are more voluminous than protodermal cells (Fig. 4F), and have thin cell wall, dense cytoplasm and evident nucleus. Immersed in the ground meristem occur procambial strands of small calibre.
The embryo axis consists of short radicle and hypocotyl, and distinguishing between them is difficult (Fig. 4D). The cotyledon node is narrow, and the epicotyls are indistinct (Fig. 4B). The plumule is undifferentiated, with only the apical promeristem notable (Fig. 4B–D).
The seed reserves consist of lipids and proteins, which are found in the cotyledons and in the embryo axis.
4 Discussion
4.1 Nucellus, endosperm, and perisperm
The ovules of the studied species are crassinucellate and have a large nucellus volume that protrudes through the micropyle, even to a small extent, as is the case of M. cordifolia and T. chamaecerasifolia. This feature is constant in the Malpighiaceae [11–12,22] and has already been highlighted in descriptions of the family [8]. The nucellus projection through the micropyle has been reported also in the ovules of Euphorbiaceae [8,23], another Malpighiales family.
All Malpighiaceae seeds that were ontogenetically investigated until now present endosperm formation [11–12,22,24–25], as observed in this study in M. cordifolia and T. chamaecerasifolia. In these species, the endosperm is formed, although it does not proliferate too much, and it is consumed in the early stages of embryo development. The literature on seed ontogeny in Malpighiaceae suggests that the presence of scarce and early reabsorbed endosperm is an apomorphy for the family, as these species are found in various informal clades as proposed by Davis and Anderson [26], including the most basal clade, which is represented by the genus Byrsonima. Moreover, Corner [8] already suggested this generalisation for Malpighiaceae.
Although the literature indicates that the endosperm is ephemeral, the absence of endosperm formation in Janusia species studied here is newly observed for the family. The absence of endosperm formation is rare and observed in a few families, including Podostemaceae, Trapaceae, and Orchidaceae [4]. In Trapaceae and Orchidaceae, the endosperm can be formed, but there are few divisions and the nuclei soon degenerate. In Podostemaceae, it is believed that the formation of the primary endosperm nucleus does not occur [4]. Based on this study, it is not possible to identify what process occurs in J. mediterranea and J. occhionii, as it was not the aim of this work to analyse the development of the female gametophyte and the initial development of the endosperm in these species. The literature reports only one work on the megagametophytes of a Gaudichaudieae species, J. guaranitica [22]. The author reported the presence of central cells and double fertilisation, as well as the presence of the endosperm. Considering these results, the absence of endosperm would not be an autapomorphy of the genus. However, it would be interesting to conduct further studies with species of the genus and tribe, as Lorenzo [22] did not make use of useful techniques that are available today and all of his results are illustrated only with drawings, hindering new interpretations on the data. With further studies, it will be possible to verify if the absence of endosperm development occurs only in one group of species within the genus Janusia, in the whole genus or in another genus of the tribe.
Although Davis and Anderson [26] have placed the tribe Gaudichaudieae (aspicarpoid clade next to the stigmaphylloid) in a less derived position, the tribe is recognised as derived in the molecular analysis of Cameron et al. [14], and its monophyly has always been recognised [14,26–27]. We believe that the absence of endosperm production may be considered derived for the family, as more basal species, such as Byrsonima intermedia [11], Banisteriopsis campestris, B. oxyclada, B. stellaris, Diplopterys pubipetala [12], and T. chamaecerasifolia (reported in this work), present the formation of endosperm. As species in a more derived clade sensu Davis and Anderson [26], such as Malpighia glabra [24] and M. cordifolia (this work), present endosperm formation, we believe that this trait appeared in Gaudichaudieae.
In the Janusia species studied here and even in M. cordifolia and T. chamaecerasifolia, which demonstrate ephemeral endosperm, it is clear that the role of nutrition to the embryo was transferred to the perisperm, which is the ontogenetic successor of the nucellus in the seeds. This transference is evident in Janusia, wherein the perisperm provides nutrition for the embryo from the outset of its development. In M. cordifolia and T. chamaecerasifolia, initially, the endosperm shares this nutritive function, but early when the endosperm is completely consumed, the perisperm provides the necessary nutrition. A similar process must occur in previously described species [11–12,24] where the authors have reported the presence of well-developed nucellus and scarce endosperm.
Among the basal angiosperms, the seeds of Nymphaeales have perisperm as the main seed reserve, and the endosperm is small, diploid and restricted to the region near the embryo, indicating that the occurrence of perisperm as the main seed reserve could be an ancestral condition for angiosperms or be an autapomorphy of Nymphaeales [13]. Independently of the interpretation, the presence of perisperm in seeds of other angiosperms lineages would be a derived condition related to the loss of endosperm function and size [13]. If it were proven that the small endosperm is the ancestral condition for angiosperms, this would indicate that there was a reversion of the state of this character in Malpighiaceae. Furthermore, in basal angiosperm seeds [13], monocots, such as Zingiberales [3] and eudicots, such as Amaranthaceae, Chenopodiaceae, Sauraceae, and Caryophyllaceae [3] have a perisperm that remains a reserve tissue for mature seeds. In Malpighiaceae, the perisperm is already consumed and seed reserve is noted in the embryo, especially in the cotyledons. The transfer of the function from the endosperm to the perisperm occurs gradually along Malpighiaceae lineage, culminating in the complete absence of endosperm in Janusia.
The occurrence of lysis in the nucellus near the megagametophyte is a common feature, and has been described for species from other families [28,29]. Analysing the ultrastructure of the nucellar cells of Tillandsia, Brighigna et al. [28] demonstrated the occurrence of programmed cell death during megagametophyte differentiation. A remarkable feature of the species of Malpighiaceae studied in this work is not only the presence of cell death in the nucellus, but especially the increase in the nucellar cells giving rise to the perisperm. Like in the megagametophyte, lysis occurs in the perisperm near to the developing embryo. In this case, lysis should be related to the supply of nutrients for the growing embryo until seed maturity. Ultrastructural studies would be interesting to verify the occurrence of programmed cell death.
4.2 Mature seed and seed coat
Seeds of Malpighiaceae are generally small and have several forms [8,11–12,25]. Even with the description of the species studied in this work, it is not possible to evaluate if seed shape would have taxonomic significance for the family.
In relation to the seed coat, we can observe a pattern of development. In all Malpighiaceae studied until now [8,11–12,25] and in this work, the integuments are not multiplicative and the mature seed coat has some collapsed and other thickened layers. The phenolic exotesta also appears to be a constant state for the family, and was registered in Byrsonima intermedia [11], Banisteriopsis campestris, B. oxyclada, B. stellaris, Diplopterys pubipetala [12], and in this work in J. mediterranea, J. occhionii, M. cordifolia, and T. chamaecerasifolia. The thickened endotesta is present in several clades of Malpighiaceae, and the absence of this layer was registered only in Banisteriopsis and Diplopterys pubipetala (stigmaphylloid clade). These observations suggest that both phenolic exotesta and thickened endotesta may be synapomorphies for the clade, but the study of species of Elatinaceae (sister of Malpighiaceae) is necessary to confirm this hypothesis.
4.3 Hypostase
The hypostase is often recognised in ovules and seeds, occurring in 81 families of dicots according to Von Teichman and Van Wyk [30]. The authors did not include Malpighiaceae in their account. There is great variability in the hypostase structure depending on the species [12], and therefore, their function is also variable. One possible function would be the storage of metabolites, such as tannins to make the ovule more resistant to pathogen attack [31] as well as to provide protection to the embryo [30]. The hypostase of the studied species fit into the lato sensu type [30], which is often found in association with extensive chalaza, as in the case of pachychalazal seeds of this work. In addition to the association with large chalaza, the hypostase is also associated with other states considered ancestral, such as bitegmic and crassinucellate ovules, with nuclear endosperm and woody plants.
Although Von Teichman and Van Wyk [30] have linked the presence of hypostase to the occurrence of other ancestral characters, Corner [30] considered the hypostase and pachychalaza as innovations that emerged at the same time in several families. In Malpighiaceae, the presence of pachychalaza was also reported in three species of Banisteriopsis and in D. pubipetala [12], but was not found in Byrsonima intermedia [11] or in Lophantera lactecens [25]. In M. glabra [24], there is post-chalazal vasculature, although the authors do not refer to the presence of pachychalaza. Presumably, the pachychalaza can act as a kind of metabolite reservoir, from the vascular bundle and adjacent regions of the chalaza, and this feature could also confer protection [32]. The pachychalaza occur in both primitive and derivative taxa and have appeared many times in the course of evolution, indicating adjustments to the dispersal and germination processes [32]. As it has been documented in just a few species of Malpighiaceae, it is likely that the character has appeared during the diversification of the family in more than one phylogenetic row.
4.4 Obturator
One aspect that draws attention in the analysed ovules is the presence of a funicular obturator, the most common type occurring in ovules [32]. It is a secretory tissue, with elongated epidermal cells of the ovary or ovule, presenting the function of guiding and favouring the pollen tube growth from the ovary wall to the micropyle [4]. It is common that the obturator cells are secretory [4,32], which appears to be the case of species studied in this work and is more evident in Janusia. A gradation in structural drafting and extension of the obturator of this species occurs, and M. cordifolia contains an obturator with a smaller extension and less differentiated cells. Tetrapterys chamaecerasifolia also demonstrates small extension and differentiation, and J. occhionii has a well-developed obturator with a secretory aspect. The same situation is observed in J. mediterranea, in which the obturator is bigger.
4.5 Final thoughts
In summary, comparing the four studied species, it is clear that the Janusia seeds present derived characters states, when compared to the seeds of M. cordifolia and T. chamaecerasifolia, such as the reduction to only one integument already in the ovule, a more elaborated obturator and endosperm absence. These data confirm the derived position of Janusia, compared to Mascagnia and Tetrapterys, as found by Cameron et al. [14]. However, these data do not allow, given the small number of sample species, considerations regarding the disputed phylogenetic position of the latter two genera, which is still unresolved, even in the most recent molecular analysis. The newly recorded observation of endosperm absence during seed development in Janusia deserves mention and indicates a clear derivation within this family, in which the other studied species have already reduced the production of this tissue with the consequent transfer of the nutritive function to the perisperm.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Doctoral grant awarded to L.S. Souto and the research grant of D.M.T. Oliveira (Process No. 309416/2011-6). We also would like to thank Dr Maria Candida Henrique Mamede and Dr Renata Sebastiani for identifying the species examined.


