1 Introduction
The House Martin Delichon urbica is an abundant species in urban areas of the Mediterranean and Europe [1–3]. Urbanization and modern buildings have probably favoured its spread in towns in the Tell and northern Sahara. In Algeria, the House Martin D. urbica is a summer visitor, arriving in spring after the Barn Swallow Hirundo rustica [1]. The diet of the House martin in Europe has been examined in Switzerland [4,5], in southern England [6], and in Poland [7]. Other studies have compared the diets of House Martins and Barn Swallows [8,9]. Among African studies, the diets of swifts (Apodidae) and swallows (Hirundinidae) have been compared during the breeding season in a South-African grassland [10]. In Algeria, several studies have focused on the diet of hirundines [11–18], but few studies have focused on the relationship between diet and food availability of D. urbica [6]. The diet of House martin can be strongly dependent on the progression of the breeding season, weather conditions, and local food resources [6]. The swallows captured the flying insects most available, according to their abundance and the facility of their capture [7].
The aim of our study was to describe the diet of House Martins in breeding colonies located in a suburban area in Pins maritimes (Algeria). Herein, we discuss the variability and differences in the composition of the diet and the selection of insects by the House Martin in relation to their availability or abundance in this area.
2 Study area
This study was conducted in the Algiers suburbs at Pins maritimes (3°09′E, 36°43′N) located between Oued El Harrach and the roadblock of Hamiz at 23 m a.s.l. (Fig. 1). The climate in this area is Mediterranean and the average monthly temperature ranges from a mean minimum of 11.6 °C in January to a mean maximum of 26.3 °C in August. The annual rainfall is 746.3 mm with a five-month drought period from May until September [19]. The vegetation is mainly dominated by trees such as Pinus halepensis, Ficus retusa, Phoenix canariensis, Washingtonia robusta, Schinus molle and Pittosporum undulatum. The understory is mainly composed of herbaceous plants such as Stenotaphrum Americana, Amarantus albus, Chenopodium album, and Euphorbia helioscopia.
Geographic location of the Pins Maritimes area (Algeria).
3 Materials and methods
The diet of the House Martin was determined by faecal analysis. Faeces were collected at Pins maritimes under occupied House Martin nests in buildings. A total of 120 faeces were collected during a six-month period between April and September 2007, with 20 faeces collected per month. The number of nests was established through direct counts at breeding sites in these buildings. In the study area, 426 occupied nests of the House Martin were recorded.
The cuticle fragments of arthropods provide sufficient indices to consider the quality and the quantity of different items found in the diet of the House Martin [6]. At the laboratory, each faecal sample was separately analysed with alcohol in a Petri dish, which made it easy to separate the various fragments (sclerotinized parts and the inorganic contents). Prey species were identified using a 20× dissecting scope.
Once the preys were identified, we carried out an estimation of their size by spreading out the various fragments (head, thorax, abdomen, mandible, wings, and legs) over graph paper. The size of insect was identified either by comparison with the insect specimens of the Pasquier and Maurel insect collections of the Department of Zoology of the Agronomic National Institute or was estimated, the size of head being assumed to correspond to between one fifth and one eighth of the body length of the insect, the thorax to be equal to one third of the body length and the abdomen to be equal to one half of the body length of the prey.
The number of individual prey items in each sample was determined based on the different parts found. Paired anatomical parts with the same features were counted as belonging to one individual. A head, thorax, abdomen, two cerci, two mandibles, two elytra, two wings, two of the same antennas, or six legs corresponded to one individual.
Prey availability was estimated by sweep netting in fallow land. The sweep netting was used to collect insects in grasses and bushes. The operation was realised early when the activity of flying insects was very weak. Samples were obtained by walking at a constant steady speed, repeatedly sweeping the net from side to side [20]. The walking speed played a significant role in the success of catches. Arthropods caught after 10 sweeps of the net were collected in plastic bags. Samples were collected on a total of seven transects per month during the breeding period of the House Martin. Arthropods were later identified and counted in the laboratory.
One dietary index, relative frequency (R.F. %) defined as the number of individuals of a species in relation to the total numbers of individuals of all species in the diet, was calculated.
Prey selection was quantified using Savage's index [21] Wi = Ai/Di, where Ai is the relative abundance of prey i in the diet of D. urbica, and Di is the relative availability of this resource in the environment. The values of Wi vary between 0 and ∞, where 1 means no selection of prey i, whereas values lower and higher than 1 show avoidance (negative preference) and selection (positive preference), respectively. This index was chosen because it is more objective than similar ones, and it is possible to verify its statistical significance with a χ2 test [22] after applying Bonferroni's adjustment (α/number of categories).
The similarity of the dietary composition between months was estimated with the Morisita index (1959) [23] modified by Horn (1966) [24]: C = 2Σxiyi/(Σxi2 + Σyi2), where C is the dietary overlap (C ranges between 0 and 1, with 1 indicating identical food composition), xi and yi denote the percentage of a given food item in the diet in different months. An Anova test was used to evaluate the monthly variation of the number of preys and the number of species per faeces; the level of significance used was P = 0.05.
4 Results
4.1 General composition of the diet
The number of prey species per faeces varied significantly between April (66 species) and June (87 species) (F = 2.85, df = 5, P < 0.01). The number of individuals of each prey species per faeces also varied significantly between months, with an average of 43.85 individuals found in September and 86 individuals found in April (F = 4.93, df = 5, P < 0.0001).
In total, 8425 items, representing 140 taxa of invertebrates were identified in the faeces of nesting House Martins (Table 1). Insects were the dominant preys (99.86%). Hymenoptera were the main preys (n = 4.780 prey items, 56.7%), followed by Coleoptera (n = 1689 prey items, 20.1%), Homoptera (n = 1193; R.F. = 14.2%) and Heteroptera (n = 457; R.F. = 5.4%).
Principal consumed prey of the House Martin, by classes and orders in the suburban area in Pins maritimes (Algiers, Algeria).
| Classes | Orders | n | R.F. % |
| Gastropoda | Pulmonea | 3 | 0.04 |
| Arachnida | Aranea | 9 | 0.11 |
| Insecta | 8413 | 99.86 | |
| Isoptera | 26 | 0.31 | |
| Mantoptera | 1 | 0.01 | |
| Orthoptera | 1 | 0.01 | |
| Dermaptera | 1 | 0.01 | |
| Embioptera | 3 | 0.04 | |
| Heteroptera | 457 | 5.42 | |
| Homoptera | 1193 | 14.16 | |
| Coleoptera | 1689 | 20.05 | |
| Hymenoptera | 4780 | 56.74 | |
| Lepidoptera | 2 | 0.02 | |
| Diptera | 260 | 3.09 | |
| Total | 8425 | 100 |
The House Martin diet showed little monthly variation, as expressed by the high similarity index of the diet composition in consecutive months (mean C = 0.70, S.D. = 0.37). Hymenopterans varied from a low of 55.0% in May to a high of 85.9% in September, mainly due to a variation in the number of D. urbica, which dominated the diet. It was followed by Coleopterans, with a minimum of 11.5% in September and a maximum of 35.6% in May. In April, Homopterans were the most numerous prey specimens, comprising 63.7% of the diet. Coleopterans and Dipterans made up 14.9% and 12.8% of the diet, respectively (Table 2).
Monthly variations of the diet of the House Martin Delichon urbica in suburban Algiers (Algeria).
| Orders | IV | V | VI | VII | VIII | IX | ||||||
| n | R.F. % | n | R.F. % | n | R.F. % | n | R.F. % | n | R.F. % | n | R.F. % | |
| Pulmonea | – | – | – | – | 3 | 0.19 | – | – | – | – | – | – |
| Aranea | – | – | 6 | 0.50 | 1 | 0.06 | – | – | 2 | 0.12 | – | – |
| Isoptera | – | – | 12 | 1.01 | – | – | – | – | – | – | 14 | 1.62 |
| Mantoptera | – | – | – | – | – | – | 1 | 0.07 | – | – | – | – |
| Orthoptera | – | – | – | – | – | – | 1 | 0.07 | – | – | – | – |
| Dermaptera | 1 | 0.06 | – | – | – | – | – | – | – | – | – | – |
| Embioptera | – | – | 2 | 0.17 | – | – | – | – | – | – | 1 | 0.12 |
| Heteroptera | 35 | 2.04 | 33 | 2.78 | 95 | 6.04 | 136 | 9.96 | 139 | 8.19 | 19 | 2.20 |
| Homoptera | 1090 | 63.67 | 55 | 4.63 | 40 | 2.54 | 5 | 0.37 | 2 | 0.12 | 1 | 0.12 |
| Coleoptera | 255 | 14.89 | 423 | 35.58 | 250 | 15.89 | 204 | 14.95 | 458 | 26.99 | 99 | 11.47 |
| Hymenoptera | 112 | 6.54 | 654 | 55.00 | 1164 | 74.00 | 1013 | 74.21 | 1096 | 64.58 | 741 | 85.86 |
| Lepidoptera | – | – | – | – | 2 | 0.13 | – | – | – | – | – | – |
| Diptera | 219 | 12.79 | 10 | 0.84 | 22 | 1.40 | 5 | 0.37 | 2 | 0.12 | 2 | 0.23 |
| Total | 1712 | 100 | 1189 | 100 | 1573 | 100 | 1365 | 100 | 1697 | 100 | 863 | 100 |
A division of the prey items into families demonstrated that the highest relative frequency prey items belonged to the family Formicidae (54.0%). The dominant species was Tetramorium biskrensis, which contributed 32.6% to the diet. It was followed by Camponotus barbaricus (6.9%) and Monomorium salomonis (5.6%) (Table 3).
Some dominant species in the diet composition of the nesting House Martins Delichon urbica in the suburban area in Pins maritimes (Algiers, Algeria).
| Order | Family | Genus (species) | Total | % |
| Homoptera | Unident. | Unident. | 1131 | 13.42 |
| Coleoptera | Scarabeidae | Pleurophorus sp. | 96 | 1.15 |
| Ptinidae | Unident. | 111 | 1.32 | |
| Alleculidae | Unident. | 206 | 2.44 | |
| Carpophilidae | Carpophilus quadri pustulatus | 116 | 1.37 | |
| Coccinellidae | Unident. | 130 | 1.54 | |
| Coccinella algerica | 107 | 1.27 | ||
| Scolytidae | Coccotrypes dactyliperda | 94 | 1.11 | |
| Silvanidae | Silvanus unidantatus | 85 | 1.01 | |
| Hymenoptera | Chalcidae | Unident. | 126 | 1.50 |
| Ichneumonidae | Unident. | 94 | 1.11 | |
| Tetramorium biskrensis | 2.747 | 32.60 | ||
| Formicidae | Monomorium salomonis | 472 | 5.60 | |
| Pheidole pallidula | 282 | 3.35 | ||
| Aphaenogaster testaceo-pilosa | 259 | 3.08 | ||
| Componotus barbaricus | 581 | 6.90 | ||
| Diptera | Unident. | Unident. | 188 | 2.23 |
| Total prey | 8.425 |
The sizes of preys ranged from 1 mm to 32 mm (Fig. 2). Preys with a size of 3 mm made up the highest percentage of the diet (42.2%) followed by preys that measured 2 mm (21.6%) and 5 mm (14.1%).
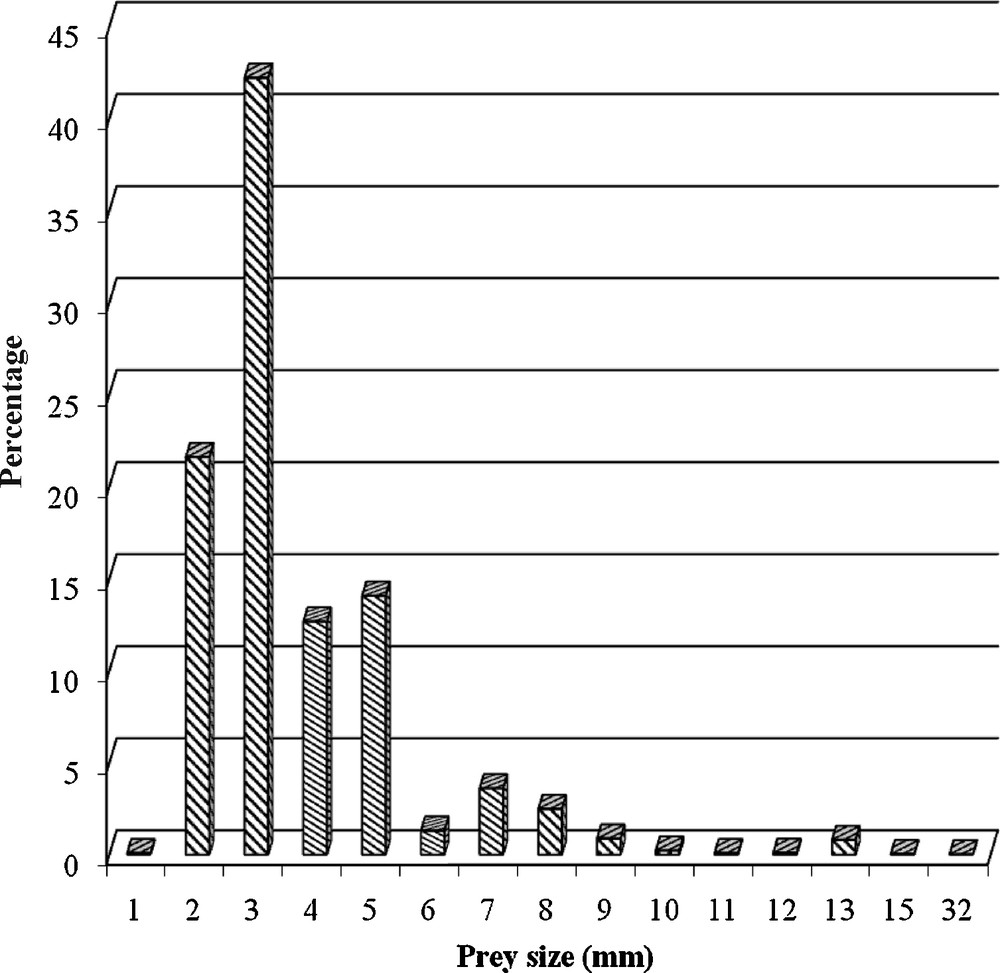
Size of preys in the diet of the House Martin Delichon urbica in the Pin Maritimes area (Algeria).
4.2 Comparison between diet and prey availability
According to the Savage index (W), and the posterior statistical comparison of the prey availability (Di) in the study area and its use (Ai), T. biskrensis, Crematogaster scutelaris, Pheidole pallidula, Diptera sp. unident., and Aphidae sp. unident. were positively selected by House Martin, whereas Aranea sp. unident., Oribates sp., Sminthurus sp. 1, Thysanourata sp. unident., Aiolopis thalassinus, Heteroptera sp. unident., Jassidae sp. unident., Aphelinidae sp. unident., Tapinoma negerrimum, and Drosophilidae sp. unident. were avoided. The consumption of other species did not show significant selection (Table 4).
Prey selection by House Martins in suburban Algiers.
| Genus (species) | A i | D i | W i | |
| Helicella sp. | 0.03 | 0.07 | 0.43 | ns |
| Cochlicella barbara | – | 1.32 | 0.00 | ns |
| Isopoda sp. unident. | – | 0.07 | 0.00 | ns |
| Aranea sp. unident. | 0.04 | 1.98 | 0.02 | * |
| Acari sp. unident. | – | 1.32 | 0.00 | ns |
| Oribates sp. | – | 9.82 | 0.00 | ** |
| Entombryidae sp. unident. | – | 0.29 | 0.00 | ns |
| Sminthurus sp.1 | – | 10.04 | 0.00 | ** |
| Sminthurus sp. 2 | – | 0.44 | 0.00 | ns |
| Thysanourata sp. unident. | – | 3.02 | 0.00 | * |
| Blattidae sp. unident. | – | 0.22 | 0.00 | ns |
| Iris oratoria | – | 0.07 | 0.00 | ns |
| Sphodromantis viridis | – | 0.07 | 0.00 | ns |
| Pezotettix giornai | 0.01 | 0.22 | 0.05 | ns |
| Aiolopis thalassinus | – | 1.47 | 0.00 | * |
| Aiolopus strepens | – | 0.07 | 0.00 | ns |
| Acrida turrita | – | 0.22 | 0.00 | ns |
| Psocoptera sp. unident. | – | 0.37 | 0.00 | ns |
| Dictyonota crassicornis | – | 0.07 | 0.00 | ns |
| Heteroptera sp. unident. | 0.02 | 3.52 | 0.01 | * |
| Ophthalmicus sp. | 0.43 | 0.22 | 1.95 | ns |
| Beritydae sp. unident. | 0.05 | 0.07 | 0.71 | ns |
| Lygaeidae sp. unident. | 0.11 | 0.07 | 1.57 | ns |
| Corysius sp. | 0.27 | 0.07 | 3.86 | ns |
| Reduvidae sp. unident. | 0.01 | 0.07 | 0.14 | ns |
| Aphidae sp. unident. | 13.42 | 10.99 | 1.22 | * |
| Fulgoridae sp. unident. | 0.04 | 0.96 | 0.04 | ns |
| Fulgora sp. | – | 0.07 | 0.00 | ns |
| Jassidae sp. unident. | 0.69 | 11.94 | 0.06 | ** |
| Psyllidae sp. unident. | – | 0.07 | 0.00 | ns |
| Attagenus sp. | – | 0.07 | 0.00 | ns |
| Tenebrionidae sp. unident. | – | 0.15 | 0.00 | ns |
| Orizaephilus surinamensis | – | 0.07 | 0.00 | ns |
| Agathidium sp. | – | 0.07 | 0.00 | ns |
| Formicomus sp. | 0.01 | 0.07 | 0.14 | ns |
| Eupurea sp. | 0.06 | 0.07 | 0.86 | ns |
| Parmulus sp. | 0.23 | 1.1 | 0.21 | ns |
| Coccinellidae sp. unident. | 0.04 | 0.07 | 0.57 | ns |
| Platylaspis luteorubra | – | 0.07 | 0.00 | ns |
| Scymnus interreptus | 0.11 | 0.15 | 0.73 | ns |
| Chaetocnema sp. | 0.11 | 0.07 | 1.57 | ns |
| Berginus tamarisci | 0.37 | 0.37 | 1.00 | ns |
| Apion aenus | 0.8 | 0.07 | 11.43 | ns |
| Eulophidae sp.unident. | – | 0.07 | 0.00 | ns |
| Miridae sp. unident. | – | 0.07 | 0.00 | ns |
| Proctotrypidae sp. unident. | – | 0.37 | 0.00 | ns |
| Dryinidae sp. unident. | – | 0.15 | 0.00 | ns |
| Chalcidae sp. unident. | 0.74 | 0.88 | 0.84 | ns |
| Braconidae sp. unident. | 0.01 | 0.07 | 0.14 | ns |
| Ichneumonidae sp. unident. | 0.01 | 0.07 | 0.14 | ns |
| Aphelinidae sp. unident. | 0.25 | 2.56 | 0.10 | * |
| Lasioglossum sp. | 0.04 | 0.07 | 0.57 | ns |
| Camponotus spissinodis | – | 0.73 | 0.00 | ns |
| Crematogaster scutelaris | 6.67 | 0.15 | 44.47 | ** |
| Crematogaster sp. | – | 0.15 | 0.00 | ns |
| Cardiacondyla sp. | – | 0.44 | 0.00 | ns |
| Tetramorium biskrensis | 32.6 | 0.29 | 112.41 | ** |
| Pheidole pallidula | 4.4 | 0.15 | 29.33 | ** |
| Tapinoma negerrimum | 3.35 | 23.15 | 0.14 | ** |
| Plagiolepis sp. | 2.96 | 2.05 | 1.44 | ns |
| Bethylidae sp. unident. | 0.02 | 0.29 | 0.07 | ns |
| Pyrallidae sp. unident. | – | 0.07 | 0.00 | ns |
| Parage aegeria | – | 0.15 | 0.00 | ns |
| Drosophilidae sp. unident. | 0.21 | 2.19 | 0.10 | * |
| Lucilia sp. | 0.09 | 0.07 | 1.29 | ns |
| Calliphoridae sp. unident. | – | 0.07 | 0.00 | ns |
| Sarcophagidae sp. unident. | – | 0.81 | 0.00 | ns |
| Cecidomydae sp. unident. | – | 0.07 | 0.00 | ns |
| Sepsis sp. | – | 1.39 | 0.00 | ns |
| Sepsis sp.1 | – | 0.07 | 0.00 | ns |
| Psychodes sp. | – | 0.07 | 0.00 | ns |
| Psychodidae sp. unident. | – | 0.59 | 0.00 | ns |
| Diptera sp. unident. | 2.56 | 0.95 | 2.69 | * |
5 Discussion
The number of prey species per faecal sample varied between 84 species in April and 111 species in June. Kisserli and Doumandji [17] found 188 species in faeces of House Martin collected in May 1994 and 82 species in April 1995 in a suburban area in Jijel (Algeria). These results were similar to those obtained in 1995 [15]. However, Daoudi et al. [14] found only 66 species in 30 House Martin faecal samples. In our study, we analyzed 120 faecal samples, which may explain why we recorded nearly twice the number of prey species in the diet as did Daoudi et al. Out of the 140 invertebrate prey species we identified, the majority (138) were insects, and insects comprised the overall highest number of preys (8413 individuals, 99.86%). The dominance of insects in the diet of House Martins has also been confirmed by Kožena [7] in Krkonoše, Poland (99.2%). Daoudi et al. [14] found insects to be the most frequently consumed preys by House Martins (99.8%) in suburban Algiers. Similar results were recorded by Farhi et al. [15] in the suburban area in Tizi Ouzou (Algeria) and by Kisserli and Doumandji [17] in a suburban area in Jijel (Algeria).
The House Martin is adapted to hunt aerial preys, and in our study winged preys comprised 99.8% of the diet. Daoudi et al. [25] reported that of the 1504 preys they identified in faeces collected in Dar El Beida (Algeria), 1490 were winged (99.1%) and 14 preys were apterous (0.9%). The dominance of winged preys in the diet was also confirmed by Benchikh et al. [18] in an Eucalyptus area of Algeria. These authors showed that the diet consisted of 3183 winged preys (99.4%) in 2000, 2318 winged preys (99.6%) in 2001, and 2938 winged preys (99.7%) in 2002. In Tizi Ouzou, similar results recorded by Farhi et al. [15] demonstrated the importance of winged preys in the diet of House Martins.
In our study, apterous preys (Aranea and Gastropoda) had a relative frequency in the diet of only 0.13%. Similar results have been reported by Gunten [4] and Kožena [7,9]. This raises the question concerning how the House Martins obtain these Arthropods? It is possible that they are preyed upon when they are carried by updrafts in the wind. Kožena [7] has also proposed that they may be captured on the ground or on walls when the birds drink or seek grill or mud to repair their nests. The parents also give young martins small snails and pieces of eggshell, which may help to break the hard exoskeletons of insects and provide calcium for their growth.
The predominant order in the House Martin diet was Hymenoptera (56.7%). They were followed by Coleoptera (20.1%), Homoptera (14.2%) and Heteroptera (5.4%). Other diet studies have also shown the dominance of Hymenopterans in the diet. Benchikh et al. [16] reported that Hymenopterans made up the highest proportion of the diet (72.99%), followed by Coleopterans (21.48%) and Heteropterous (4.42%) in a Eucalyptus area of Algeria. Also Daoudi et al. [14], in Dar El Beida (Algeria) showed that Hymenoptera (85.7%) represented the most common insect preys in Dar Elk Beida (Algeria). In Tizi Ouzou (Algeria), Farhi et al. [15] also found that Hymenoptera (69.0%) was most commonly eaten, followed by Coleoptera (21.8%), Heteroptera (5.1%), Diptera (2.4%), and Homoptera (1.2%). Kisserli and Doumandji [17] found Hymenoptera to be the most frequently consumed preys by House Martins (2964 individuals, 57.1%), followed by Coleoptera (1825 individuals, 35.2%), and Heteroptera (320 individuals, 5.8%). These results resembled those obtained by Algerian authors in different areas (see Table 5). However, in other studies in Europe, Homopterans and Dipterans have been shown to be the dominant preys in House Martin diets. In Switzerland, Gunten [4] found Dipterans (45.4%) and Homopterans (33.1%) were the most commonly eaten preys by House Martins (Table 3). Similar results are recorded by Bryant [6] in South England, where 59.5% of the diet of House Martins consisted of Dipterans, followed by Homopterans (17.8%), Hymenopterans (10.6%), and Coleopterans (5%). In contrast, Kožena [7] reported that Homoptera formed the highest percentage of the diet of House Martins (55.7%), followed by Dipterans (32.7%), Coleopterans (4.3%), and Hymenopterans (2.9%) in Poland. In our study, Homopterans were most frequently consumed only during April when the made up 63.7% of the diet. The low rate of ant consumption during April could be explained by absence of winged ants. The swarming period of ants started in May and June in Algeria. Doumandji [26] reported that 22.2% of the diet of Barn Swallows consisted of ants and they choose ant species according to their swarming periods: Tapinoma simrothi in May, Cataglyphis bicolor in May and June, P. pallidula in June, Crematogaster scutellaris and Aphaenogaster sp. in August.
Percentage of insect prey in the diet of House Martin in different regions.
| Country | Switzerland | England | Poland | Algeria | ||||
| Locality Orders |
Lake of Thun | South of England | Krkonoše | Tizi Ouzou | Eucalyptus | Jijel | Dar El Beïda | Pins maritimes |
| Odonata | – | – | – | – | – | 0.06 | – | – |
| Mantoptera | – | – | – | – | – | – | – | 0.01 |
| Orthoptera | – | – | – | 0.11 | 0.01 | 0.04 | – | 0.01 |
| Dermaptera | – | – | – | – | 0.09 | 0.02 | 0.26 | 0.01 |
| Embioptera | – | – | – | – | – | – | – | 0.04 |
| Psocoptera | – | – | – | – | – | 0.09 | – | – |
| Hemiptera | 7.2 | 0.2 | 0.3 | 5.05 | 4.42 | 5.82 | 4.71 | 5.45 |
| Homoptera | 33.1 | 17.8 | 55.7 | 1.18 | 0.44 | 1.04 | 0.17 | 14.22 |
| Coleoptera | 1.6 | 5.0 | 4.3 | 21.78 | 21.48 | 35.18 | 8.65 | 20.14 |
| Hymenoptera | 2.6 | 10.5 | 2.9 | 68.96 | 72.99 | 57.14 | 85.53 | 56.99 |
| Nevroptera | – | – | – | 0.13 | – | – | – | – |
| Lepidoptera | – | – | – | 0.03 | 0.06 | 0.02 | – | 0.02 |
| Diptera | 45.4 | 59.5 | 32.7 | 2.29 | 0.50 | 0.67 | 0.51 | 3.10 |
| Another order | 10.1 | 7.0 | 4.1 | 0.47 | 0.01 | – | 0.17 | – |
| Source | Gunten (1961) [4] | Bryant (1973) [6] | Kožena (1975) [7] | Farhi et al. (2003) [15] | Benchikh et al. (2005) [16] | Kisserli and Doumandji (2005) [17] | Daoudi et al. (2002) [14] | Present study |
The dominant species in the diet of House Martins in our study were T. biskrensis (32.6%), C. barbaricus (6.9%), and M. salomonis (5.6%). The dominance of ant species in the diet was also confirmed by Farhi et al. [15] in suburban Tizi Ouzou (Algeria), where T. biskrensis and M. salomonis made up 18.4% and 18.2% of the diet, respectively. Similar results were found by Benchikh et al. [16] in a suburban Eucalyptus area south of Algiers, where the diet consisted of 33.4% M. salomonis, 15.9% P. pallidula, and 12.9% T. biskrensis. Ant preys were also the most preferred by House Martins in our study with species such us T. biskrensis, C. scutelaris, and P. pallidula having similarity indices exceeding 0.90.
The sizes of preys varied between 1 mm and 32 mm. Kožena [7] indicated that the size of prey consumed by House Martin fledglings varied between 1 and 13 mm in Poland. However, the adults took preys ranging in size between 1 to 15 mm, with an average size of 3.5 mm. The author also noted that 82.3% of the preys captured by the House Martin have a size lower than 4 mm. Benchikh et al. [27] reported that House Martins took preys between 1.5 and 18 mm in length. In our study, preys measuring 3 mm in length made up 42.2% of the diet, followed by preys measuring 2 mm in length (21.6%). Similar results were recorded by Kožena [28] in Poland, where preys measuring 2 and 3 mm in length represented 22.3 and 34.6% of the diet, respectively. Many papers dealing with the diet of the House Martin or other hirundines link prey selection with the abundance, biomass, and size of the available insects. Our results show that the size of insects captured by sweep netting varied between 1 to 50 mm, with the dominance of insects less than 8 mm (90%). The average size of prey taken by the House Martin is similar than the average size of prey available in a given habitat. The small preys are elevated by air and wind, what they explain their capture by the House Martin.
The diet of House Martins in Pins maritimes is based primarily on the Hymenopteran prey, especially ants. This diet seems to be typical of House Martins in Algeria. These results resemble those of swallow diets in tropical areas (Turner and Rose [29]; Fry [30]), but differ from swallow diets in temperate areas (Gunten [4]; Bryant [6]; Kožena [7]). The high availability of the ants could explain their high predation by House Martin. The diet composition of D. urbica is dependent on the availability of preys in the environment.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
We are grateful to the two anonymous reviewers and to Dr. Marc Béchard for improving and editing the English text.
Appendix 1 Diet composition of the nesting House Martins Delichon urbica in the suburban area in Pins maritimes (Algiers, Algeria).
| Genus (species) | n | % | Genus (species) | n | % |
| Helicella sp. | 3 | 0.03 | Coreus sp. | 6 | 0.07 |
| Dysderidae sp. unident. | 9 | 0.10 | Metaconthus sp. | 5 | 0.06 |
| Calotermes flavicollis | 14 | 0.17 | Lygaeidae sp. unident. | 80 | 0.95 |
| Reticulitermes lucifugus | 12 | 0.14 | Lygaeus militaris | 65 | 0.77 |
| Mantis religiosa | 1 | 0.01 | Lygaeus equastris | 22 | 0.31 |
| Pezotettix giornai | 1 | 0.01 | Corysius sp. | 23 | 0.27 |
| Labia minor | 2 | 0.02 | Oxycarenus sp. | 13 | 0.15 |
| Embioptera sp. unident. | 2 | 0.02 | Nysius sp. | 78 | 0.93 |
| Heteroptera sp. unident. | 2 | 0.02 | Gonianotus sp. | 4 | 0.05 |
| Scutelleridae sp. unident. | 3 | 0.04 | Pyrrhocoris apterus | 4 | 0.05 |
| Odontoscelis sp. | 48 | 0.57 | Capsidae sp. unident. | 2 | 0.02 |
| Ancyrosoma albolineatum | 1 | 0.01 | Anthocoridae sp. unident. | 1 | 0.01 |
| Pentatomidae sp. unident. | 12 | 0.13 | Ploearea sp. | 15 | 0.18 |
| Sciocoris marginatus | 2 | 0.02 | Homoptera sp. unident. | 1131 | 13.42 |
| Aelia acuminata | 3 | 0.03 | Aphidae sp. unident. | 3 | 0.04 |
| Strachia sp. | 18 | 0.21 | Fulgoridae sp. unident. | 3 | 0.04 |
| Sehirus sp. | 9 | 0.10 | Jassidae sp. unident. | 76 | 0.90 |
| Carpocoris fuscispinus | 2 | 0.02 | Coleoptera sp. unident. | 20 | 0.24 |
| Ophthalmicus sp. | 36 | 0.43 | Ophonus sp. | 3 | 0.03 |
| Peribalus sp. | 1 | 0.01 | Acupalpus sp. | 2 | 0.02 |
| Bembidion sp. | 4 | 0.05 | Alleculidae sp. unident. | 206 | 2.44 |
| Microlestes sp. | 1 | 0.01 | Anthicus floralis | 3 | 0.04 |
| Pleurophorus sp. | 96 | 1.15 | Anthicus instabilis | 13 | 0.15 |
| Trechus sp. | 5 | 0.05 | Formicomus sp. | 1 | 0.01 |
| Elateridae sp. unident. | 12 | 0.14 | Oedemera sp. | 5 | 0.06 |
| Dermestes sp. | 8 | 0.09 | Carpophilus quadri pustulatus | 116 | 1.37 |
| Histeridae sp. unident. | 1 | 0.01 | Cyllodes sp. | 5 | 0.06 |
| Cantharidae sp. unident. | 2 | 0.02 | Eupurea sp. | 19 | 0.23 |
| Lytta sp. | 1 | 0.01 | Parmulus sp. | 1 | 0.01 |
| Dolichosoma melanostoma | 1 | 0.01 | Nitidula sp. | 7 | 0.08 |
| Lichenum pulchellum | 2 | 0.02 | Trachys pygmaeus | 1 | 0.01 |
| Crypticus sp. | 1 | 0.01 | Anthaxia sp. | 3 | 0.04 |
| Ptinidae sp. unident. | 111 | 1.32 | Coccinellidae sp. unident. | 130 | 1.54 |
| Staphylinus sp. | 7 | 0.08 | Coccinella algerica | 107 | 1.27 |
| Oxytelus sp. | 11 | 0.13 | Adonia variegata | 7 | 0.08 |
| Astenus sp. | 28 | 0.33 | Adalia decimponctata | 1 | 0.01 |
| Philonthus sp. | 5 | 0.06 | Scymnus interreptus | 9 | 0.11 |
| Stenus sp. | 1 | 0.01 | Scymnus apetzoides | 6 | 0.07 |
| Quedius sp. | 11 | 0.13 | Oenopia doublieri | 1 | 0.01 |
| Xantholinus sp. | 5 | 0.06 | Rhizobius chrysomeloides | 5 | 0.06 |
| Lathrobium sp. | 1 | 0.01 | Tytthaspis phalerata | 1 | 0.01 |
| Onthophylus sp. | 3 | 0.03 | Myrrha octodecemlineata | 11 | 0.13 |
| Platysthatus sp. | 1 | 0.01 | Chrysomelidae sp. unident. | 30 | 0.35 |
| Aphthona sp. | 3 | 0.03 | Silvanus unidantatus | 85 | 1.01 |
| Pachnephorus sp. | 9 | 0.11 | Apion aenus | 68 | 0.80 |
| Chaetocnema sp. | 44 | 0.52 | Larinus sp. | 3 | 0.04 |
| Cassida sp. | 9 | 0.11 | Hymenoptera sp. unident. | 28 | 0.33 |
| Podagrica fuscipes | 4 | 0.04 | Chalcidae sp. unident. | 126 | 1.50 |
| Bruchidae sp. unident. | 31 | 0.37 | Braconidae sp. unident. | 8 | 0.09 |
| Bruchidius sp. | 8 | 0.09 | Ichneumonidae sp. unident. | 94 | 1.11 |
| Callosobruchus maculatus | 4 | 0.05 | Aphelinidae sp. unident. | 13 | 0.15 |
| Curculionidae sp. unident. | 69 | 0.82 | Apoïdea sp. unident. | 1 | 0.01 |
| Sitona sp. | 17 | 0.20 | Halictidae sp. unident. | 3 | 0.04 |
| Ceuthorrhynchus sp. | 8 | 0.10 | Lasioglossum sp. | 61 | 0.72 |
| Baridius quadriticollis | 60 | 0.71 | Tetramorium biskrensis | 2747 | 32.60 |
| Baridius cerelucins | 5 | 0.06 | Monomorium salomonis | 472 | 5.60 |
| Brachyderes sp. | 30 | 0.35 | Pheidole pallidula | 282 | 3.35 |
| Nanophyes sp. | 1 | 0.01 | Tapinoma negerrimum | 35 | 0.42 |
| Metallites sp. | 1 | 0.01 | Cataglyphis bicolor | 12 | 0.15 |
| Otiorrhynchus sp. | 1 | 0.01 | Messor sp. | 21 | 0.25 |
| Coccotrypes dactyliperda | 94 | 1.11 | Aphaenogaster testaceo-pilosa | 259 | 3.08 |
| Staenopterus sp. | 2 | 0.02 | Plagiolepis sp. | 3 | 0.04 |
| Cnemidotus sp. | 13 | 0.15 | Componotus barbaricus | 581 | 6.90 |
| Olibrus sp. | 17 | 0.20 | Crematogaster scutellaris | 5 | 0.06 |
| Bostrychidae sp. unident. | 38 | 0.45 | Formicidae sp. unident. | 11 | 0.13 |
| Berginus tamarisci | 3 | 0.03 | Vespoîdea sp. unident. | 3 | 0.03 |
| Bethylidae sp. unident. | 1 | 0.01 | Lepidoptera sp. unident. | 56 | 0.66 |
| Sphegidae sp. unident. | 10 | 0.12 | Diptera sp. unident. | 188 | 2.23 |
| Betteloîdea sp. unident. | 1 | 0.01 | Drosophilidae sp. unident. | 9 | 0.10 |
| Chrysidae sp. unident. | 1 | 0.01 | Lucilia sp. | 8 | 0.09 |
| Total | 8.425 | 100 |


