1 Introduction
The tribe Scotonycterini includes three species of fruit bats (Chiroptera, Pteropodidae) restricted to evergreen tropical rainforests of Africa and characterized by white fur patches on the nose and behind the eyes [1]: Scotonycteris zenkeri Matschie, 1894 (Zenker's fruit bat), Scotonycteris ophiodon Pohle, 1943 (Pohle's fruit bat), and Casinycteris argynnis Thomas, 1910 (short-palated fruit bat). The geographical distribution of S. zenkeri overlaps with the two large blocks of African dense moist forests – the Upper Guinea Forest in west Africa and the Congo Basin Forest in central Africa (Fig. 1A; [2]). These two forest blocks are separated in west Africa by the Dahomey Gap, a savannah corridor that extends from central Ghana, through Togo, Benin, and western Nigeria [3]. The two other species within this tribe have less widespread distributions: S. ophiodon is also found in both west and central Africa (Fig. 1A; [4]), but is only known from 16 localities [5], which do not include the swamp forest around the Nigeria Delta or central and eastern portions of the Congo Basin; and C. argynnis is endemic to the Congo Basin Forest (Fig. 1A; [6]).
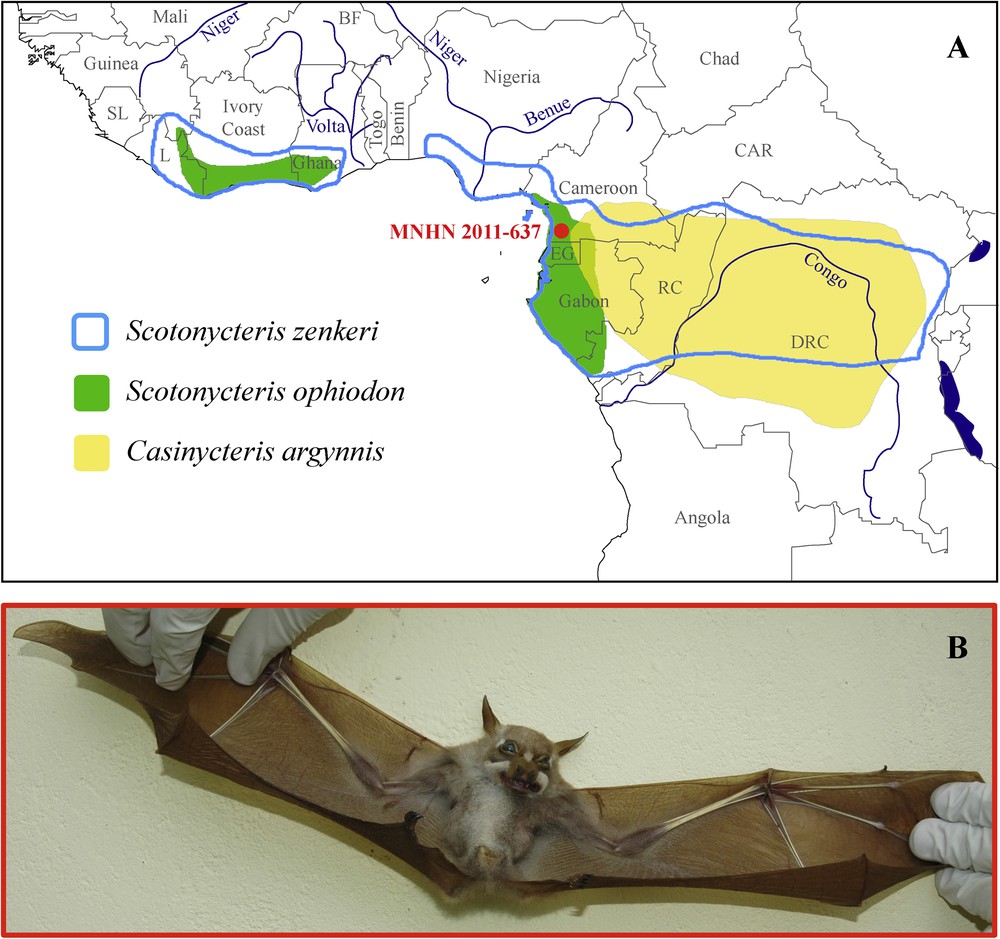
A. (Colour online) Geographic distribution of the three species of the tribe Scotonycterini (modified from the maps provided by the IUCN [2,4,6]). B. Photograph of the holotype of Casinycteris campomaanensis sp. nov. (MNHN 2011-637) collected near the village of Nkoélon-Mvini (South Region, Cameroon).
Morphologically, the three species of Scotonycterini can be easily distinguished from each other based on two standard body size measurements, body mass (W) and forearm length (FA): S. zenkeri is the smallest species (W: 16–27 g; FA: 45.0–56.4 mm), S. ophiodon is the largest species (W: 65–95 g; FA: 74.6–87.6 mm), and C. argynnis is intermediate (W: 26–33 g; FA: 49.8–63.5 mm) [7]. There is sexual dimorphism in all of these species, with females being larger than males (on average W: 20% heavier; FA: 10% longer). A few discrete characters can also be used to differentiate the three species [1,7]. The wing membrane finger joints are brown in S. zenkeri, whereas they are yellowish in the two other species. In S. ophiodon, the canines have a second inner cusp and the ear tips are slightly pointed, whereas in the other two species the cusp is lacking and they have rounded ears. In C. argynnis, the postdental palate is markedly short, whereas in the two other species it extends posteriorly beyond the upper molars.
In November 2007, an adult female Scotonycterini was collected near the Nkoélon-Mvini village close to the Campo-Ma’an National Park, in the southwestern corner of Cameroon (Fig. 1A). At first glance, the general appearance of the animal was similar to that of C. argynnis, but its body mass was 49 g, which is intermediate between female C. argynnis (28–34 g) and S. ophiodon (75–88 g), and its FA was 73.2 mm, which is larger than female C. argynnis (54.4–63.5 mm), but slightly smaller than female S. ophiodon (75.3–87.6 mm) (Fig. 2A; data from Bergmans [7]). Later examinations revealed craniodental differences between the Nkoélon-Mvini village animal and the other members of the Scotonycterini. In addition, its mitochondrial cytochrome b gene sequence differs significantly from that of the three other species of Scotonycterini. It is therefore described herein as new species.

(Colour online) Differences between females of the four species of Scotonycterini based on morphological characters. The morphological data were extracted from Bergmans [7], in which geographical variation was analyzed for three species of Scotonycterini based on minimum and maximum values: Scotonycteris zenkeri (blue circles), S. ophiodon (green circles), and Casinycteris argynnis (yellow circles). The red circle corresponds to the holotype of C. campomaanensis sp. nov. collected near the village of Nkoélon-Mvini (MNHN 2011-637). A. Forearm length (FA, measured in mm; abscissa) as compared to body mass (W, taken in g; ordinate). B. Palatal length (PL) as a percentage of greatest skull length (GSL) (abscissa) as compared to zygomatic width (ZW) as percentage of GSL (ordinate). Masquer
(Colour online) Differences between females of the four species of Scotonycterini based on morphological characters. The morphological data were extracted from Bergmans [7], in which geographical variation was analyzed for three species of Scotonycterini based on minimum and maximum ... Lire la suite
2 Materials and methods
2.1 Field survey
The field survey was conducted in the Campo-Ma’an rain forest located in the South Region of Cameroon. Bats were captured with Ecotone mist nets (Poland) around the village of Nkoélon-Mvini (02°23.831′ N, 10°02.691′ E, 117 m; Fig. 1A) between 18 and 25 November 2007. Bats were measured, photographed and, for members of the family Pteropodidae, species were identified using the dichotomous key of Bergmans [1]. The unidentified specimen described below was collected in the same immediate zone as five other fruit bat species: Eidolon helvum (Kerr, 1792), Epomops franqueti (Tomes, 1860), Megaloglossus woermanni Pagenstecher, 1885, Myonycteris torquata (Dobson, 1878), and Scotonycteris zenkeri. Reference specimens from this survey were deposited in the Muséum national d’histoire naturelle, Paris (MNHN) and other material used in this study is housed in the Zoologisches Museum Berlin (ZMB).
2.2 Morphological measurements
The body mass (W) of captured bats was measured using a PESOLA Micro Line 100 g or a PESOLA Medio Line 300 g. The following external measurements were taken on recently euthanized specimens using a digital calliper to the nearest 0.1 mm: FA, the forearm length (from the elbow to the wrist with both joints folded); EAR, ear length (from the base of the ear to the distal tip of the pinna); HF, hind foot length (from the heel to the tip of the longest toe, including the claw); TIB, tibia length (from the knee to the ankle); 2DM, 3DM, 4DM and 5DM, which correspond to the lengths of 2nd, 3rd, 4th and 5th metacarpals taken from the wrist to the end of the respective metacarpals; 1D1P, 1D2P, 2D1P, 2D2P, 3D1P, 3D2P, 4D1P, 4D2P, 5D1P, and 5D2P, which correspond to the lengths of the first and second phalanges of the respective 1st, 2nd, 3rd, 4th and 5th digits.
Two-dimensional images of the cranium and mandible were taken with a digital microscope in dorsal, lateral and ventral views using a ruler marked off in millimetres. The photographs were used under ImageJ 1.46r [8] to measure craniodental characters to the nearest 0.01 mm for comparisons with the data provided by Bergmans [7] for the three species of Scotonycterini. Abbreviations and definitions for craniodental measurements include BCH, braincase height (from the base of the auditory bullae to the highest part of the skull); BCW, braincase width (greatest width of the braincase); C1–C1, greatest width measured across the outer borders of the upper canines; C1–C1, greatest width measured across the outer borders of the lower canines; C1–M1, maxillary tooth row length (from the anterior edge of the upper canine to the posterior edge of the upper molar); C1–M2, length of mandibular toothrow (from the anterior edge of the canine to the posterior edge of the 2nd lower molar); CBL, condylobasal length (from the distal edge of the occipital condyles to the anterior edge of the incisors); GSL, greatest skull length (from the posterior edge of the skull to the anterior edge of the incisors); IOW: interorbital width (least width of the interorbital constriction); M1–M1, greatest width measured across the outer edges of the upper molars; M2–M2, greatest width measured across the outer edges of the last lower molars; ML, mandible length (from the outermost part of the symphysis to the most posterior part of the articular condyle); PL, palatal length (from the anterior palatal emargination to the midpoint of posterior palatal emargination); POW, postorbital width (least width of the postorbital constriction); ZW, zygomatic width (greatest width of the skull across the zygomatic arches). All toothrow measurements were taken from an alveolar position. Two percentages were also calculated to allow comparisons with the data provided by Bergmans (1990) – PL × 100/GSL and ZW × 100/GSL.
2.3 Molecular analyses
A pectoral muscle sample of the odd specimen from near Nkoélon-Mvini (MNHN 2011-637) was preserved in 95% ethanol. Total genomic DNA was extracted using QIAGEN DNeasy Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol with the final volume of 100 μL eluted DNA in AE buffer. The mitochondrial cytochrome b (cytb) gene was amplified and sequenced with the following set of oligonucleotide primers: 5′-GAA AAA YCA CCG TTG TAY TTC AAC TA-3′ (CB-GLU-CH2) and 5′-CCC TTY TCT GGT TTA CAA GAC C-3′ (CB-LTHR-CH). The quality of 5′ and 3′ ends of the cytb gene was improved using the two following sets of primers published in Hassanin et al. [9]: U102/L402 and U162/L482.
A small portion of skin was sampled from the holotype of S. ophiodon (ZMB 50001). Since this specimen was preserved in alcohol during more than 100 years (from 1899 to 2013), DNA degradation took place and it was not possible to amplify long PCR fragments (> 300–400 nt). Therefore, seven sets of primers were used to amplify and sequence overlapping fragments of the cytb gene:
- • CB-GLU-CH2 and 5′-CAT ATC CTA TRA AGG CTG TTG C-3′ (CB-L372-EM);
- • 5′-CAG GAY TAT TCC TRG CAA TAC A-3′ (CB-U162-EM) and L402;
- • 5′-CT ACA YGC YAA CGG AGC ATC-3′ (CB-U264-SC) and 5′-CC AAT ATA GGG RAT AGC TGA TAG-3′ (CB-L447-SC);
- • 5′-CCA TGA GGC CAA ATA TCA TT-3′ (CB-U420-SC) and 5′-GGA TTA TRT CTR YGT CTG ATG-3′ (CB-L633-SC);
- • 5′-GAC AAA GCY ACC YTA ACA CG-3′ (CBF2-U531-SC) and 5′-GRA TRG CGT ATG CAA ATA GGA-3′ (CB-L820-SC);
- • 5′-GAC CTA CTA GGA GAC CCA GA-3′ (CB-U762-SC) and 5′-CAG GTT AGT GTR AGA AGG TC-3′ (CB-L990-SC);
- • 5′-CAA RCT YGG AGG AGT VCT AGC-3′ (CB-U879-SC) and CB-LTHR-CH.
The polymerase chain reactions (PCR) were carried out in a volume of 20 μL containing 3 μL of PCR buffer 10X with MgCl2, 2 μL of dNTPs (6.6 mM), 1 μL of each of two primers (10 μM) and 0.1 μL of Taq polymerase (2.5 U, Qiagen, Hilden, Germany). The PCRs were run using the C1000 Touch thermal cycler (BIO-RAD) as follows: 4 min at 94 °C; the denaturation/annealing/elongation process was set with five cycles of 30 s at 94 °C, 60 s at 60 °C, and 60 s at 72 °C, followed by 30 cycles of 30 s at 94 °C, 60 s at 50 °C, and 60 s at 72 °C. Final elongation followed for 7 min at 72 °C. PCR products were purified using ExoSAP Kit (GE Healthcare, Buckinghamshire, UK) and then sequenced in both directions using an automated DNA Sequencer (Applied Biosystems 3100). These two last steps were performed at the Centre national de séquençage (Genoscope), Évry, France. Sequences were edited and assembled using Sequencher 5.1 (Gene Codes Corporation).
The cytb sequences newly generated were compared to those available in the EMBL/Genbank/DDBJ nucleotide database for the family Pteropodidae. The Bayesian approach was used to reconstruct phylogenetic relationships. The three outgroup species used to root the pteropodid tree, i.e., Artibeus jamaicensis, Hipposideros armiger and Megaderma lyra, were chosen on the basis of previous molecular studies [10,11]. DNA sequences were aligned on Se-Al v2.0a11 [12]. The cytb dataset represents a total alignment of 1,140 nucleotides and 76 taxa. The best-fitting model of sequence evolution was selected under jModelTest 2.1.4 [13] using the Akaike information criterion. Bayesian analyses were then conducted using the selected GTR + I + G model on MrBayes v3.2.1 [14]. The posterior probabilities (PP) were calculated using four independent Markov chains run for 10 000 000 Metropolis-coupled MCMC generations, with tree sampling every 1000 generations, and a burn-in of 25%. Mean pairwise distances were calculated with PAUP version 4b10 [15] using Kimura's two-parameter (K2P) model.
3 Results
3.1 Systematic description
Casinycteris campomaanensis sp. nov.
Holotype: MNHN 2011-637 (field number: C07–41), adult female, in alcohol, skull removed, collected on 21 November 2007 by Alexandre Hassanin. Body mass = 49 g. External measurements (in mm) of the holotype include: FA = 73.2, EAR = 22.3, HF = 15.0, TIB = 30.6, 1D1P = 12.3, 1D2P = 17.5, 2DM = 37.0, 2D1P = 9.2, 2D2P = 5.5, 3DM = 54.6, 3D1P = 34.8, 3D2P = 45.0, 4DM = 52.7, 4D1P = 27.6, 4D2P = 28.0, 5DM = 55.1, 5D1P = 24.7, and 5D2P = 26.0. Skull measurements include: BCH = 13.26, BCW = 15.42, C1–C1 = 6.71, C1–C1 = 4.31, C1–M1 = 10.34, C1–M2 = 10.37, CBL = 32.11, GSL = 32.26, IOW = 6.47, M1–M1 = 12.19, M2–M2 = 12.16, ML = 23.67, PL = 14.76, and ZW = 23.16. The sequence of the mitochondrial cytb gene has been deposited in the EMBL/GenBank/DDBJ nucleotide databases with accession number KJ145797.
Type locality: Village of Nkoélon-Mvini, Campo-Ma’an area, South Region, Cameroon, 02°23.831’ N, 10°02.691’ E, 117 m above sea level.
Etymology: The specific epithet refers to the Campo-Ma’an area, where the holotype was collected. I suggest ‘Campo-Ma’an fruit bat’ as the English common name and ‘Casinyctère de Campo-Ma’an’ as the French common name.
Diagnosis: A medium-sized fruit bat of the tribe Scotonycterini with a forearm length of 73.2 mm, body mass of 49 g, and a GSL of 32.26 mm. The PL represents 45.75% of the GSL (Fig. 2B). The general dorsal fur coloration of the living animal is rusty brown, with the ventrum paler, and several white markings on the head, including a white dorsal spot on the rostrum, a white chevron emerging from the outer corner of the eyes, a broad white band around lips, and a rather indistinct white tuft at the anterior base of the ear. The soft palate is composed of two series of transverse ridges (Fig. 3): the first series includes five thick, prominent and undivided interdental ridges, which are generally medially arched anteriorly; the second series contains seven thin, irregular and serrate postdental ridges with the two last ridges medially divided. In occlusal view, the first lower premolars are smaller than the incisors (Fig. 4).

(Colour online) Palatal ridges of the holotype of Casinycteris campomaanensis sp. nov. (MNHN 2011-637; photograph taken by Alexandre Hassanin). Abbreviations: C = canine, I = incisor, M = molar and P = premolar.
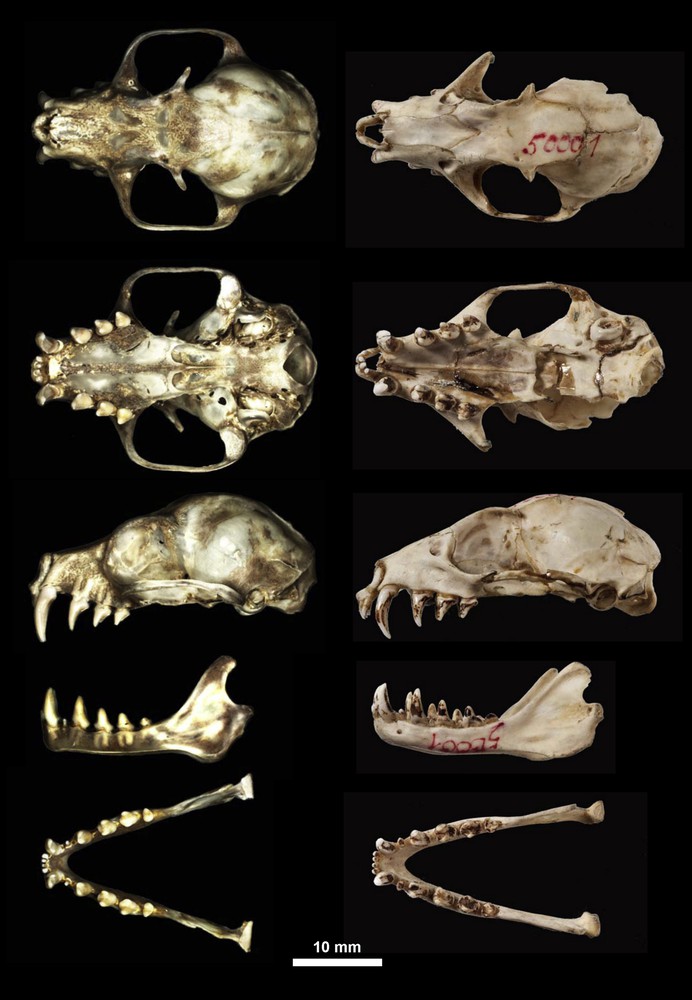
(Colour online) Skull of the holotype of Casinycteris campomaanensis sp. nov. (left column, MNHN 2011-637; photographs taken by Alexandre Hassanin) compared with the holotype of Scotonycteris ophiodon (right column, ZMB 50001; photographs taken by Carola Radke).
Description: The dorsal pelage is dense, soft and woolly. The general coloration is rusty brown, with hairs being three-coloured: dark brown basally, pale in the medially and rusty brown proximally. The ventrum pelage is shorter and paler than the dorsum, with the throat, breast and belly being whitish. Flanks are brown. The head has a complex pattern: the pelage is brown with several white markings, including on the lips and cheeks, a patch on the nose, a chevron emerging from the outer corner of the eyes, and a rather indistinct tuft at the anterior base of the brown ears. The rostrum skin coloration for the most part is greenish yellow, with the exception of the nostrils, which are with a distinct reddish colour. Upper and lower eyelids are also greenish yellow.
The wing membranes are brown with contrasting yellow finger joints. The fifth metacarpal (5DM = 55.1) slightly exceeds the length of the third and fourth metacarpals (3DM = 54.6; 4DM = 52.7), while the second metacarpal is shorter (2DM = 37.0). The first phalanx of the third digit (3D1P = 34.8) is longer than that of the fourth and fifth digits (4D1P = 27.6; 5D1P = 24.7), while that of the second digit is shorter (2D1P = 9.2). The second phalanx of the third digit (3D2P = 45.0) is longer than that of the fourth and fifth digits (4D2P = 28; 5D2P = 26.0), while that of the second digit is shorter (2D2P = 5.5). The plagiopatagium is attached to the first toe. There is no visible tail.
The skull is robustly built (Fig. 4). It has a GSL of 32.26 mm and a CBL of 32.1 mm. The zygomatic width is relatively broad (72% of GLS) and the zygomatic arches are abruptly expanded. The braincase is rounded (BCH = 13.26; BCW = 15.42). The rostrum is short and broad. The bony palate is arched medially, with a deep groove along the median palatine suture. A postdental palatum is present, tapering backwards, and ending with a long posterior spine.
As in other species of Scotonycterini, there are 28 teeth with the following dental formula: I1 I2 C1 P3 P4 M1/I1 I2 C1 P1 P3 P4 M1 M2 (Fig. 4). The maxillary tooth rows diverge antero-posteriorly (C1–C1 = 6.71; M1–M1 = 12.19), as do the mandibular tooth rows (C1–C1 = 4.31; M2–M2 = 12.16). The upper canines are conspicuously longer than other teeth, curve slightly backwards and have a faint vertical groove on the antero-medial surface. There is a pronounced diastema between C1 and P3. In lateral view, P3 extends beyond P4, and P4 is longer than M1. In occlusal view, upper premolars and molars appear roundish, and all have a secondary inner cusp. Lower canines are less developed than the upper canines but have a small secondary inner cusp. Lower first premolars are notably reduced in size and smaller than the incisors. In lateral view, P3 extends beyond P4, P4 is longer than M1, and M1 is longer than M2. In occlusal view, all lower cheek teeth (except the small P1) appear bifurcated with two distinct cusps on the inner and outer surfaces of each tooth.
Habitat:Casinycteris campomaanensis sp. nov. was collected in an area with low undulating hills and plains, ranging between sea level and 500 m, which characterizes the zone around the village of Nkoélon-Mvini. The study area is bordered to the west by the Atlantic Ocean, to the east by mountainous reliefs that reach up to 1100 m, and to the south by the Ntem River. The local natural habitat belongs to the Atlantic Equatorial coastal forests, an ecoregion characterized by exceptionally high levels of species richness and endemism [16]. The study area has a typical equatorial climate, with two dry seasons (November-March and July to mid-August) and two wet seasons (April–June and mid-August to October). In the Campo-Ma’an area, mean monthly temperatures are consistently around 25 °C, and mean annual rainfall is higher in the western part (2,800 mm in Campo) than in the eastern part (1,670 mm in Ma’an) [17]. The local habitat types change from mangrove or coastal forest in the western zone, through the endemic lowland evergreen forest dominated by Caesalpinioideae (around Nkoélon-Mvini), to the submontane forest on central hilltops and mixed evergreen and semi-deciduous forests in the drier eastern zone [17].
3.2 Molecular phylogeny of the family Pteropodidae
The Bayesian tree inferred from the analysis of 76 cytb gene sequences is presented in Appendix 1, the subtree of Fig. 5 focusing on the subfamily Epomophorinae (see below). The family Pteropodidae was found to be monophyletic (PP = 1), but the most basal relationships are not stable (PP < 0.5) (Appendix 1), suggesting that the different lineages diverged from each other in a relatively short period. The results of Fig. 5 show that the tribe Scotonycterini is monophyletic (PP = 1) and belongs to a large clade corresponding to the subfamily Epomophorinae sensu lato (PP = 0.94), which comprises the tribes Epomophorini (PP = 1; genera Epomophorus, Epomops, Hypsignathus, Micropteropus and Nanonycteris), Eonycterini (PP = 1; genus Eonycteris), Myonycterini (PP = 0.43; genera Myonycteris and Megaloglossus), Rousettini (PP = 1; genus Rousettus), and Stenonycterini (containing only Stenonycteris lanosus). Within the tribe Scotonycterini, Casinycteris argynnis and C. campomaanensis are sister species (PP = 1). The genus Scotonycteris is found to be paraphyletic, as the holotype of S. ophiodon (ZMB 50001) is grouped with the two species of Casinycteris (PP = 1), whereas S. zenkeri appears as an early offshoot.
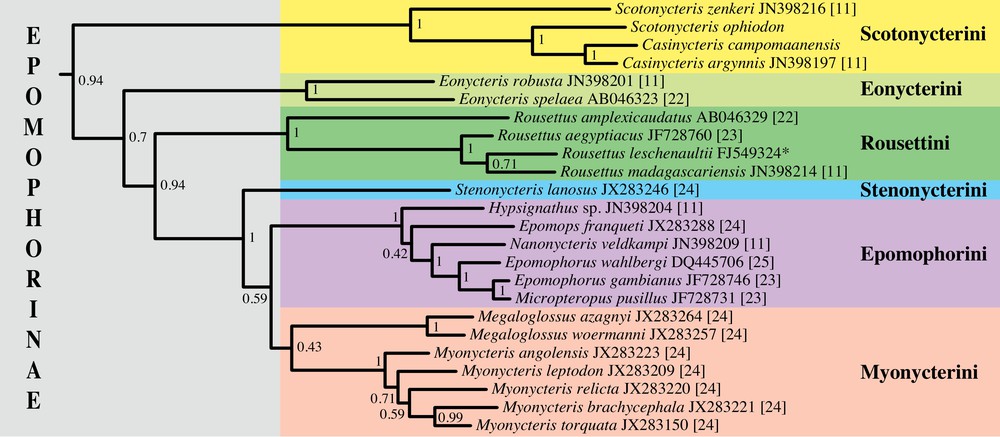
(Colour online) Molecular phylogeny of the subfamily Epomophorinae as inferred from mitochondrial sequences of the complete cytochrome b gene (1140 nucleotides). In this study, the cytochrome b gene was sequenced for the holotypes of Casinycteris campomaanensis sp. nov. (MNHN 2011-637) and Scotonycteris ophiodon (ZMB 50001) (accession numbers KJ145797 and KJ145798). All other sequences were extracted from the EMBL/GenBank/DDBJ nucleotide database. The accession number of each sequence is indicated after the species name, followed by the bibliographic reference [11,22–25]. The asterisk is used for unpublished sequences. The tree was reconstructed using Bayesian inferences. The values at the branches represent posterior probabilities (see Section Materials and methods for details). Masquer
(Colour online) Molecular phylogeny of the subfamily Epomophorinae as inferred from mitochondrial sequences of the complete cytochrome b gene (1140 nucleotides). In this study, the cytochrome b gene was sequenced for the holotypes of Casinycteris campomaanensis sp. nov. (MNHN 2011-637) ... Lire la suite
Pairwise nucleotide distances, as estimated with the K2P model, indicate that the four species of Scotonycterini have divergent cytb sequences: 4.4% between C. campomaanensis and C. argynnis; 8.4% between C. campomaanensis and S. ophiodon; 14.7% between C. campomaanensis and S. zenkeri; 7.7% between C. argynnis and S. ophiodon; 13.7% between C. argynnis and S. zenkeri; and 14.4% between S. ophiodon and S. zenkeri.
4 Discussion
The cytb gene phylogeny (Fig. 5) suggests that the classification of the tribe Scotonycterini needs revision, specifically with the paraphyletic nature of the genus Scotonycteris. To rectify this situation, it is recommended that S. ophiodon should be placed in Casinycteris, containing C. argynnis and C. campomaanensis, resulting in Scotonycteris being monospecific with S. zenkeri. These taxonomic changes are also corroborated by morphological characters (see below).
In all species of Scotonycterini, the head pelage is rusty brown with white fur patches on the nose and behind the eyes. Three species, C. campomaanensis, C. argynnis and S. ophiodon, have greenish yellow skin on the snout and eyelids, and yellow finger joints contrasting with the brown wings. On the basis of its colour pattern, S. ophiodon differs from S. zenkeri, whereas it shares similarities with the two species of Casinycteris. Overlying these morphological characteristics on the molecular tree topology of the family Pteropodidae indicate that these colour features are synapomorphies.
As shown in Fig. 2A, the differences in body size, as revealed by comparison between FA and body mass, separate females of the four species of the tribe Scotonycterini. Scotonycteris zenkeri differs from other Scotonycterini by its smaller body size. On the basis of body mass, C. campomaanensis occupies an intermediary position between C. argynnis and S. ophiodon. Regarding FA, C. campomaanensis is similar to the smallest specimens of S. ophiodon. The intraspecific variation in body size appears more important in S. ophiodon than in S. zenkeri or C. argynnis (Fig. 2A). As described by Bergmans [7], S. ophiodon may consist of several different morphological lineages. For this reason, morphological and molecular comparisons were made between the holotypes of S. ophiodon (ZMB 50001) and C. campomaanensis (MNHN 2011-637). Indeed, both are adult females possessing forearms of almost equal length (75 and 73.2 mm, respectively). In addition, the holotype of S. ophiodon was collected in 1899 at Bipindi, a town about 100 km from the type locality of C. campomaanensis. Both morphological and molecular data show notable differences between the two holotypes, confirming that they belong to distinct species. As shown in Fig. 4, the skull morphology of S. ophiodon is very different from that of C. campomaanensis, with the former having a less rounded braincase, more elongated rostrum, and less pronounced zygomatic arches. The cytb gene of these two species differs by more than 8.4%, further lending support to their distinctiveness.
In parallel to the cytb data, skull morphology of C. campomaanensis is similar to C. argynnis. Both species share a domed braincase. In proportion to the GSL, the WZ of C. campomaanensis falls within the range of measurements presented by Bergmans [7] for specimens of C. argynnis (71.8 and 70.5–75.3%, respectively; Fig. 2B). The zygomatic arches are abruptly expanded in both species. The teeth have similar size and shape, but the first lower premolars are smaller than the lower incisors in C. campomaanensis. Another noticeable difference concerns the bony palate. As illustrated in Andersen [18] and Happold [19], C. argynnis exhibits the unique peculiarity of having practically no postdental palate. Although reduced in comparison to S. ophiodon and S. zenkeri, the postdental palate of C. campomaanensis extends well beyond the upper molars (Fig. 4) and measures 4.12 mm, which represents 27.9% of the total palatal length. For comparison, the postdental palate of the S. ophiodon holotype represents 46.3% of the total palatal length, and that of a Cameroon specimen of S. zenkeri (MNHN 2011-682) represents 35.4%.
Differences in the soft palate morphology is a common method for species identification of pteropodids [1,18], as is the case for the four species of Scotonycterini. The soft palate of C. campomaanensis has two series of palatal ridges: the first includes five thick, prominent and undivided interdental ridges and the second contains seven thin, irregular and serrate postdental ridges (Fig. 3). In contrast, the soft palate of C. argynnis has three or four thick interdental ridges, followed by 13–16 crowded, irregular, thin and serrate postdental ridges [7] and that of S. ophiodon has six thick undivided interdental ridges followed by 10–13 narrow serrated postdental ridges, some divided some complete and others incomplete, with irregular connections between the ridges [20]. In S. zenkeri, the soft palate is composed of four thick interdental ridges, the first three undivided and the fourth sometimes medially divided, and 6–9 thin, serrated and irregular ridges [21].
5 New classification
Tribe Scotonycterini Bergmans, 1997: Beaufortia 47:69.
Scotonycteris Matschie, 1894: Sitzb. Ges. Naturf. Fr. Berlin, p. 200.
- Scotonycteris zenkeri Matschie, 1894: Sitzb. Ges. Naturf. Fr. Berlin, p. 202.
- (type locality: Yaoundé, Cameroon).
Casinycteris Thomas, 1910: Ann. Mag. Nat. Hist., ser. 8, 6:111.
- Casinycteris argynnis Thomas, 1910: Ann. Mag. Nat. Hist., ser. 8, 6:111.
- (type locality: Bitye, Cameroon).
- Casinycteris campomaanensis Hassanin, 2014, new species.
- (type locality: Nkoélon-Mvini, Cameroon).
- Casinycteris ophiodon(Pohle, 1943): Sitzb. Ges. Naturf. Fr. Berlin 1942: p. 78 [publ. 1943].
- (type locality: Bipindi, Cameroon).
6 Conclusion
The current geographic ranges of the four recognized Scotonycterini species (Fig. 1A) include rainforests of southern Cameroon, which would indicate that this area has played an important role in the diversification of the tribe. The analysis presented herein has shown that the characters used to define Casinycteris campomaanensis have been important to better understand the evolution of the tribe Scotonycterini. In the classification presented herein, the genus Casinycteris is diagnosed by several external features, such as the greenish yellow skin on the snout and eyelids, and the yellowish finger joints. Casinycteris campomaanensis and C. argynnis share a massive and broad skull with a rounded braincase and pronounced zygomatic arches. The most striking differences between these two species are their body size, their soft palate morphology, and the extreme reduction of the postdental palate in C. argynnis.
Disclosure of interest
The author declares that he has no conflicts of interest concerning this article.
Acknowledgments
I am very grateful to the Institut de recherche pour le développement (IRD) in Yaoundé, Gilles Etoga (WWF), Elomo Molo, Flobert Njiokou and Luther Tchakep for permission to carry out fieldwork and to export specimens. Fieldwork was supported by grants from the PPF ‘Etat et structure phylogénétique de la biodiversité actuelle et fossile’. I thank the following individuals who helped with the collecting of fruit bats in Cameroon: Jérôme Fuchs, Elysée Ganago, Innocent Medjo, Henri Nzonang and Anne Ropiquet. I also thank Nora Lange, Carola Radke, Lisa Kluckert and Frieder Mayer at the Museum für Naturkunde (Berlin, Germany), who sent a tissue sample and images of the Scotonycteris ophiodon holotype. I also acknowledge Steven M. Goodman for his helpful comments on the manuscript. Laboratory work was supported by the MNHN, CNRS and ATM ‘PPF taxonomie moléculaire, DNA Barcode et gestion durable des collections’, and ‘Consortium national de recherche en génomique’, which is part of agreement No. 2005/67 between the Genoscope and the MNHN on the projects ‘SpeedID’ and ‘Bibliothèque du Vivant’.


