1 Introduction
Apollo butterfly, Parnassius apollo (Lepidoptera: Papilionidae), is a relic of glacial fauna in Europe. Once relatively common, since the first half of the 20th century its extinction has been documented in numerous sites (e.g., [1–4]). Among many confirmed and suspected factors causing negative impacts on Apollo populations throughout Europe, weather catastrophes are considered very influential on butterfly populations [5].
Apollo declines are still being observed despite the fact that numerous legislation measures and conservation efforts have taken place (see [2,4] and references therein for details). In the Pieniny Mountains – a small range at the Polish-Slovakian border – a large metapopulation of P. apollo ssp. frankenbergeri Slaby existing in the beginning of 20th century steeply decreased and split into smaller and local demes more vulnerable to various negative impacts. Following weather anomalies in 1957 and 1961, severe breakdown of the remaining demes was observed and eventually all but one deme was extinct by the mid-1980s. Because a recovery plan was launched in 1990, the last deme of this Apollo subspecies was saved from extinction [6]. The recovery plan enabled research on its feeding preferences, physiology of digestion, and the energy budget of final larval instars, in search of possible ‘weak points’ and useful hints for protective measures [7–10].
Effect of ambient temperature on the rate of Apollo development was only minimally studied prior to this research [11,12]. We were interested whether the degree-day concept (e.g., [13,14]) could provide useful data concerning the developmental time of Apollo final stages. Such information seems quite important nowadays, after the recovery plan in the Pieniny Mts. was ended in 2010 and all active measures strengthening the population were stopped. Regular observations of Apollo population in the Pieniny Mts. revealed periods of stability as well as a rapid decrease by about ¾ of the observed population size between 2003 and 2004 [4]. These observations were done on Apollo adults, so it is important to possibly anticipate expected events that may result in a decrease of the population's size. Among previous developmental stages, last-instar larvae are good candidate for such anticipated actions. Due to their large size, intense consumption and time of development, they are relatively easy to spot on their food-plant, so they can be occasionally collected and breed in the semi-natural conditions in case of expected severe weather anomaly.
2 Materials and methods
2.1 Insects
P. apollo (L.) ssp. frankenbergeri Slaby larvae were obtained from a semi-natural breeding colony, set up in Pieniny National Park for purposes of a recovery plan for this subspecies in the Pieniny Mts. [6]. Just after moulting, fifth-instar (L5) larvae were placed individually in 0.4 dm3 plastic containers with ventilation holes and transparent walls. Due to asynchronous moulting, the larvae in the experimental groups differed in their individual age on a given day. The containers were grouped in large glass insectaria (c.a. 0.6 m3) kept outdoors to provide nearly natural temperature, solar radiation, and humidity variation. Pupated individuals were placed in muslin cages to enable freshly emerging adults to properly stretch their wings. Then, after sex determination and marking each adult with identity mark, some of them were kept in the colony for breeding, while the rest were released into the wild. Data for this study were obtained from rearing experiments carried out in May–June 1996, 1997, and 2003. In 1997, due to a different experimental set up, the observations and measurements ended at the pupal stage; then we could not identify the adult for particular larvae. For the purpose of this study, previously obtained data were recalculated and combined with meteorological data that allowed us to make new conclusions regarding Apollo development. Our data were based upon a time period of rapidly increasing Apollo deme size from 1996 and 1997 with the highest number of adult individuals observed in natural habitat sites in 2002–2003, followed by a significant decrease in the deme size in 2004 (see [4] for details).
2.2 Food utilization and energy budget
Larvae were fed ad libitum with freshly collected leaves and sprouts of their food-plant witch's moneybags (Hylotelephium telephium). Fresh leaves were weighed before being provided to the larvae. Every 2 days, the food was replaced, the plant remains and faeces were collected, and the larvae were weighed. The collected material was dried in 60 °C to obtain the dry weight. The dry weight of consumed food was calculated from a correlation between fresh and dry weight of control samples of the leaves (y = 0.0726x + 9.0689, r = 0.948, P < 0.05, n = 15). Food utilization by single larva was measured gravimetrically [15]. The energy content of dry matter was calculated by combustion in adiabatic digital calorimeter KL-12M (PRECYZJA-BIT, Poland) in 3-MPa pure oxygen atmosphere (resolution of temperature change at 0.001 °C).
The components of the energy budget were denoted according to Petrusewicz and Macfadyen [16], and indices of the budget were adopted from Waldbauer [15], with slight modifications. They can be briefly summarized as follows [J/individual/instar]:
We also calculated the following indices of energy budget:
AD = (A/C) · 100% [%/ind./instar], where AD means approximate digestibility,
ECI = (P/C) · 100% [%/ind./instar], where ECI means efficiency of ingested food conversion into body substrate,
ECD = (P/A) · 100% [%/ind./instar], where ECD means efficiency of assimilated food conversion into body substrate.
2.3 Calculation of developmental time
The developmental time of individual Apollo larvae was calculated according to degree-days (°D) single sine method [14,17], using the following formula:
Daily temperature (the lowest and the highest) and rainfall data, recorded by a weather station located nearest to the colony (c.a. 6 km), were obtained from the scientific division of Pieniny National Park (Fig. 1).
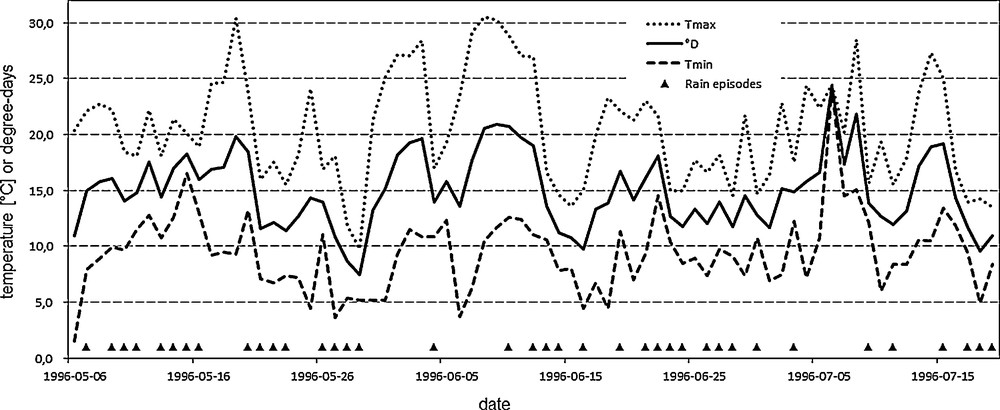
Fluctuations of minimal (Tmin) and maximal (Tmax) daily temperatures and calculated amount of degree-days (°D: continuous middle line) during development of last-instar apollo larvae in 1996 (relations between extreme daily temperatures and degree-days had similar pattern in 1997 and 2003, then were omitted here). Small filled triangles denote days with rainy episodes. Meteorological data were obtained from Pieniny National Park.
There is a lack of laboratory data on Apollo development at various temperatures, therefore developmental thresholds are unknown. Based on morphological and biological features of the larvae, dark coloration and the feeding activity of hatched individuals under the snow cover [18,19], we assumed 0 °C as the lower developmental threshold for the calculation of developmental time of the later larval instars and pupa. On the other hand, older larvae and adults are heliobionts that actively feed at temperatures even as high as 35 °C (personal observations). This was also partially confirmed by biochemical assays carried out in our laboratory with the highest Q10 values for trypsin and α-glucosidase activities in Apollo larvae stated in the 25 °C–35 °C range [9,10]. All recorded temperature data for the analysed developmental periods fell within the 0 °C–35 °C range, thus we could use a simple formula for degree-days calculations.
2.4 Statistical analysis
Statistical elaboration of the obtained data was performed with StatSoft, Inc. STATISTICA (data analysis software system), version 10. ANOVA and T Tukey's test for unequal sample size, applied to a post hoc analysis (α = 0.05), allowed a comparison of energy budgets and total developmental time. Correlation analysis and homogeneity of slopes (Generalized Linear Models) tests (α = 0.05) were applied to compare the development of males and females during a particular year and among the years.
3 Results
Apollo larvae feed on H. telephium leaves in a broad range of temperature conditions, although they prefer warm, sunny days. Rainfalls almost entirely stopped their feeding (personal observations during the experiment). Elaboration of raw meteorological data revealed that in 1996, 1997, and 2003, the mean difference between maximal and minimal daily temperatures was 11.1, 11.1, and 13.9 °C, respectively, while the maximal difference reached 22.8, 20.1, and 28.0 °C. During the examined years, the last-instar Apollo larvae on average consumed different amounts of their food-plant leaves, hence the amounts of energy required to complete their instar and pupate stages were also different. In 1996, the larvae consumed the highest amounts of food and energy −34.2 kJ. In 1997 and in 2003, it was 78% and 55% respectively of the amount of 1996 (Table 1). Lower consumption was accompanied by decreased assimilation that resulted in approximate digestibility 5.5 and 19.7 percentage points lower in 1997 and 2003 respectively, in comparison with 1996. However, the larvae that consumed and assimilated the least energy from their food allocated relatively more energy into body mass (higher ECD values) (Table 1).
Parameters (mean ± SD) of energy budget [kJ per individual] and indices [% per individual] for the last-instar P. apollo larvae.
| Parameters & indices | 1996 n = 24 |
1997 n = 15 |
2003 n = 19 |
| C | 34.2a ± 17.18 | 26.6a,b ± 8.67 | 18.7b ± 4.08 |
| A | 23.1a ± 16.23 | 15.9a,b ± 7.02 | 8.2b ± 2.30 |
| P | 8.0a ± 1.05 | 4.0b ± 1.31 | 4.1b ± 0.71 |
| R | 15.1a ± 16.07 | 12.0a,b ± 6.69 | 4.0b ± 2.06 |
| AD | 63.4 | 57.9 | 43.7 |
| ECI | 27.0 | 15.9 | 22.8 |
| ECD | 46.0 | 28.2 | 53.9 |
Components of the energy budget in larvae that pupated into males did not differ significantly from that in larvae developing into female adults, despite females were consuming and assimilating about 25–30% more energy than males. It was determined that female larvae spent the energy on maintenance costs, since the allocation of energy into tissue production was nearly the same in both sexes. This tendency was observed across both years examined (Table 2).
Mean values of energy budget parameters [kJ per individual] and indices [% per individual] for the last-instar P. apollo larvae (male and female larvae separated).
| Parameters & indices | Male larvae | Female larvae | ||
| 1996 n = 11 |
2003 n = 13 |
1996 n = 13 |
2003 n = 6 |
|
| C | 28.4 ± 9.26 | 16.9 ± 2.70 | 39.1 ± 20.92 | 22.6 ± 3.97 |
| A | 18.2 ± 9.34 | 7.3 ± 2.11 | 27.2 ± 19.78 | 10.2 ± 1.34 |
| P | 7.7 ± 1.00 | 4.0 ± 0.59 | 8.2 ± 1.07 | 4.7 ± 0.76 |
| R | 10.5 ± 9.95 | 3.4 ± 2.08 | 19.1 ± 19.38 | 5.5 ± 1.13 |
| AD | 61.5 | 43.0 | 65.0 | 45.3 |
| ECI | 29.6 | 23.7 | 24.7 | 20.9 |
| ECD | 51.1 | 57.5 | 41.7 | 46.2 |
Despite higher energy consumption and assimilation by Apollo larvae in 1997 in comparison with 2003 (lower consumption in comparison with 1996), we observed the longest mean duration time of the instar that year (Table 3). On the other hand, completion of the last-instar required the same number of degree-days, irrespectively of the year (Table 3). Detailed analyses revealed that female larvae had slightly longer instar duration than male larvae in 1996 (Table 4).
Mean duration [days] of the last-instar P. apollo larvae, calculated degree-days and means of rainy days during the instar.
| Parameters | 1996 n = 24 |
1997 n = 15 |
2003 n = 19 |
| Instar duration | 17.3a ± 2.39 | 21.7b ± 3.22 | 16.1a ± 3.69 |
| Degree-days | 261.0a ± 26.56 | 261.7a ± 36.84 | 246.9a ± 46.19 |
| Rainy days | 9.7a ± 2.46 | 13.3b ± 1.67 | 8.0a ± 2.74 |
Mean duration [days] and corresponding degree-days of the last-instar P. apollo larvae (male and female larvae separated).
| Parameters | Male larvae | Female larvae | ||
| 1996 n = 11 |
2003 n = 13 |
1996 n = 13 |
2003 n = 6 |
|
| Instar duration | 15.5a ± 1.4 | 15.6a ± 3.9 | 18.9b ± 1.8 | 17.0a ± 3.4 |
| Degree-days | 240.1a ± 12.8 | 241.9a ± 48.2 | 278.8b ± 21.7 | 257.9a ± 43.4 |
Discrepancy between longer duration time of L5 larvae in 1997 and similar degree-days requirements as seen in the preceding year or 2003 may result from much higher rainfall intensities during that stage of Apollo development. Available meteorological data did not allow accurate calculation of rain episodes duration, hence we could only calculate the number of rainy days. Despite this inaccuracy, we found a significant positive correlation between the number of rainy days (x) and duration of the instar (y):
Moreover, various consumption patterns during the instar also suggest the influence of external factors on the feeding intensity of the Apollo larvae in the examined years (Fig. 2).
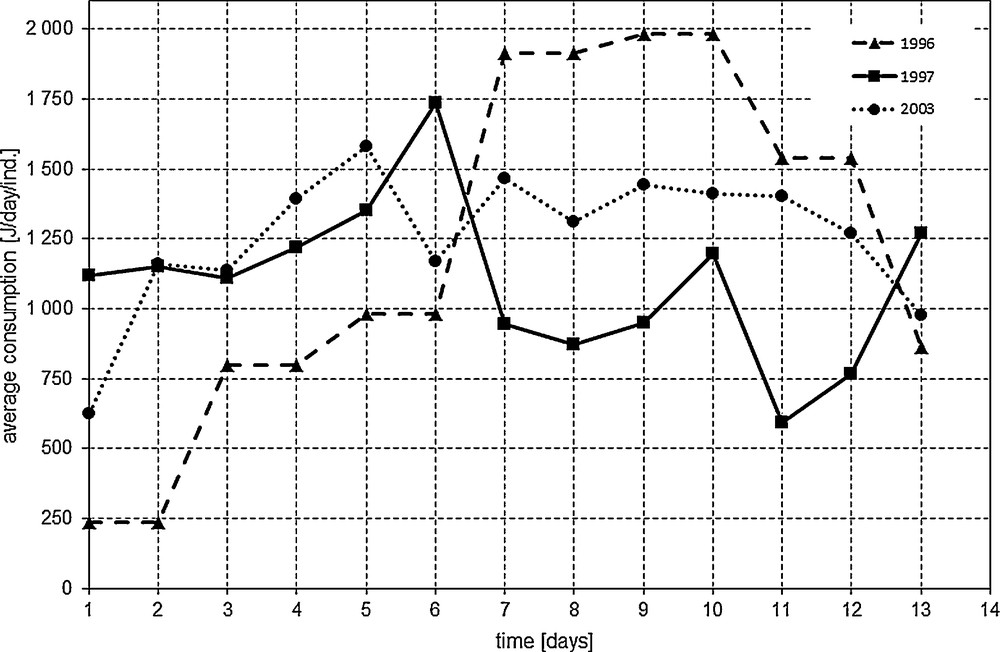
Average consumption of fifth instar Parnassius apollo larvae [J/day/ind.] during consecutive days since the beginning of the instar in 1996, 1997, and 2003. Significant differences between average consumption in particular days among the years were as follows: 1st and 11th days: 1996 vs. 1997 & 1997 vs. 2003; 2nd day: 1996 vs. 1997 & 1996 vs. 2003; 4th day: 1996 vs. 2003; 7th and 8th days: 1996 vs. 1997.
Fresh body weight of particular individuals positively correlated with the duration of the last larval instar. Logarithmic transformation of the growth curves resulted in linear functions that were compared by homogeneity of slopes test. Transformed growth curves for male larvae were parallel to curves for female larvae both in 1996 and in 2003 (Table 5). Moreover, male larvae that developed in 1996 were heavier in comparison to larvae developing in 2003. Hence, growth of male larvae appeared to be variable among the analysed years.
Increase of fresh weight during the last-instar of P. apollo larvae.
| No. | Year and sex | Regression equation | r | Signif. |
| 1. | 1996 F | y = 284.4 + 576.5x | 0.87 | |
| 2. | 1996 M | y = 280.3 + 551.0x | 0.89 | a |
| 3. | 1996 M + F | y = 280.8 + 566.0x | 0.88 | C |
| 4. | 1997 M + F | y = 274.6 + 409.1x | 0.82 | D |
| 5. | 2003 F | y = 282.4 + 482.5x | 0.84 | |
| 6. | 2003 M | y = 230.6 + 420.9x | 0.87 | b |
| 7. | 2003 M + F | y = 240.5 + 443.7x | 0.84 | D |
There was no significant difference among years found for female larvae. However, pooling of the data for both sexes confirmed that in 1996 Apollo larvae grew bigger than in the two other years (Table 5, Fig. 3). The obtained results allowed describing growth of female Apollo larvae (y) as a function of instar duration (x) by one common equation, irrespectively of the year:
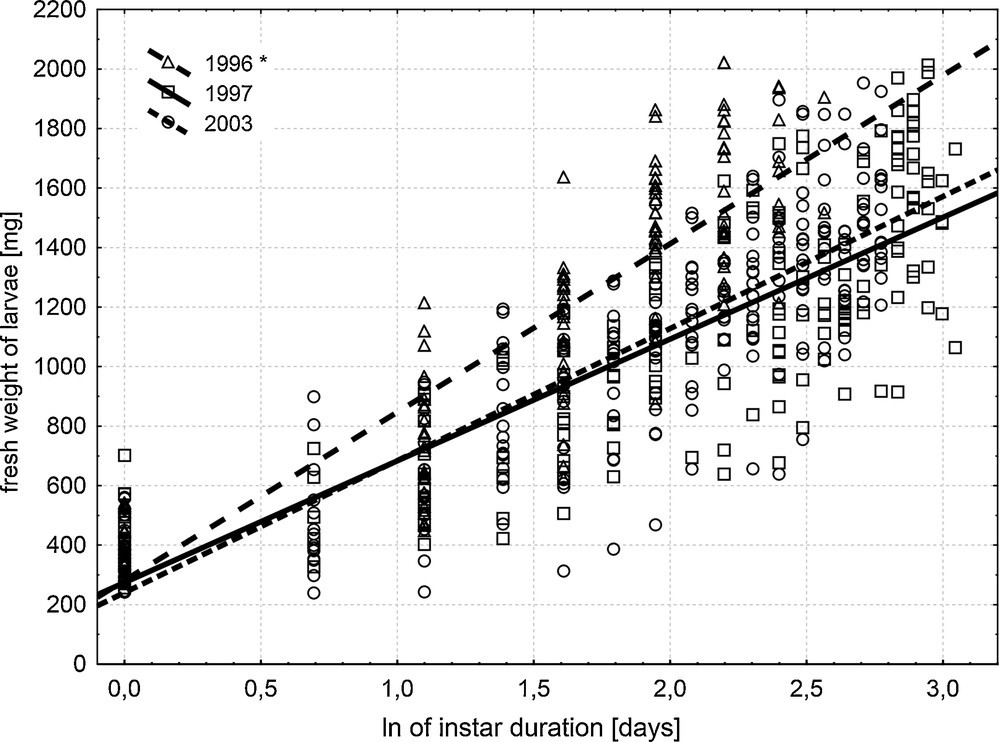
Correlation between days after moult and fresh body weight [mg] in the last-instar Parnassius apollo larvae of both sexes, examined in 1996, 1997, and 2003. *Regression line for 1996 significantly differed from the lines for 1997 and 2003.
4 Discussion
Progress in the growth and development of insects occurs only in ranges of physiological temperatures limited by lower and upper developmental thresholds [20]. These data led to elaboration of degree-day (°D) concept commonly applied in Integrated Pest Management (IPM) practice for effective pest control [21]. We are not aware of any attempt of applying this concept to describe temperature-dependent development of endangered insect species, probably due to restrictions or limitation in obtaining the living material.
Effects of ambient temperature on butterflies’ development were documented for numerous species both in the laboratory and in field studies [22]. There are large fluctuations of daily temperatures as well as seasonal variation in mountain habitats and this may cause species-specific changes in food consumption and utilization, and consequently in growth performance and developmental time [15,23,24]. Besides the effects of mean temperatures, the variation in temperature can also influence the rate of development [25,26]. Studies on some lepidopteran species revealed that developmental time of the caterpillars was more affected by temperature change than were the consumption rate and the weight gain [24].
P. apollo is a sun-loving species that minimally tolerates prolonged rainfalls and the accompanying low temperatures. In such unfavourable conditions, both larvae and adults decrease their locomotion and feeding activity, and can even die [12] (personal observations). In the Pieniny Mts., Apollo preferably inhabits open calcareous slopes with southern exposure [27]. Nevertheless, large variations of daily temperatures are typical during the development of the final larval stages. Fluctuations of daily temperatures resulted in difference of duration time of the Apollo last-instar larvae; nevertheless, larvae required the same number of degree-days to complete the instar. Prolonged rain episodes (rainy days) appeared to be major factor that delayed pupation of the larvae. This raises a question if weather conditions influenced the total amounts of food ingested and assimilated by the last-instar larvae. We calculated that surviving larvae consumed about 18.7 ± 4.08 kJ/ind. on average in 2003, but about twice as much in 1996. The mean temperature (hence daily amount of degree-days) was similar in the studied time-periods of these 2 years, then greater consumption and assimilation in 1996 than in 2003 resulted in higher body mass of the larvae. Moreover, there was no common consumption pattern during the instar because different amounts of food were ingested by larvae on particular days of the instar when analysed years were compared (Fig. 2).
Adult butterfly viability, performance, and fecundity depend on energy and nutrients stores accumulated during larval stage. Prolonged development may increase the risk of death and disturb phenological events. It has been documented that despite differential reproduction costs to males and females, the differences in the energy budget between the sexes during larval stage are small [15,24]. Similar observations were documented for Apollo and this allowed us to pool the data obtained for male and female larvae into one group for a particular year.
Weight gain of Apollo larvae during the last-instar can be described by a logarithmic equation. Double logarithmic transformation of these data resulted in linear equations, and it appeared that regression lines for males and females were parallel in a particular year. The lack of statistical difference in body weight between males and females in 1996 and in 2003 allowed us to combine data for a particular year to obtain a single regression line (Table 5, Fig. 3).
Comparison of these regression lines for particular years revealed no differences between larval growth in 1997 and 2003, but the growth curve obtained in 1996 differed from the others. Detailed analysis revealed that different – much higher – growth occurred only in males. Then it appeared that common growth formula for last-instar larvae could be calculated for females, but not for males. Smaller weight of grown male larvae in 2003 in comparison with individuals from 1996 is an interesting observation, since there were no differences in duration time or mean number of rainy days during larval development. The Apollo rearing procedure adopted during the recovery plan provides some clues for explanation of this observation. Females used for founding the next colony generation were randomly selected from the colony individuals. They were mated in natural conditions with ‘wild’ males including those males released from the colony several days earlier. Whether this indicates possible decrease of male size in the Apollo population inhabiting the Pieniny Mts. needs to be confirmed by comparative measurements. However, lower indices of energy budget measured in male Apollo larvae in 2003 in comparison with 1996 also suggest possible changes in larval metabolism, although interannual weather-dependent variation cannot be excluded.
Despite limitations of available living individuals, our results proved the degree-day concept useful for predicting Apollo development. Further studies involving, e.g., adapted thermoimaging methods (e.g., [28]) could provide better insight into a direct dependence of locomotory and feeding activity of the larvae on their body temperature and could allow determination of developmental thresholds with limited individuals available.
The obtained results on the dependence of Apollo development on weather condition can appear useful in preventing unexpected and dangerous population decrease due to weather anomalies. They provide a tool for the prediction of the adults’ appearance based on field observation of the last-instar larvae combined with actual and foreseen weather conditions. In case of expected weather anomaly, the population can be supported by semi-natural rearing. They also suggest the importance of continuous monitoring of Apollo population in the Pieniny Mts. unless the population is large and strong enough to withstand local weather anomalies without any protective measures.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


