1 Introduction
Coastal environments adjacent to human and industrial settlements are heavily influenced by direct anthropogenic activities [1,2]. Toxic heavy metals such as cadmium (Cd) and lead (Pb) are considered among the most important pollutants. The natural concentrations of these elements in an ecosystem can vary by anthropogenic mobilization. These compounds, widely distributed in the environment, are used in industries and are present in most wastewaters discharged into the sea [3].
The availability of heavy metals to the biomass of a polluted region is the prime concern both in terms of the prediction of the effects of metal pollution on an ecosystem and in terms of possible human health risks [4,5]. Trophic transfer of metals is increasingly recognized as an important pathway for metal accumulation in marine invertebrates and fishes [6]. In suspension feeding marine bivalves, both aqueous and dietary exposures represent dominant routes for metal uptake and accumulation [7,8].
Mussels are highly suspension feeders, ingesting plankton, material resuspended from sediments, and aggregates consisting of high-molecular-weight substances, detritus, faecal pellets, and microorganisms [9,10]. As adults, they need to filter large volumes of water to consume sufficient amounts of particulate matter to grow, reproduce, and survive. For this reason, mussels are used as sentinel organisms to assess the toxicological effects induced by the anthropogenic activities [11–13].
Metallothionein (MT) induction as a response to metal exposure is well documented, and thus MTs have been proposed as biomarkers of trace metal pollution in numerous species from different zoological groups [14–17]. Mussels of the genus Mytilus are considered the main marine invertebrate candidates for monitoring use based on MTs as biomarkers [18–22]. In these animals, MT synthesis may vary considerably among tissues [23]; the most marked induction by metals was observed in the digestive gland during Cd exposure both in laboratory experiments and in the field [24,25]. It has been observed a seasonal trend of MT contents in the digestive gland, maybe related to the reproductive cycle and the growth rate of mussels [26,27]. However, many studies confirmed the reliability of using the digestive gland as the best biological matrix for MT determination in mussels [28–30].
The coastal areas of the Campania region (Tyrrhenian Sea, Southern Italy) consist of ecosystems that are subject to intensive anthropic pressures, such as unsustainable fishing and inputs of environmental contaminants; nevertheless, many mussels are caught or cultivated in this area. Recently, we have investigated the bioaccumulation of Cd, Pb and MT in digestive gland and mantle of Mytilus galloprovincialis mussels farmed in this area. Our data demonstrated that the amount of both metals in the digestive gland was greater than in the mantle, whereas the metallothionein preferentially accumulated in the mantle [22,31]. We hypothesized that the MT content in the mantle, i.e. a tissue involved in important functions such as the secretion of the shell, the accumulation of reserve substances and the development of gonads [32–34] might be probably associated with the physiological function of the tissue, rather than with exposure to heavy metals [31].
In an attempt to correlate seasonal tissues conditions with accumulation of heavy metals and MT synthesis, we determined in this work the seasonal patterns of accumulation of Cd, Pb and MT in the digestive and reproductive glands of wild M. galloprovincialis specimens from coastal environments surrounding Naples.
2 Material and methods
2.1 Sampling of mussels
Adult native mussels M. galloprovincialis (5.0 ± 1.0 cm shell length) were collected from natural banks at four different locations along the Campanian coast of the Tyrrhenian Sea. The selected sampling sites were, from north to south: Torregaveta pier (site 1), Capo Miseno rocks (site 2), Naples port area (site 3), and Torre del Greco port area (site 4). For seasonal variations, samples (n = 12 for each sampling) were taken at each site in the winter of 2011 and the spring, summer, and autumn of 2012. The bivalves were transported to the laboratory in plastic buckets containing seawater from the corresponding sampling site at approximately 4 °C and used within 3 h of collection. Shells were opened and soft tissues were blotted to remove excess water and weighed. Then, gonads and digestive glands were excised, weighed, and processed for analyses.
The gonadosomatic index (GSI) was calculated as the ratio of mussel gonad weight to body weight.
Twenty mussels collected in the summer of 2012 at site 4 were transferred to the laboratory and placed in 15-L glass-holding aquaria filled with filtered, aerated artificial seawater. Bivalves were maintained in aquaria under a natural photoperiod with constant temperature (20 ± 1 °C); food was given daily as a mixture of lyophilized microalgae. After 40 days, mussels were processed as described above.
2.2 Cadmium and lead determination
Metals were analysed in seawater samples collected from the appropriate sites. After collection into pre-cleaned high-density polyethylene bottles, the seawater samples were acidified to a pH value lower than 2.0 by adding concentrated nitric acid in order to avoid metal adsorption onto the inner bottle walls and then filtered through a 0.45-μm polycarbonate membrane Nucleopore filter (Millipore). Tissue samples were prepared according to Trinchella et al. [31]. Briefly, approximately 0.2 g of fresh tissue (digestive gland and gonad) was digested in 2 mL of concentrated ultrapure HNO3 (Fluka) at 70 °C for 30 min and centrifuged for 15 min at 12,000 g. Cadmium and lead contents in seawater samples and in the tissues supernatants (n = 3) were determined by flame atomic absorption, using a Varian A220 atomic spectrophotometer, according to the manufacturer's conditions and with background correction. Working standards in 0.2% v/v HNO3 were prepared daily by diluting known aliquots of the stock solution to the appropriate volume. The detection limit of metals in different samples was determined from the standard additions curve for each metal. It was based on the usual definition of the concentration of the analyte yielding a signal equivalent to three times the standard deviation of the reagent blank signal (n = 5). The estimated detection limit was 15 ng/g for Pb and 8 ng/g for Cd. Quality assurance procedures included the internal calibration with an appropriate standard reference material (BCR/278R mussel tissue, IRMM). The values obtained for the standard reference material were always within the 95% confidence interval of certified values. NaCl interference did not compromise Cd and Pb determination; the background signal was compared to that obtained by performing the analysis on the artificial seawater.
2.3 Metallothionein determination
Metallothioneins were partially purified and spectrometrically determined in digestive and reproductive gland samples, as previously described [31]. Briefly, tissue samples were homogenised in three volumes of homogenising buffer with 0.1% β-mercaptoethanol after ethanol/chloroform extraction. After incubation with 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB), the sample's absorbance was read at 412 nm. Metallothionein concentration was measured using reduced glutathione as a reference standard, and expressed in μg/g tissue (wet weight). Calculations assumed 21 cysteine residues per molecule and a molecular weight of 7100 Da [35]. However, it should be noted that MT content in the tissues could be overestimated, as this method, which is based on the determination of sulfhydryl groups, is not specific only for metallothioneins, but also for metallothionein-like proteins.
2.4 Statistical analysis
Differences between groups of mussels according to the date or the site of sampling were evaluated by analysis of variance (ANOVA), and post hoc comparisons were assessed by the multiple-range Fisher's LSD test. Significant differences were *P < 0.05; **P < 0.01; ***P < 0.0001. These tests were carried out using a standard statistical package (StatView 5.0 software). Correlation coefficients (ρ) were determined using Excel.
3 Results
The cadmium content in seawater samples from the four different sites was of about 2.5 μg/L. This value did not change during the year. The value was approximately the same for the inter-shell seawater. The content of lead in both seawater and inter-shell water was lower than the detection limit of the AA spectrophotometer in all the samples examined.
Metals and metallothioneins contents were determined in digestive and reproductive glands of every single specimen collected at the different sampling sites during the four seasons. Samples showed measurable amounts of metals and metallothioneins; only in a few cases, the Pb content was below the detectable limit of the AA spectrophotometer. The statistical analysis carried out on the set of measurements collected during the year at the different sampling stations indicated a higher accumulation of Cd (P < 0.0001) and Pb (P = 0.0057) in the hepatopancreas with respect to gonads (Fig. 1), whereas the differences in MT levels in the two glands examined were only weakly significant (P = 0.05) (Fig. 1). Among the different stations, no difference in Cd contents was found; on the contrary, significant differences were found in Pb content (Fig. 1). In particular, specimens from the site 2 showed the highest content of Pb (P < 0.0001). As regarding MT content, Fisher's post hoc analysis showed statistically differences only between specimens from the sites 1 and 2 (P = 0.031).
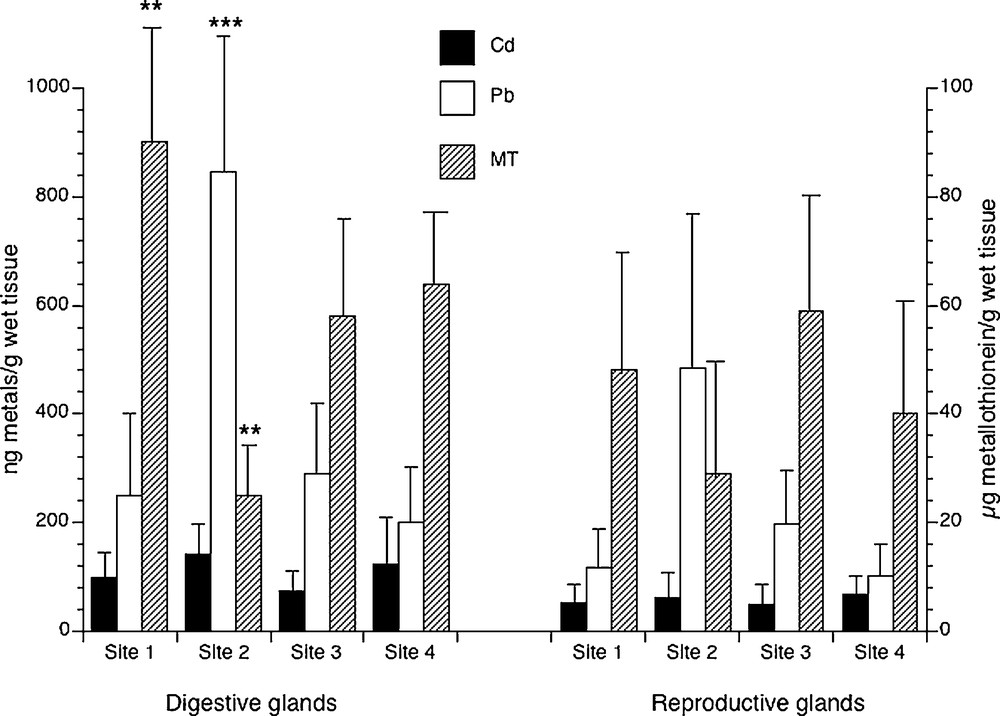
Cadmium (Cd), lead (Pb) and metallothionein (MT) contents in digestive (left) and reproductive (right) glands of Mytilus galloprovincialis specimens from four different mussel natural banks. Values are expressed as mean ± S.D. (n = 48). Site 1, Torre Gaveta; site 2, Capo Miseno; site 3, Naples port area; site 4, Torre del Greco port area. ANOVA, Fisher's LSD, **P < 0.01; ***P < 0.0001.
Statistical analyses were then carried out on values of heavy metals and MT concentration determined in digestive and reproductive glands of mussels from the different sites collected at the different seasons. Considering the whole study, seasonal changes were significant for all the three variables studied in each organ and in each geographical group of mussels.
As regarding Cd, the lowest rate of accumulation was found in spring in all the sites examined and in both glands (Fig. 2A and B); more differences were found in the other seasonal periods among the different sites. For the digestive gland, specimens from site 1 showed the highest content of Cd in summer; this value was at least 3-fold larger than those found in the same tissue both in the other sites and in the different seasonal periods examined (Fig. 2A). Specimens from site 2 showed the largest accumulation of Cd in autumn, whereas those from site 3 accumulated Cd mostly in winter; finally, specimens from site 4 showed a significantly higher Cd content in summer and autumn (Fig. 2A).
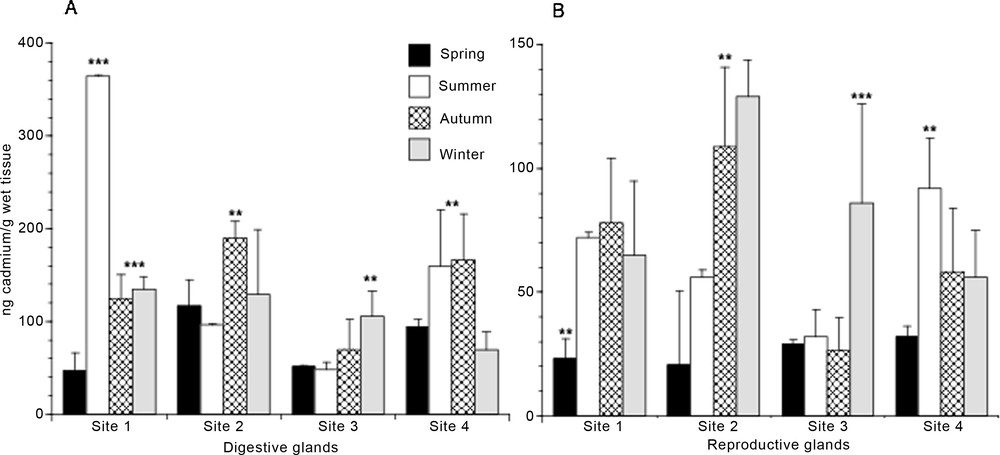
Seasonal cadmium content in digestive (A) and reproductive (B) glands of Mytilus galloprovincialis specimens from four different mussel natural banks. Values are expressed as mean ± S.D. (n = 12). Site 1, Torre Gaveta; site 2, Capo Miseno; site 3, Naples port area; site 4, Torre del Greco port area. ANOVA, Fisher's LSD, *P < 0.05; **P < 0.01; ***P < 0.0001.
In the reproductive gland, the largest Cd content was found in the specimens from site 2 during autumn and winter; a significantly higher amount of Cd was then found during winter for site 3 and during summer for site 4 (Fig. 2B).
Results depicted in Fig. 3A (hepatopancreas) and 3B (gonad) demonstrated that the Pb concentration in the two tissues examined varied greatly throughout the year at all the sites considered in the analysis. Generally, the trend of the Pb content in these tissues exhibited the following increasing order: summer < spring < autumn < winter. Only specimens collected at the Torregaveta pier (site 1) showed a very low Pb content in both digestive and reproductive glands also in autumn. Specimens from the Torre del Greco port area (site 4) showed the lowest Pb content, whereas specimens from Capo Miseno rocks (site 2) evidenced the highest content.
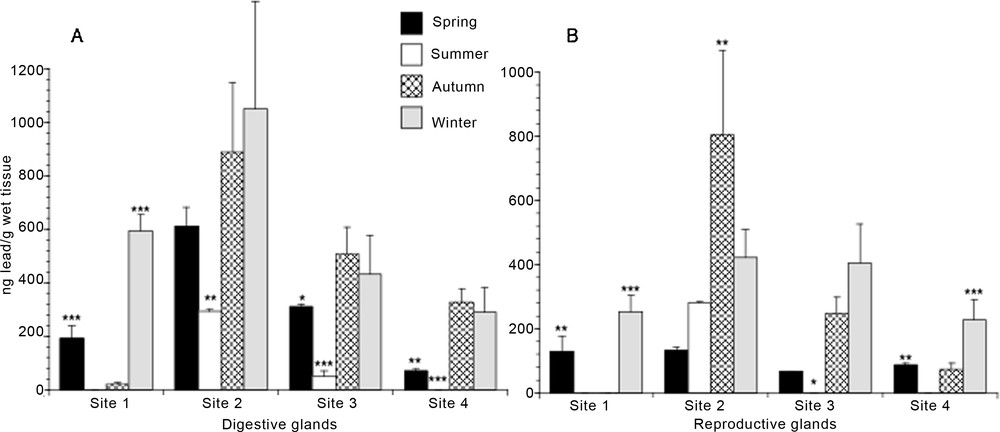
Seasonal lead content in digestive (A) and reproductive (B) glands of Mytilus galloprovincialis specimens from four different mussel natural banks. Values are expressed as mean ± S.D. (n = 12). Site 1, Torre Gaveta; site 2, Capo Miseno; site 3, Naples port area; site 4, Torre del Greco port area. ANOVA, Fisher's LSD, *P < 0.05; **P < 0.01; ***P < 0.0001.
More pronounced differences are observed in the seasonal distribution of MT, both in tissues and in the sites considered (Fig. 4A and B). In the hepatopancreas, the MT content was lower in winter, followed by values determined in autumn. Generally, the highest MT contents were found in spring and summer. Results depicted in Fig. 4A also showed that specimens from site 2 had the lowest MT content, whereas specimens from site 1 showed the highest MT content, especially in spring, summer, and autumn. In the reproductive glands (Fig. 4B), the highest MT level was found in spring in all the mussels examined; this value dramatically decreased in the summer season, then increased slowly during the autumn and winter periods. No great differences in the MT amount were found among sites; the most abundant accumulation of MT was found during the spring season in the glands of specimens from site 1. Indeed, the MT content determined in gonads of these mussels was approximately two-fold higher when compared with the MT amount measured on the same organ and in the same period in the mussels collected in the other stations.
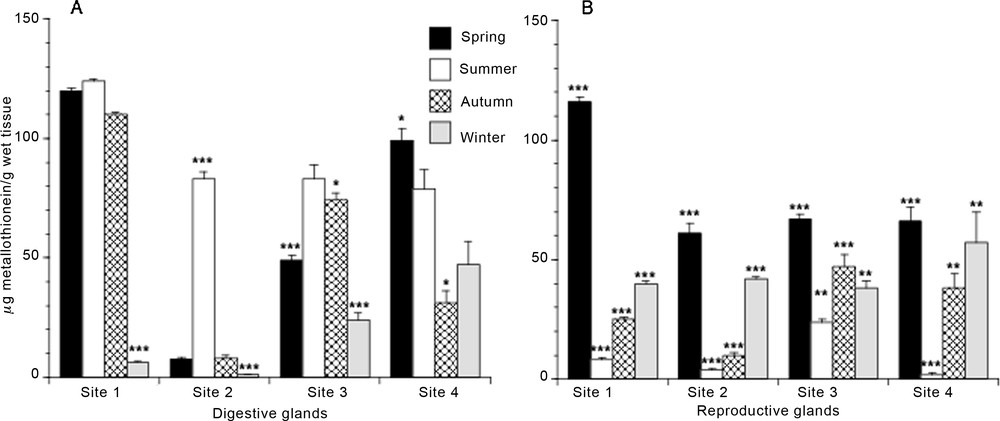
Seasonal metallothionein content in digestive (A) and reproductive (B) glands of Mytilus galloprovincialis specimens from four different mussel natural banks. Values are expressed as mean ± S.D. (n = 12). Site 1, Torre Gaveta; site 2, Capo Miseno; site 3, Naples port area; site 4, Torre del Greco port area. ANOVA, Fisher's LSD, *P < 0.05; **P < 0.01; ***P < 0.0001.
The results of correlation analyses indicate that the MT content was inversely correlated to the Cd content in the reproductive gland (ρ = –0.56), while only a weakly positive correlation was indicated for MT and Cd content in hepatopancreas (ρ = 0.082); on the contrary, the MT content seemed to be strongly inversely correlated to the Pb amount in the hepatopancreas (ρ = –0.83) and not in the reproductive gland (ρ = –0.14).
As temporal changes in the MT concentrations observed in gonads may be linked to changes in gonads’ conditions, the relationship between MT content and the gonadosomatic index (GSI) was examined. We report in Table 1 the seasonal variation of the GSI. As expected for M. galloprovincialis specimens collected in the Mediterranean Sea, GSI was high in all the mussels considered, regardless of their site of origin, immediately before the spawning period (March–April); the index dramatically decreased during the spawning period (from June to October), and increased again during the resting period (winter season). Relationships between metallothionein content and gonadosomatic index were determined applying statistical analysis on data for each one of the seasons, regardless of the sampling site of mussels. Significant positive correlations were found between the two parameters (ρ > 0.7), except for the winter season, where a negative correlation was observed (ρ = –0.91). On the contrary, no correlation was found between metal contents and GSI (ρ = 0 for both metals).
Seasonal gonadosomatic index (fraction of gonad tissue mass in the total soft tissue mass, %) of Mytilus galloprovincialis specimens from four different mussel natural banks. Values are expressed as mean ± S.D. (n = 12). Site 1, Torre Gaveta; site 2, Capo Miseno; site 3, Naples port area; site 4, Torre del Greco port area.
| Site | Seasons | |||
| Spring | Summer | Autumn | Winter | |
| Torregaveta (pier, 1) | 39 ± 15 | 23 ± 6 | 15 ± 5 | 37 ± 10 |
| Capo Miseno (rocks, 2) | 30 ± 12 | 28 ± 10 | 13 ± 3 | 32 ± 12 |
| Naples (port area, 3) | 30 ± 3 | 20 ± 6 | 16 ± 3 | 31 ± 10 |
| Torre del Greco (port area, 4) | 24 ± 4 | 16 ± 4 | 19 ± 7 | 23 ± 5 |
Metal and MT contents in tissues from mussels collected in summer at the Torre del Greco port area and maintained for 40 days in metal free seawater were: 160 ± 30 ng/g of Cd and 3.7 ± 0.3 μg/g of MT in the digestive gland; 101 ± 20 ng/g of Cd and 3.8 ± 0.4 μg/g of MT in the reproductive gland. The Pb content was below the detection limit in both tissues.
4 Discussion
The seasonal bioaccumulation of cadmium and lead in digestive and reproductive glands of the mussel Mytilus galloprovincialis was studied in relation to four different locations along the Campanian coast of the Tyrrhenian Sea. Two sites correspond to port areas, at high anthropogenic impact; the other two sites (Torregaveta and Capo Miseno) are more touristic areas. Nevertheless, the Cd and Pb contents in seawater were similar for the four different sites and did not fluctuate during the year. Also the metal contents in the two tissues examined did not widely differ among the locations. As expected, the metal levels were significantly higher in the digestive gland with respect to the reproductive gland; indeed, the digestive gland of molluscs has been known as a target organ for pollution effects, because it primarily accumulates contaminants and plays a major role in xenobiotic metabolism, immune defence, homeostatic regulation, and detoxification [36–38]. The average amounts of Cd and Pb were 0.13 μg/g and 0.45 μg/g in the hepatopancreas and 0.06 μg/g and 0.2 μg/g in the reproductive gland. These values are similar to those observed in mussels collected at aquaculture farms located along the Campania coast [22,31], and are significantly lower than those determined on M. galloprovincialis individuals that did not come from the Mediterranean Sea [39,40]. Noteworthy, these animals show high biomagnification, especially for the accumulation of lead, whose concentration in inter-shell water is below the instrumental detection limit; in the light of this statement, one should conclude that in these animals dietary exposures represent dominant routes for metal uptake and accumulation, although it has been demonstrated that, in other marine organisms, the Pb ingested along with the food is poorly absorbed [41,42]. However, the levels of the two metals were lower than the limits established by the European Food Safety Authority [43], which are 1 μg/g wet tissue for Cd and 1.5 μg/g for Pb.
In all the samples examined, also the amounts of MT were quite low, comparable with values determined in wild molluscs inhabiting both anthropogenic and naturalistic sites located along the Italian coast of the Mediterranean Sea and with values registered in farmed animals [22,31,44,45]. Differently from metals, the average MT content was of about 44 μg/g wet tissue in both tissues examined and no positive correlation was found between metals and MT concentrations in tissues. It has been postulated that in invertebrates heavy metals detoxification is mainly due to sequestration systems and insolubilization processes [15,46] rather than MT binding; in addition, there is growing evidence demonstrating the role played by phytochelatins in detoxification pathways also in animal species that are widely used in environmental toxicity testing and environmental monitoring, such as the earthworm Lumbricus rubellus and the oyster Crassostrea gigas [47,48].
This study lasted from spring to winter, allowing investigation of the potential effect of season (anthropogenic activities, climatic factors, reproduction) on both metal and MT concentrations and their relationships.
The effect of seasons on metal concentrations in mussels showed similar seasonal patterns between the sites, regardless of their anthropogenic impacts, with the lowest values recorded during the summer period for Pb and during the spring season for Cd. Anyway, there were also some interaction between site and season; in fact, to the undetectable Pb level measured in the autumn in the mussels from site 1 corresponds the highest level of Pb determined in the same period for the animals of site 2. Such observation can be attributed to differences in seasonal changes in metal exposure between these two locations. However, it has been reported that the seasonal changes in metal concentrations in mussels can result from changes in animal physiology, rather than changes in metal exposure conditions [39]. Many studies demonstrated that seasonal changes in food availability, temperature or reproduction were often correlated with changes in metal concentrations in mussel tissues [39,49,50].
As regards MTs, in the digestive glands, the lowest level was found in winter, in agreement with previous investigations [51–53]. In the reproductive gland, for all the locations analysed, the MT amount was strictly correlated with the reproductive status of the gonads, being maximum before spawning, then dropped to its lowest levels and later on increased during the winter stasis. Similar data were found also in mussels from other species and other geographical locations [54,55].
Interestingly enough, the cadmium ions measured in tissues from mussels relayed for 40 days demonstrated that the metal accumulated was not easily excreted; indeed, Cd and Pb concentrations in digestive and reproductive glands were similar to that determined in mussels before the relaying period. Conversely, the MT content dramatically decreased after mussels relay. This is most evident in the hepatopancreas, since the MT content in the gonad was very low in the seasonal period considered.
These data reinforce the hypothesis according to which in mussels heavy metals form insoluble precipitates and that the MT are engaged in other important cytoprotective functions, in addition to metal binding. The less stressful and more controlled conditions experienced by relayed mussels lead to the decrease of MT cellular concentration.
In conclusion, the Pb and Cd contents in mussels from the Gulf of Naples do not represent a risk to human health, even in the period of their maximum accumulation, which do not depend on the harvesting or farming area, but rather on the seasonality. Data further demonstrate that MT functions go beyond metal detoxification, thus opening new scenarios for their functions in invertebrates. In this regard, it should be emphasized that the MT content could be modulated by trace metals such as zinc and copper, whose role is closely linked to many physiological and reproductive processes. Finally, the relaying of mussels before marketing could improve the animals stress conditions, but would seem to have a slight effect on metal excretion, at least for the relaying period considered in this work.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.


