1 Introduction
The ultrastructural features of spermatozoa, such as the number and structure of the flagella, the presence or absence of mitochondria and the arrangement of microtubules, have been widely used as systematic characters because spermatozoa possess morphological characteristics that are more likely to be conserved than more traditional macroscopic characters [1]. Indeed, the use of spermiogenesis and spermatozoan ultrastructural features has been considered to be useful for resolving phylogenetic relationships within Platyhelminthes [2–11].
In light of their importance, several ultrastructural descriptions have been completed on various platyhelminth species, particularly free-living flatworms [3,12–17]. However, even though freshwater planarians feature relatively high diversity, data on the ultrastructural characteristics of spermatogenesis of this group are relatively scarce and are restricted to only a few species [12,18–20]. In particular, among the dendrocoelids, only one species, Dendrocoelum lacteum, has been studied [21]. Therefore, and given that based on molecular data the phylogeny of platyhelminthes is currently controversial, additional research in this group is surely needed. It is known that freshwater planarians are hermaphroditic [22]. With the present study of Dendrocoelum constrictum, we have followed the spermatogenesis and have established the structure of the spermatozoon. This study represents the first ultrastructural investigation of the spermatogenesis of a subterranean freshwater planarian, D. constrictum. The oogenesis of the species has been described previously and has greatly improved our knowledge of the cellular features of free-living flatworms group reproductive biology, particularly freshwater planarians [23]. In fact, oocyte maturation is characterized by a marked growth of the cytoplasm because of the accumulation of cytoplasmic organelles and spherical globules that migrate toward the cortical ooplasm, forming a continuous monolayer. These spherical globules have been suggested as cortical granules rather than eggshell globules [23].
2 Materials and methods
2.1 Source of specimens
Sexually mature specimens of D. constrictum were collected from the Ain Sobah spring in northwestern Tunisia, as described previously [23,24]. Samples were collected between January and May during the rainiest season, a time when D. constrictum is abundant and sexually mature.
2.2 Light microscopy
Specimens were fixed in Bouin's fluid and were preserved in 70% alcohol. Histological sections were prepared at intervals of 7 μm and stained in Mallory-Cason stain.
2.3 Transmission electron microscopy (TEM)
Worms were fixed overnight at 4 °C in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2). After fixing, the material was post-fixed in 1% osmium tetroxide in the same buffer for 2 h at room temperature, dehydrated using ascending grades of ethanol series, impregnated in propylene oxide and resin mixture, and embedded in pure resin. Ultrathin sections of silver shades (60–70 nm) were cut using an ultra-microtome (Leica, UCT) equipped with a diamond knife; sections were then placed on copper grids and stained with uranyl acetate (20 min) and lead citrate (5 min). Stained sections were observed with a TEM (JEOL JEM-1011) operating at 80 kV. Both the micrographs and electron micrographs were digitized using Adobe Photoshop by adjusting the contrast and the brightness balance.
3 Results
3.1 Light microscopy
Testes are situated on either side of the body; they are essentially dorsal in position, but with a few, distinctly ventral follicles. The testes extend from the level of the ovaries to the posterior end of the body (Fig. 1A).
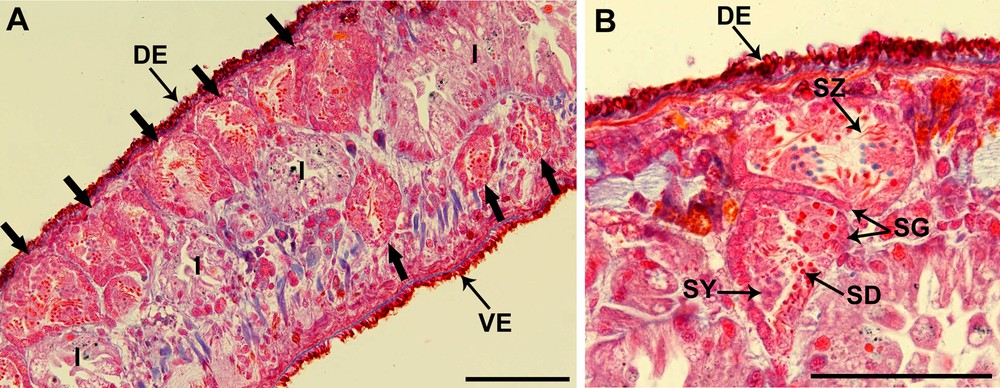
(Color online.) Light microscopic view of Dendrocoelum constrictum testes (Mallory-Cason staining). A. Sagittal section of a specimen showing that the localization of the testes (large arrows) is primarily dorsal (DE) and a slightly ventral (VE). B. Two testes with different stages of spermatozoon maturation: from the periphery to the central part of the testis we find spermatogonia (SG), a cluster of distinctly larger primary spermatocytes (SY), secondary spermatocytes, spermatids (SD) and spermatozoa (SZ). I: intestine. Scale bar = 100 μm.
Spermatogonia are arranged in a regular layer located peripherally (Fig. 1B). Toward the central region of the testis, there are distinct clusters of cells containing clumps of spermatocytes, spermatids and spermatozoa that can be distinguished on the basis of features of the nucleus. Spermatids are easily identified with their dark and round nuclei due to their high levels of compact chromatin, while the mature spermatozoa have elongated dark nuclei and are present in the lumen of the testis (Fig. 1B).
3.2 Electron microscopy
3.2.1 Spermatogenesis
Cell development begins at the periphery of the testes and as cells proceed through spermatogenesis, the spermatogenic stages shift toward the center of the testicular lumen (Fig. 2A and B).
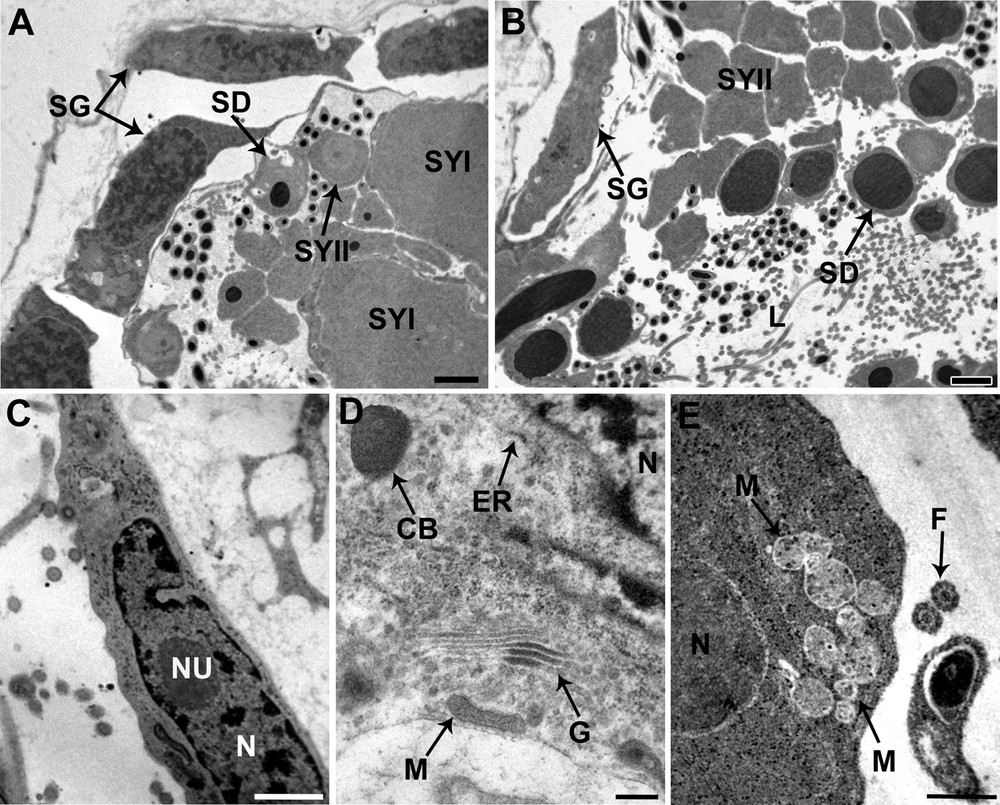
Spermatogenesis of Dendrocoelum constrictum under TEM. A. Spermatogonia (SG) situated at the periphery of the testicular wall, and the enlarged primary spermatocytes (SYI) and secondary spermatocytes (SYII). SD: spermatid; Scale bar = 1 μm. B. Spermatids (SD) are characterized by a condensed nucleus surrounded by a reduced cytoplasm, lying close to the lumen of the testis, whereas the spermatozoa are scattered throughout the lumen. We can also see the spermatogonia (SG) situated at the periphery and more internal the secondary spermatocytes (SYII). Scale bar = 2 μm. C. Detail of a spermatogonium with a characteristically large nucleocytoplasmic ratio, nucleus (N) with nucleolus (NU) and numerous ribosomes. Scale bar = 1 μm. D. Detail of the cytoplasm from a secondary spermatocyte distinguished by the prominent development of mitochondria (M), Golgi complexes (G), endoplasmic reticulum (ER) and chromatoid bodies (CB). Scale bar = 2 μm. E. An early spermatid characterized by an ovoid nucleus (N) and numerous mitochondria (M) which begin to cluster at this stage. Scale bar = 0.5 μm. L: lumen; F: flagellum.
The spermatogonia form a peripheral layer of elongated cells approximately 6 μm in diameter. They are characterized by a high nucleus to cytoplasm ratio; their nuclei are eccentrically located and have well-developed nucleoli and granular chromatin with clumps of heterochromatin scattered throughout a granular nucleoplasm (Fig. 2C). The reduced cytoplasm is filled with free ribosomes, many of which occur in clusters or rosettes. Mitochondria, endoplasmic reticulum and Golgi complexes were not observed.
Primary spermatocytes are obtained through mitotic division of the spermatogonia. The ratio of cytoplasmic to nuclear volume of these cells is so high that they are some of the largest cells formed during spermatogenesis (Fig. 2A). Secondary spermatocytes have a cytoplasm characterized by the presence of Golgi stacks and rough endoplasmic reticulum (Fig. 2D). The Golgi stacks produce small vesicles containing a dense, homogeneous substance. The chromatoid body, an electron-dense roughly subspherical mass of material, has also been observed (Fig. 2D). Ribosomes are scattered through the cytoplasm, sometimes assembled in clusters. Mitochondria are scattered with dense matrices.
Secondary spermatocytes, obtained through meiotic division of primary spermatocytes, are smaller than the latter and have nuclei that contain condensed nucleolus (Fig. 2A and B).
Each early spermatid has round or an ovoid shape with a large spherical nucleus (Fig. 2E). Several mitochondria with lightly staining matrices are visible in the cytoplasm of early spermatids, together with granular ER and clusters of ribosomes. The mitochondria begin to cluster at this stage, which indicates that they may fuse into a single mitochondrion.
Each spermatid, originally round with a round nucleus, becomes elongated during spermiogenesis (Fig. 3A). The beginning of spermiogenesis is marked by the formation of a differentiation zone situated at the periphery of each spermatid (Fig. 3B). Early spermatids, which are round in shape, show a protrusion that contains two centrioles, which are placed symmetrically and joined by a banded structure termed the intercentriolar body (Fig. 3B and C). Each centriole gives rise to a flagellum that grows externally to the emerging median cytoplasmic process, perpendicular to the axis of the protrusion, and in opposite directions (Fig. 3C). The emergence of both flagella occur at the same level (Fig. 3D). Later on, the nucleus elongates and the apical region of the differentiation zone shows the presence of an electron-dense material, a ring of arching membranes is formed at the base of the differentiation zone and cortical microtubules that border the periphery of the differentiation zone (Fig. 3E and F). The elongation continues until the ring of arching membranes is strangled and the newly formed spermatozoon is released from the general cell mass (Fig. 3F). The following stage (Fig. 3G) is characterized by a rotation of the centrioles, which are now parallel to each other but still perpendicular to the elongation axis of the spermatid. Finally, the elongated nucleus, which is characterized by a fibrillar helical structure, migrates toward the median cytoplasmic process after flagellar rotation, pushing ahead the two flagella (Fig. 3H). The complete migration of mitochondria occurs at a final stage of spermiogenesis after nuclear migration is complete. The residual cytoplasm left after the release of the newly formed spermatozoon, consists of a large vacuole together with some degenerating cytoplasmic elements (Fig. 3I).
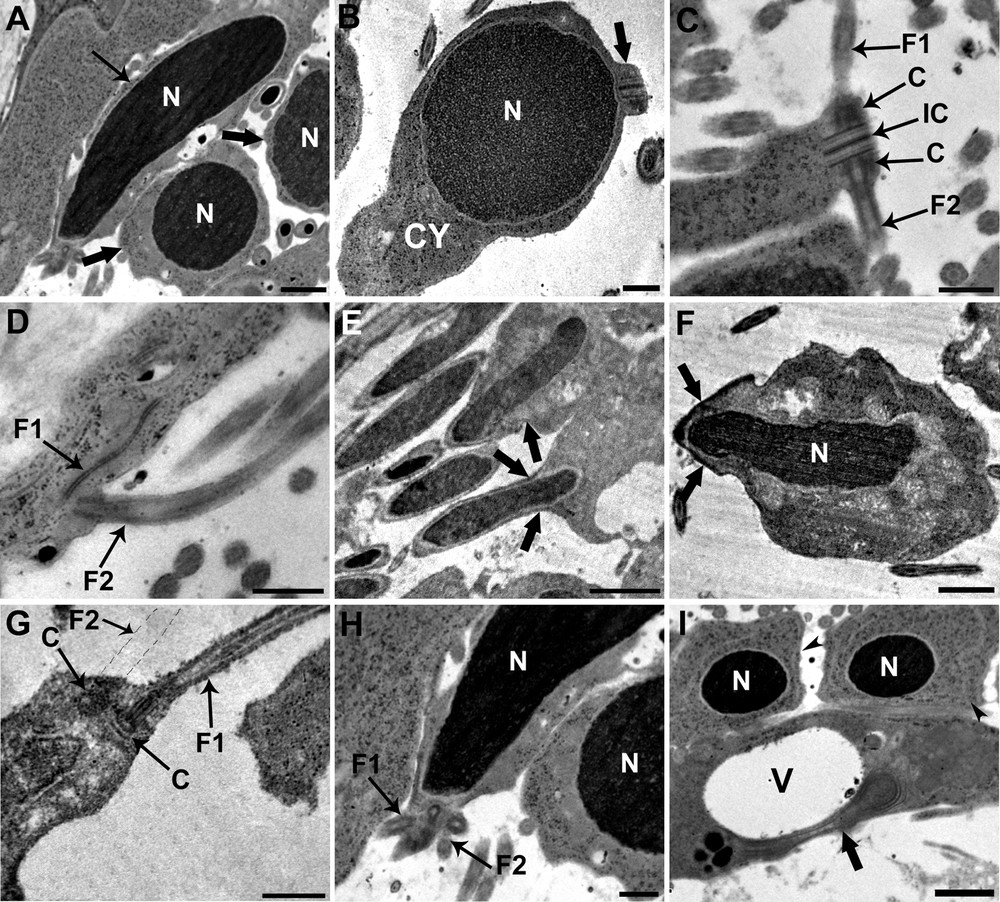
Electron micrographs showing different stages of spermiogenesis. A. Each spermatid, originally round in shape with a round and condensed nucleus (N) (large arrow), becomes elongated (arrow). Scale bar = 1 μm. B. Longitudinal section of an early spermatid showing the rounded and condensed nucleus (N) that occupies the distal end of the cell; a differentiation zone appears distal to the nucleus as a small protrusion of cytoplasm (C) (large arrow). Scale bar = 2 μm. C. Cross-section of the differentiation zone showing the intercentriolar body (IC) between the two centrioles (C) to support the two flagella (F1 and F2). Both flagella grow in opposite directions outside the spermatid. Scale bar = 0.5 μm. D. Longitudinal section of the flagella (F1 and F2) showing their emergence that occur at the same level. Scale bar = 0.5 μm. E. Elongation of the nucleus and formation of the arching membranes (large arrow) at the base of the differentiation zone. Scale bar = 2 μm. F. Elongation continues until the ring of arching membranes is strangled, and the newly formed spermatozoon is released from the general cell mass (large arrow). Scale bar = 1 μm. G. Longitudinal section of differentiation zone, after flagellar rotation, showing flagella (F1 and the emplacement of F2) parallel to the spermatid axis containing the central structure. Scale bar = 0.5 μm. H. The elongated nucleus (N) migrates toward the median cytoplasmic process pushing ahead the two flagella (F1 and F2). Scale bar = 0.5 μm. I. The residual cytoplasm (large arrow) after the release of the newly formed spermatozoon consists of a large vacuole (V) together with some degenerating cytoplasmic elements. We can also see two spermatids (arrow heads). Scale bar = 1 μm.
3.2.2 Spermatozoon
Based on transverse and longitudinal sections (Fig. 4A and B) examined by electron microscopy, the sections show the nucleus alone, the nucleus accompanied by the giant mitochondrion, or the mitochondrion alone depending on the level examined. To reconstitute the organization of the spermatozoon in D. constrictum, we distinguish the three following regions.
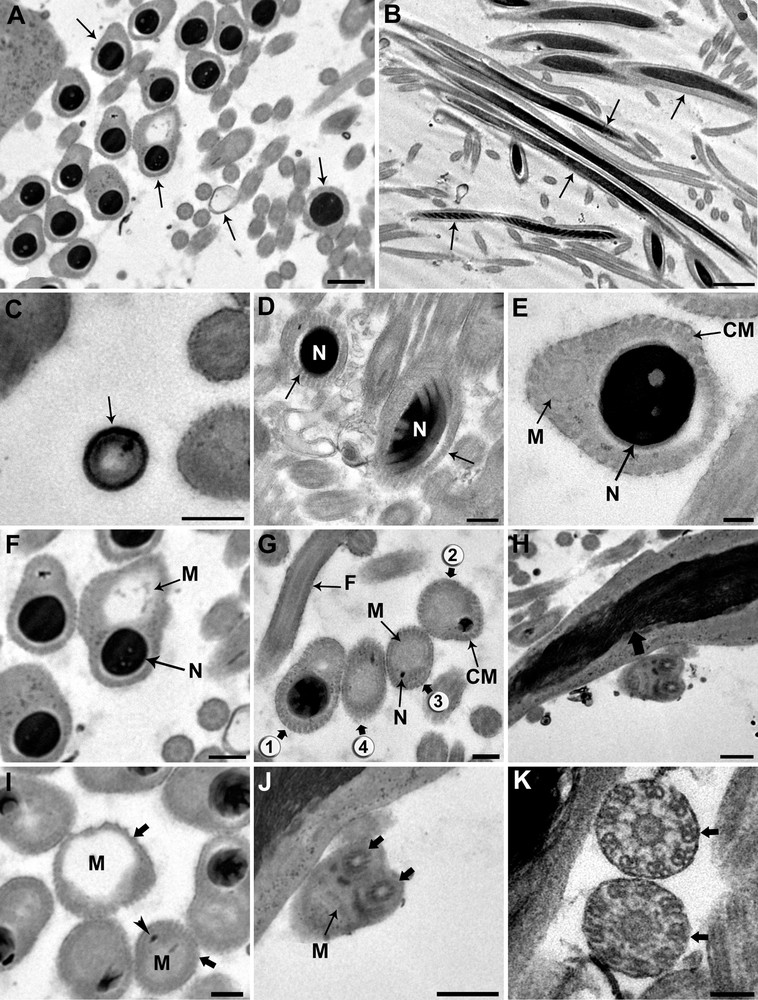
Ultrastructural organization of the mature spermatozoon of Dendrocoelum constrictum. A–B. Transverse and longitudinal sections of the mature spermatozoon at different levels (arrows). A: Scale bar = 0.5 μm, B: Scale bar = 2 μm. C. Transverse section of the anterior extremity of a spermatozoon, devoid of a nucleus and microtubules, exhibits a specific external ornamentation of the plasma membrane (arrow). Scale bar = 0.2 μm. D. Cross-section of the posterior area of region I in which we only see the nucleus (N) and no mitochondrion appears (arrow). Scale bar = 2 μm. E. Cross-section of the anterior area of region II showing both nucleus (N) and mitochondrion (M). At this level, the section of the condensed nucleus is larger than that of the mitochondrion. Scale bar = 0.1 μm. F–G. From the anterior to the posterior area of region II of the spermatozoon, the mitochondrion (M) begins to widen whereas the nucleus (N) becomes narrower until it disappears (spermatozoa section stages from 1 to 4). F: Scale bar = 0.5 μm, G: Scale bar = 0.2 μm. H. Longitudinal section of the spermatozoon showing an elongated and helical structures of the nucleus (large arrow). Scale bar = 0.5 μm. I. Cross-section of the anterior area of region III showing the enlarged profile of the mitochondrion (M) and the disappearance of the nucleus (large arrows). Arrow head is showing the trace of the nucleus. Scale bar = 0.2 μm. J. Transverse section showing the insertion level of the flagella (large arrows) at the posterior part of spermatozoon. Note the reduced section of the mitochondrion and the disappearance of the cortical microtubules. Scale bar = 0.5 μm. K. The axonemal pattern in D. constrictum (large arrows). The flagellar microtubules are arranged in a 9 + ‘1’ structure. Scale bar = 2 μm. CM: cortical microtubules.
Region I corresponds to the anterior portion of the spermatozoon. The extreme tip of this region is characterized by lightly staining content devoid of the nucleus, mitochondrion and microtubules and exhibits a specific external ornamentation of the plasma membrane (Fig. 4C). The posterior part of this region is characterized by the presence of the nucleus where it reaches its maximum width and fills the entire section of the spermatozoon (Fig. 4D). The nuclear chromatin is densely packed. The microtubules, numbering between 40 and 45, lie immediately beneath the plasma membrane and circumscribe the nucleus in this region.
Region II corresponds to the middle of the spermatozoon. It represents a wide region because it is characterized by the presence of the nucleus accompanied by the mitochondrial rod (Fig. 4E). From the proximal to the distal part of the spermatozoon in this region, the mitochondrion begins to widen, whereas the nucleus becomes narrower until it disappears completely (Fig. 4F and G). In longitudinal section, the chromatin appears to form a helical pattern (Fig. 4H). The number of cortical microtubules decreases from 40 to 35–30 along the anterior-posterior axis in this region.
Region III represents the posterior part of the spermatozoon. It is characterized by the disappearance of the nucleus and thus contains only the mitochondrial rod and 10–30 microtubules (Fig. 4I). The posterior part of the spermatozoon contains only the mitochondrial rod whereas the peripheral area is devoid of cortical microtubules and most likely represents the exiting point of the flagella (Fig. 4J). The flagellar microtubules are arranged in a 9 + ‘1’ structure (Fig. 4K). A diagram showing the organization of the mature spermatozoon of D. constrictum has been proposed from the ultrastructural sections (Fig. 5).
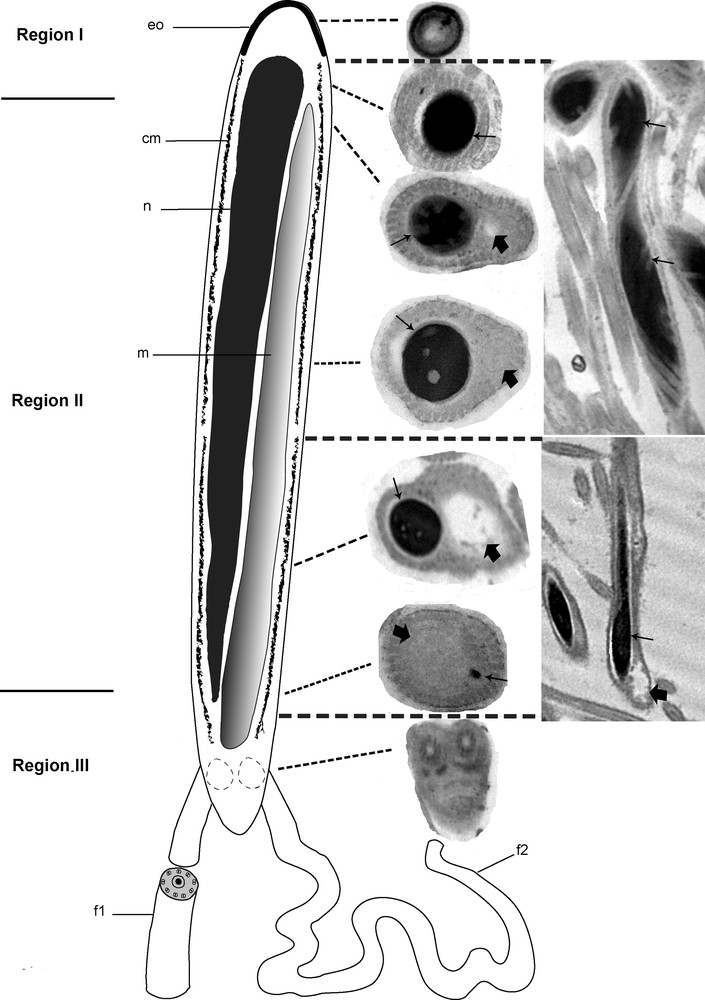
Diagram showing the organization of the mature spermatozoon of Dendrocoelum constrictum reconstructed from ultrastructural sections. cm: Cortical microtubules; eo: external ornamentation; f1: flagellum 1; f2: flagellum 2; m: mitochondrion; n: nucleus. Arrows are showing the nucleus whereas the large arrows are showing the mitochondrion.
4 Discussion
Spermatogenesis, spermiogenesis and the basic structure of spermatozoa from D. constrictum is similar to that of other freshwater planarians, including Dendrocoeulum lacteum, Polycelis tenuis, Polycelis nigra, Schmidtea mediterranea, and Dugesia sicula. However, D. constrictum has some characteristics that have not yet been described for other freshwater planarians.
The spermatozoa of D. constrictum consist of two main divisions as described in many triclads [18,19,22,25,26]. The proximal division consists of a condensed elongated nucleus and a giant mitochondrion, whereas the distal process primarily contains the mitochondrion. The length of the nucleus within the spermatozoa may be used to distinguish the two suborders of the Polyclad ftaworms [27]. In fact, the cotylean species have a nucleus that extends through most of the spermatozoon in contrast to the acotyleans that have their nucleus located in the posterior part of the spermatozoon. Moreover, the numbers of mitochondria can be of phylogenetic value since it can increase up to more than two for some species of Platyhelminthes [16,28–30] but might disappear completely in cestodes [31]. In Polyclad ftaworms, the spermatozoa have many mitochondria along with small and large dense bodies arranged in a specific pattern [27].
The spermatozoa of D. constrictum possess two axonemes with a 9 + ‘1’ pattern. This structure is always observed in most flatworms [15,19,22,25,31–33]. The only exceptions to this are the Acoel with a 9 + ‘2’ arrangement [17,21] and the Digenea with a 9 + 0 pattern [34]. In contrast to the spermatozoa of S. mediterranea in which the second flagellum appears some distance from the first, indicating that their emergence from the spermatozoon does not occur at the same level, both free flagella of D. constrictum are subterminal and emerge together from one side of the spermatozoon. The number of microtubules and their arrangement vary remarkably between groups and, in some cases, allow closely related species to be distinguished [4,5,7,35–38]. The spermatozoa of D. constrictum have an increased number of cortical microtubules, reaching a maximum number of 40–45 in the anterior and middle part of region I. The number of microtubules then decreases until they disappear toward the posterior extremity of the spermatozoon. This type of organization has been observed in many species of digenes [8,28,39] but, to our knowledge, has never been reported in freshwater planarians. In fact, in S. mediterranea, the number of cortical microtubules increased from the anterior extremity of the spermatozoon to the middle region, and then decreased to the tail [12]. In Dugesia sicula, a single row of peripheral longitudinal microtubules with a maximum number of 40 surrounds the nucleus and the mitochondrion along the entire sperm shaft [20]. They can disappear entirely in some species of Nemertodermatides and Acoeles [40,41], and in some Digenes and Monogenea [34,42,43].
To our knowledge, the external ornamentation has never been reported in planarians. It has commonly been reported in Digenean species [44–46]. Its localization can be in the anterior extremity of the spermatozoon or at a more posterior level and not around the anterior part of the two axonemes [28]. Studies that could demonstrate the importance of these ornamentations are needed to further investigate the role they may play.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project NoRGP-VPP-254.


