1 Introduction
The hormurid genus Hormiops Fage, 1933 was described from three specimens collected by the Russian zoologist Constantine Dawydoff on the Vietnamese Côn Sơn Island, formerly known as Poulo Condore and part of the Côn Đảo Archipelago. The validity of this monotypic genus has been controversial since 1989. Lourenço [1] considered that Hormiops should be placed in the synonymy of Liocheles Sundevall, 1833. Fet [2] subsequently suggested that Hormiops davidovi Fage, 1933 (Fig. 1A, C) was a junior synonym of Liocheles australasiae (Fabricus, 1775), arguing that the former was found within the distribution range of the latter. Lourenço and Monod [3] reinstated the genus, but their decision was based solely on the examination of the type material and not on a phylogenetic analysis. Prendini [4] deemed the diagnostic characters insufficient for a generic distinction, and synonymized Hormiops with Liocheles again, but he did not provide phylogenetic evidence to support the taxonomic change. However, contrary to Fet [2], Prendini [4] recognized Liocheles davidovi (Fage, 1933) [= H. davidovi Fage, 1933] as a valid species. Hormiops was finally reinstated as a valid genus by Monod and Prendini [5] based on a proper phylogenetic framework.

(Colour online.) Habitus of the hormurid genus Hormiops Fage, 1933. Hormiops davidovi Fage, 1933, male (A) and female (C). Hormiops infulcra sp. nov., male (B) and female (D).
Hormiops is so far only known from two groups of granitic islands of the South China Sea, i.e. the Côn Đảo Archipelago near the southern tip of Vietnam and the Seribuat Archipelago off the southeastern coast of Peninsular Malaysia. Specimens from Pulau Tioman, the largest island of the Seribuat Archipelago [6], were first mentioned and identified as H. davidovi by Kovařík [7]. However, about 700 km of open sea separate Tioman from Côn Sơn, suggesting that the specimens found on the Malaysian island most likely belong to a distinct species [8]. Field surveys were conducted in both archipelagos in order to collect fresh material to clarify the status of the Tioman populations. Examination of the scorpions collected during these expeditions and comparison with H. davidovi type material confirmed that the Tioman specimens belong to an unknown species, described here as Hormiops infulcra sp. nov. (Fig. 1B, D). The fresh specimens also enable us to thoroughly examine characters previously unknown or inadequately studied, such as cuticle ornamentation, hemispermatophores, and book lungs. Based on these new data and on observations made in the field, a revised diagnosis of the genus, a determination key to the Australasian hormurid genera, as well as a description of the ecomorphotype and habitat of these scorpions, both previously unknown, are provided. Furthermore, a biogeographic hypothesis is proposed to explain the disjunct distribution of the genus in Southeast Asia, based on the current phylogeny of the Indo-Pacific hormurids [5] and on a synthesis of Sundaland geomorphological history.
2 Material and methods
2.1 Fieldwork
Most specimens examined were collected during field surveys in Vietnam and Malaysia. Scorpions were collected during the day by inspecting rock crevices and exfoliations, and at night with ultraviolet (UV) light [9] using a portable Maglite lamp equipped with a UV led retrofit (Xenopus Electronix, Austin, TX, USA).
2.2 Georeferencing
Exact geographical coordinates of collecting localities were recorded using a portable GPS device (Garmin E-trek Summit). Only coarse data, rounded to the nearest 10 seconds, are provided in the present publication following the recommendations of Chapman and Grafton [10]. Geographical coordinates for records without GPS data were traced by reference to gazetteers and the Geonet Names server (http://earth-info.nga.mil/gns/html/index.html) and are given between brackets.
2.3 Abbreviations
Collections containing material examined in the present study are abbreviated as follows: MHNG, Muséum d’histoire naturelle (Geneva, Switzerland); MMUM, Manchester Museum, University of Manchester (Manchester, U.K.); MNHN, Muséum national d’histoire naturelle (Paris, France); RMBR, Raffles Museum of Biodiversity Research (Singapore).
2.4 Examination and dissection
Specimens were examined with a Zeiss Stemi SV8 stereomicroscope. Hemispermatophores were dissected from adult male specimens using microsurgical scissors and forceps immediately after specimens were euthanized. Paraxial organ tissues were then removed manually with forceps. Dissecting the specimens as early as possible ensure that paraxial organ tissues have not stiffened yet and can be removed more easily without damaging the hemispermatophores. This is particularly recommended for small, weakly sclerotized hemispermatophores like those of Hormiops.
2.5 Morphological terminology
Morphological terminology follows Stahnke [11] for pedipalp segmentation, Vachon [12] for trichobothrial patterns, Couzijn [13] for leg segmentation, Lamoral [14] and Monod and Volschenk [15] for hemispermatophore, and Prendini [4] for carapace sulci and sutures, and pedipalp and metasomal carinae.
2.6 Photographs and illustrations
High-resolution images of diagnostic characters were taken under long-wave UV and visible light with a custom-built stacking system at the MHNG. Zerene Stacker (Zerene Systems, Richland, WA, USA) was used to fuse images taken at different focal planes into a single image with a greater depth of field. Line drawings of hemispermatophores were produced using a camera lucida mounted on the stereomicroscope. Pencil sketches were subsequently inked and scanned for further processing and editing. Illustrations and photographs were edited (background removal and contrast adjustment) in Adobe Photoshop CS5 and plates prepared with Adobe illustrator CS5 (both from Adobe systems, San Jose, CA, USA).
2.7 Phylogenetic analysis
Hormiops infulcra sp. nov. was added to the dataset of Monod and Prendini [5]. Based on new observations of the metasomal segment I, two characters of the previous matrix (98, 99) were merged into one (101), and three new characters (93–95) were coded for each taxa. The matrix was compiled with Mesquite version 2.75 [16] and is provided in the supplementary material, as well as the list of characters and character states.
One hundred and nine of the 141 discrete characters were coded into binary states and 32 were coded into multistates. Evolutionary transformation sequences could not be inferred for eight multistate characters (7, 48, 59, 76, 88, 128, 134) which were treated as nonadditive (unordered) [17]. The remaining 26 multistate characters (4, 9, 10, 12, 23, 32, 33, 34, 41, 43, 46, 51, 52, 63, 67, 71, 81, 82, 101, 106, 107, 117, 123, 126, 127, 132), discretized continuous variation reflecting a linear evolutionary transition, were treated as additive (ordered) [18]. Characters were not weighted a priori.
Twenty-five uninformative characters (3, 6, 12, 19, 22, 42, 47, 50, 53, 56, 59, 61, 62, 65, 68, 72, 82, 85, 96, 97, 109, 112, 115, 119, 141), detected with the “mop uninformative chars” function in Winclada version 1.00.08 [19], were excluded from the analysis. The analysis and tree statistics are thus based on 121 phylogenetically informative characters.
Parsimony analysis was conducted with new technology heuristic search strategies, i.e. sectorial searches, tree-drifting and tree-fusing [20], implemented in TNT version 1.1 [21,22]. An equal weighting (EW) analysis was initially performed with 100 random taxon addition replicates as starting points. Each replication was initially auto-constrained with Wagner and previously inferred trees. Constrained and random sectorial searches with 200 parsimony ratchet [23] iterations, 50 iterations of tree drifting and 10 rounds of tree fusing were performed at each replication. The maximum number of trees held in memory was initially set to 10,000. The cladogram presented here was generated as a metafile from TNT and subsequently edited in Illustrator CS3 (Adobe Systems, San Jose, CA, USA).
A sensitivity analysis (sensu Wheeler [24]) was undertaken in order to assess the robustness of clade support and the stability of tree topologies under different weighting regimes. Implied weighting (IW) analyses [25,26] with concavity (K) values ranging from 1 to 10 were performed on the dataset. A preferred hypothesis was selected from among the equally parsimonious alternatives, according to three criteria: maximum fit, minimum length, maximum congruence with the topological result of the sensitivity analysis. The tree was collapsed under “rule 1” [27,28].
The relative degree of support for each node was assessed for the phylogeny selected with symmetric resampling (SR) frequencies [29]. 10,000 pseudoreplicates were used to calculate the frequencies with the probability to increase and decrease character weight set to the default value (P = 0.33). Suboptimal trees were generated from 10 random addition sequences submitted to tree-bisection-reconnection (TBR) branch-swapping and up to 10 trees were kept per replication. Frequencies were reported on the preferred tree as GC (‘Group present/Contradicted’) scores.
2.8 Reproductive biology
Several males and females of both species were kept in captivity in order to conduct observations on their sexual behaviour and gestation periods. Temperatures were maintained between 25 and 30 °C without seasonal changes. Each specimen was housed individually in plastic containers and fed 2–4 crickets every two weeks. Several females, gravid when collected, gave birth in the laboratory. Mating experiments were performed by placing female/male pairs in a larger plastic container. The bottom of this “arena” was covered with a thin sheet of cork bark, moistened on one side in order to create a humidity gradient across the enclosure. Inseminated females were maintained under the same laboratory conditions until they gave birth.
3 Systematics
Family: Hormuridae Laurie, 1896
Determination key to Australasian hormurid genera
1. Carapace with three pairs of lateral ocelli and distinct anterior furcated suture and sulci. Metasomal segment I not flattened dorso-ventrally (as high as wide, and about the same width and height as following segments); ventrolateral and ventro-submedian carinae parallel to the longitudinal axis of the segment; ventro-submedian carinae not fused distally, distinct along total length of segment (Fig. 2C–E). Telson equal or slightly longer than metasomal segment V.......................................2.

Ventral aspect of metasoma in the genera Hormiops Fage, 1933, Hormurus Thorell, 1876 and Liocheles Fabricius, 1889. A. Hormiops davidovi Fage, 1933. B. Hormiops infulcra sp. nov. C. Liocheles sp., W Java, Indonesia. D. Hormurus ischnoryctes Monod & Prendini, 2013. E. Hormurus longimanus (Locket, 1995). Abbreviations: mg (medial granules, ventro-submedian carinae), pg (posterior granules, lateral and ventro-submedian carinae), spg (subposterior granules, ventro-submedian carinae).
Carapace with two pairs of lateral ocelli and with anterior furcated suture and sulci vestigial or absent. Metasomal segment I flattened dorso-ventrally (wider than high, wider than following segments, lower than following segments); ventrolateral and ventro-submedian carinae converging to the same point near the posterior margin of the segment; ventro-submedian carinae distinct in anterior half, fused into a single carina in posterior half (Fig. 2A–B). Telson shorter than metasomal segment V....................................... Hormiops.
2. Retrolateral surface of chela manus: Esb proximal, aligned with Eb series....................................... Liocheles
Retrolateral surface of chela manus: Esb distal to Eb series, at least midway between Eb series and Est.......................................Hormurus
Genus: Hormiops Fage, 1933
Remark: A revised and updated diagnosis of the genus is warranted by the discovery of the new species H. infulcra, which does not share several characters of H. davidovi that were given in the previous diagnosis of the genus [5]. Moreover, the types series of Hormiops davidovi suffered from a prolonged stay in substandard preservative. The specimens have lost their pigmentation and fluorescence, preventing a thorough examination of cuticular macrosculptures. The availability of fresh material collected in the course of this study allowed an accurate study and imaging of these structures by means of UV lighting and led to the discovery of important additional diagnostic characters for the carination of the metasomal segment I.
Diagnosis (modified and completed from Monod and Prendini [5]): Hormiops differs from Hormurus and Liocheles in the following combination of characters. The carapace is flat in Hormiops, whereas the median ocular tubercle is at least slightly raised in Hormurus and Liocheles. The anterior furcated suture and sulci are vestigial or absent in Hormiops, but present in Hormurus and Liocheles. Two pairs of lateral ocelli are present in Hormiops, whereas three pairs are present in Liocheles and Hormurus species except the troglobite, H. polisorum (Volschenk, Locket & Harvey, 2001). Lateral transverse sulci are absent or at most very shallow on the mesosomal tergites in Hormiops, but present in Hormurus and Liocheles. The metasomal segment I is flattened dorso-ventrally (as wide as high, and wider than following segments) in Hormiops (Fig. 2A–B), whereas it is as high as wide, and about the same width as following segments in Hormurus and Liocheles (Fig. 2C–E). Metasomal segments II–V are laterally compressed (higher than wide) in Hormiops, whereas they are rounded in Hormurus and Liocheles. The ventrolateral and ventro-submedian carinae of metasomal segment I converge to the same point near the posterior margin of the segment in Hormiops (Fig. 2A–B), whereas they are parallel to the longitudinal axis of the segment in Hormurus and Liocheles (Fig. 2C–E). The ventro-submedian carinae of metasomal segment I are distinct in anterior half, fusing into a single carina in posterior half in Hormiops (Fig. 2A–B), whereas they are not fused distally and distinct along the total length of the segment in Hormurus and Liocheles. The telson is shorter than metasomal segment V in Hormiops, whereas it is equal to or slightly longer in Hormurus and Liocheles.
Hormiops differs further from Hormurus in the following characters. The proximal part of the finger is smooth and trichobothria db, dsb and dst are not surrounded by granules delimiting smooth depressions in Hormiops, whereas the surface of the finger is granular with db, dsb and dst located in distinct, smooth depressions in Hormurus. Pedipalp chela trichobothrium Esb is proximal and aligned with the Eb series in Hormiops, whereas Esb is distal to the Eb series, usually situated midway between the Eb series and Est or close, and sometimes slightly distal to Est in Hormurus. Posterior spiniform granules on the ventrolateral carinae of metasomal segment II, present in Hormiops (Fig. 2A–B), are absent or vestigial in Hormurus (Fig. 2D–E).
Hormiops differs further from Liocheles in the following characters. Pronounced posterior spiniform granules, absent or vestigial on the ventrosubmedian carinae of metasomal segments I and II in Hormiops (Fig. 2A–B), are present in Liocheles (Fig. 2C). The apex of the capsular lamella of the hemispermatophore is aligned with or proximal to the base of the laminar hook in Hormiops (Fig. 3F–I), whereas it is aligned with or slightly distal to the apex of the hook in Liocheles.

(Colour online.) Diagnostic characters for males of Hormiops davidovi Fage, 1933 (A, B, D, F, G) and Hormiops infulcra sp. nov. (C, E, H, I). Pedipalp chela, retrolateral aspect, illustrating dentate margin of chela fingers (A, B, C). Left pectine (D, E). Left hemispermatophore, dorsal aspect (F, H) and detail of capsular region, ventral aspect (G, I). (A, D, F, G) MHNG, VMI-12/04. (B) MHNG, VMI-12/07. (C, E, H, I) Holotype (MHNG, VMI-12/14). Abbreviations: bl (basal lobe), blf (basal lobe, fixed finger), bnm (basal notch, movable finger), cl (capsular lamella), dl (distal lobe), ebp (ental basal process), ml (median lobe), slm (suprabasal lobe, movable finger), snf (suprabasal notch, fixed finger). Scale, 1 mm (A–E), 0.5 mm (F–G).
Reproductive biology: Under laboratory conditions, the gestation period in both species varies between 7 and 9.5 months. Three females of H. davidovi gave birth seven, eight and nine months after the copulation respectively and two females of H. infulcra sp. nov. after 8.5 and 9.5 months. The size of broods varies between seven and 30 juveniles. Eleven H. davidovi females initially collected gave birth to 7, 9 (2×), 12 (2×), 13 (2×), 14, 20, 23, and 30 juveniles, respectively. Broods of the eleven initially gravid H. infulcra sp. nov. females comprised 8, 9, 10 (3×), 15, 17, 23, 24, and 27 juveniles (2×), respectively. The three H. davidovi females fecundated in the laboratory gave birth to 12, 16 and 19 juveniles respectively, and the two H. infulcra sp. nov. to 15 and 18.
The sex ratio of the broods is not provided because the first instars are two small to be sexed with accuracy without causing stress and thus significantly increasing the relatively high mortality rate in the first stages of development. Moreover, broods were kept communally in order to increase survival rate and, although juveniles of both species are relatively tolerant toward individuals of the same brood, occasional cases of cannibalism do occur. Therefore, once the specimens were large enough to be sexed (third-fourth instars), it was not possible to determine the initial male/female ratio.
Remark: A manuscript including complete descriptions and imaging of both species has been submitted for publication in a taxonomic journal and should be available in the coming months.
Hormiops davidovi Fage, 1933
H. davidovi Fage [30]: 32–33, fig. 1, 2, pl. I, figs a–c; Fage [31]: 181; Kästner [32]: 234, fig. 215; Takashima [33]: 94, 95; Fage [34]: 71; Vachon [12]: fig. 80; Kovařík [35]: 132; Lourenço and Monod [3]: 343, 344, figs 1–4.
Hormiops davydovi [misspelling]: Monod and Prendini [5]: Fig. 1A; 15–16, 24–25, 34.
Liocheles australasiae: Fet [2]: 395.
L. davidovi: Prendini [4]: 72; Monod and Volschenk [15]: 686.
Syntypes: Vietnam, Poulo Condore [= Côn Sơn Island, N08°42’00” E106°36’00”], S off the coast of Vietnam; II.1930/IV.1931; in forest, under stones, M. C. Dawydoff, 1 ♂, 1 ♀, 1 juv. (MNHN-RS 0562).
Other material: Vietnam, Poulo Condore Island [= Côn Sơn Island, N08°42’00” E106°36’00”], M. Germain, 1 ♀, 11 juv. (MNHN-RS 0499). Côn Đảo NP, Côn Sơn Island, track to Ong Dung Beach, 8.I.2012, rainforest, in rock crevices (granitic boulders), L. Monod, 1 ♂, 3 ♀♀ (MHNG VMI-12/01). Côn Đảo N.P., Côn Sơn Island, track to Soy Ray plantation, N08°41’ E106°35’, 50–180 m, 9.I.2012, rainforest, in rock crevices, L. Monod, 4 ♂♂, 4 ♀♀, 20 juv. (MHNG VM-12/02). Côn Đảo N.P., Côn Sơn Island, track to Dat Tham Beach, N08°42’ E106°35’, 150 m, 10.I.2012, rainforest, in rock crevices, L. Monod, 2 ♂♂, 4 ♀♀ (MHNG VMI-12/04). Côn Đảo N.P., Côn Sơn Island, track to Dam Tre Bay, N08°44’ E106°39’, 15–70 m, 11.I.2012, rainforest, in rock crevices, L. Monod, 1 ♂, 5 ♀♀, 2 juv. (MHNG VIM-12/07). Côn Đảo N.P., Ba Island, N08°38’ E106°33’, 60 m, 12.I.2012, rainforest, in rock crevices, L. Monod, 1 ♀ (MHNG VMI-12/10).
Distribution and ecology: H. davidovi is only known from, and probably endemic to, the Côn Đảo Archipelago, a group of granitic islands near the southern tip of Vietnam. Specimens were collected from narrow rock crevices of granitic outcrops in primary evergreen forests. The habitat and habitus are consistent with the lithophilous ecomorphotype [36].
Conservation status: the known populations of H. davidovi are located on several protected islands that are part of the Côn Đảo National Park. Although this species is currently not threatened by habitat destruction, the land area of the islands on which it was found is small (less than 80 km2). Because it is thus vulnerable to potential threats from tourism and loss of habitat in the future, it is recommended that H. davidovi be placed in the IUCN Red List of near threatened species [37].
Hormiops infulcra sp. nov.
H. davidovi [misidentification]: Kovařík [7]: 57–58, figs 1–7.
Holotype: W MALAYSIA, PAHANG, Pulau Tioman, track from Genting to Paya, N02°46’ E104°07’, 70 m, 25.I.2012, rainforest, in rock crevices, L. Monod, ♂ (MHNG VMI-12/14).
Paratypes: Same data as holotype, 3 ♂♂, 2 ♀♀, 5 juv. (MHNG VMI-12/14). Pulau Tioman, foothills of Gunung Kajang, N02°47’ E104°07’, 60 m, 1–2.X.2001, rainforest, in rock crevices, L. Monod, 10 ♂♂, 13 ♀♀, 46 juv. (MHNG). Pulau Tioman, track near Mukut, N02°44’ E104°07’, 115 m, 23.I.2012, rainforest, in rock crevices, L. Monod, 4 ♂♂, 7 ♀♀, 14 juv. (MHNG VMI-12/12). Pulau Tioman, track near Nipah, N02°45’ E104°07’, 25 m, 24.I.2012, rainforest, in rock crevices, L. Monod, 4 ♂♂, 2 ♀♀, 2 juv. (MHNG VMI-12/13). Pulau Tioman, track from Japamala Resort to Lanting, N02°44’ E104°07’, 65 m, 27.I.2012, rainforest, in rock crevices, L. Monod, 2 ♂♂, 1 ♀♀ (MHNG VMI-12/15).
Additional material: Pulau Tulai [N02°54’44” E104°06’26”], 23.VIII.2003, P. K. L. Ng et al., 1 ♀, 4 juv. (RMBR ZRC.ARA.456), 1 juv. (RMBR ZRC.ARA.457).
Etymology: the name infulcra is a constructed from the Latin words “in” [not, without] and the nominative plural of “fulcrum”. The epithet is an invariable noun in apposition and refers to the absence of fulcra in the pectines.
Diagnosis: H. infulcra sp. nov. differs from H. davidovi by the following combination of characters. H. infulcra sp. nov. is distinctly smaller and slightly lighter in colour than H. davidovi. The prosomal carapace of males is slightly less densely granular in H. infulcra sp. nov. than in H. davidovi. The externoventral carinae of the pedipalp femur is granular in H. infulcra sp. nov., whereas it bears coarse spiniform granules in H. davidovi. The dorsoexternal carina of the pedipalp patella in H. infulcra sp. nov. is costate-granular and more distinct than the faint costate ridge observed in H. davidovi. The digital carina of the pedipalp chela is granular in H. infulcra sp. nov., whereas it is costate in H. davidovi. In males of H. infulcra sp. nov. the dentate margins of the fixed and movable chela fingers are linear (Fig. 3C), whereas most males of H. davidovi possess a well-developed suprabasal lobe on the movable finger and a corresponding suprabasal notch on the fixed finger (Fig. 3A). The telotarsi IV has four proventral macrosetae in H. infulcra sp. nov., whereas they bear five in H. davidovi. The average number of pectinal teeth is six for males and five for females in H. infulcra sp. nov., whereas males and females of H. davidovi usually have seven and six pectinal teeth, respectively. The pectinal fulcrae are absent in H. infulcra sp. nov. (Fig. 3E), but present in H. davidovi (Fig. 3D). The tergites of males are less granular medially in H. infulcra sp. nov. than in H. davidovi. Moreover, in H. infulcra sp. nov. males, the posterior margins of tergites I-V are smooth medially and the median ridges are sometimes smooth as well, whereas tergites are completely granular in H. davidovi males. The metasoma is finely and sparsely granular in H. infulcra sp. nov. whereas it is smooth or nearly so in H. davidovi. The ventrosubmedian carinae of metasomal segment II have no granules posteriorly in H. infulcra sp. nov. (Fig. 2B), whereas they bear two small posterior spiniform granules in some specimens of H. davidovi (Fig. 2A). The hemispermatophore distal lamina is straight and only slightly longer than the basal part in H. infulcra sp. nov. (Fig. 3H), whereas it is slightly curved and longer than the basal part in H. davidovi (Fig. 3F).
Distribution and ecology: H. infulcra sp. nov. is only known from two islands of the Seribuat Archipelago (Rompin District, Pahang State, Peninsular Malaysia) and is probably endemic to this group of granitic islands. On Pulau Tioman, scorpions were collected in primary rainforests, for the most part in narrow crevices of granitic outcrops. Few specimens were found under the bark and in holes of fallen logs or standing trees, but these were most likely accidental occurrences as previously noted for the Australian Hormurus longimanus (Locket, 1995) [38]. H. infulcra sp. nov. is common on Pulau Tioman. Crevices inspected were usually inhabited by several specimens, suggesting high population densities. Although these scorpions are primarily stenotopic rock dwellers, the limited quantity of crevices in granite probably constrains specimens to seek alternative refuges when populations grow too large. The habitat and habitus of this species are consistent with the lithophilous ecomorphotype [36]. H. infulcra sp. nov. was found in syntopy (sensu Rivas [39]) with Liocheles australasiae.
Conservation status: Most known populations of H. infulcra sp. nov. are situated in protected rainforests of the Pulau Tioman Wildlife Reserve. Although this species is currently not threatened by habitat destruction, the land area of the islands on which it is found is small (133.6 km2). H. infulcra sp. nov. is thus deemed vulnerable to potential threats in the future, especially from tourism and loss of habitat, and it is recommended that it be placed in the IUCN Red List of near-threatened species [37].
Remarks: The salticid Bavia sexpunctata (Doleshall, 1859) (Fig. 4A) was collected together with the scorpions on Pulau Tioman. The spider was moving in a way similar to that of a scorpion, not using its first pair of legs to walk, but rather holding them up in the air like a scorpion holds its pedipalps. Although about half of the size of an adult H. infulcra sp. nov., the habitus of the spider is reminiscent of that of an adult H. infulcra sp. nov. male with the typical elongated pedipalps of the genus. Several Asian salticid genera, i.e. Bavia Simon, 1877, Viciria Thorell, 1877, Stagetillus Simon, 1885 and Thianitara Simon, 1903, possess a comparable habitus and when threatened, some species have been observed to adopt a defence posture resembling that of a scorpion with pedipalps outstretched and metasoma raised (Fig. 4B). It is possible that these strange salticid spiders mimic scorpions; however, further studies are necessary to confirm this.

(Colour online.) A. Bavia sexpunctata (Doleshall, 1859), male, Pulau Tioman, Seribuat Archipelago, Pahang, Malaysia (MMUM, G7549.1). B. Unidentified salticid spider (male) from Sungai Congkak Recreational Forest (Hulu Langat, Selangor, Malaysia) in defensive position. Scale, 2.5 mm.
4 Biogeography of Hormiops
A recent biogeographic analysis of Indo-Pacific hormurid scorpions [5] suggests that hormurids colonized Laurasia from Gondwanaland via the Apulian microplate about 130 Ma ago, establishing a lineage in the northern hemisphere distinct from the African stock. These Laurasian scorpions, ancestors of the extant Australasian genera Hormiops, Hormurus and Liocheles, subsequently reached equatorial latitudes and were probably well established in Southeast-Asian monsoon and perhumid ecosystems by the Mid–Late Cretaceous.
The monophyly of Hormiops and its sister relationship to Liocheles are unambiguous (Fig. 5), suggesting that this is a rather ancient lineage. Hormiops has, so far, never been recorded from continental Southeast Asia, it is only known from two archipelagos in the South China Sea, whereas Liocheles is widespread on the mainland. Field work and examination of museum collections indicates that this distribution is most likely not a sample bias. However, it is not clear whether the known extant populations of Hormiops are the remnants of a former, more widespread distribution, or whether this disjunction is the result of in situ speciation on one of the archipelagos followed by dispersal to the second across the South China Sea.
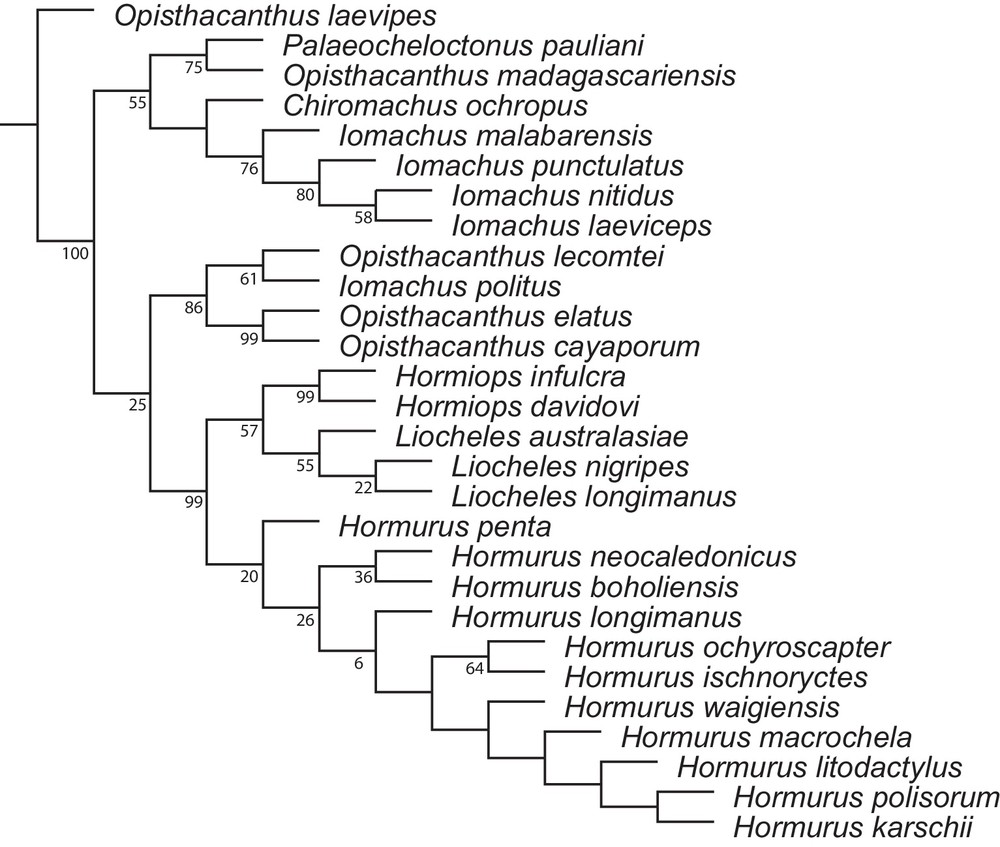
Most parsimonious tree (MPT) obtained by cladistic analysis of Indo-Pacific hormurid scorpion phylogeny under implied weighting (IW) with K = 10 that minimize length and maximize fit and branch support (length, 348 steps; Fi, 88.27%; average branch support, 46,8%). Zero length branches are collapsed under “rule 1”. Symmetric resampling frequencies (GC scores) of nodes indicated below corresponding branches.
Judging from the phylogenetic position of Hormiops (Fig. 5; [5]), from ecological observations in the field, and from the current knowledge of Sundaland geomorphological history during the Late Cretaceous and Caenozoic, the first scenario is regarded here as the preferred hypothesis. The evolution and distribution through time of Hormiops are considered here to be intricately correlated with the geomorphological history of Sundaland during the Late Cretaceous and Caenozoic. They probably resulted from drastic changes of palaeotopography and successive contractions/expansions of megathermal forests across the region. An ongoing biogeographic analysis based on molecular dating and ancestral areas reconstructions (Monod and Prendini, in prep.) will allow confirming whether the hypothesis formulated here is correct.
During the course of the present study, a detailed and comprehensive synthesis of Sundaland palaeogeography and palaeoenvironments from the Cretaceous onward was compiled from numerous publications on geology, palynology and palaeoclimatology. The evolution of Sundaland geomorphology through time involved numerous and complex events. Presenting all information necessary to fully understand these processes in the main text would make it difficult to read. Therefore, an overview is provided in the supplementary material and can be easily referred to by the readers.
4.1 Occurrence of ancient arachnid lineages on the Côn Đảo and Seribuat Archipelagos
The Côn Đảo and Seribuat Archipelagos are remnants of a Late Cretaceous cordillera extending along the eastern Sundaland margin [40], and due to their continental origin, these islands harbour an array of arachnids belonging to primitive lineages. Five ancient taxa unrelated to Hormiops are known from the rainforests of Pulau Tioman [41–44], i.e. Chaerilus sejnai Kovařík, 2005, a scorpion of the basal family Chaerilidae Pocock, 1893 [45]; Oncopus tiomanensis Schwendinger & Martens, 2004, a humicolous harvestmen (Opiliones), representative of the Sandokanidae Özdikmen & Kury, 2005, a basal family of the sub-order Laniatores [46–48]; two undescribed species of the oldest opilionid sub-order Cyphophthalmi Simon, 1879 [47,48]; Liphistius tioman Platnick & Sedgwick, 1984, a burrowing spider of the archaic sub-order Mesothelae Pocock, 1892 [45,49]. Two chaerilid scorpions are thus far also reported from perhumid habitats of the Côn Đảo archipelago [8,50], i.e. Chaerilus phami Lourenço, 2011 and Chaerilus terueli Kovařík, 2012.
These primitive arachnids have putatively very limited dispersal capacities [51,52], a character shared by both Hormiops species. H. davidovi and H. infulcra sp. nov. are lithophilous species and, like many other Australasian hormurids [5,15,38,53], these stenotopic scorpions are arguably poor dispersers with a low tolerance to other substrates [36]. All these rainforests-restricted taxa, Hormiops included, can be considered as palaeoendemic elements, remnants of Sundaland's south-eastern mountains biota, and most likely evolved in response to the same vicariance events. Divergence of the two Tioman styllocellid mite-harvestmen from their mainland counterparts at about 65 Ma [44] is congruent with this hypothesis and confirms the antiquity of these opilionids and their presence on the island before the formation of the Gulf of Thailand and of the South China Sea.
4.2 Occurrence of Hormiops on the islands
Both the Côn Đảo and Seribuat Archipelagos are at least 80 Ma old [54–58] and were arguably part of ancient palaeomountains that existed from the Late Cretaceous until the Mid Eocene [40,56,59–74]. Therefore, when these islands became isolated from the mainland (probably in the Eocene–Oligocene) due to the subsidence of extensive land areas, the formation of various sedimentary basins and increasing marine transgressions [63,66,72,74–84] across the South China Sea region, they undoubtedly already carried an established flora and fauna, including hormurid scorpions. The presence of other hormurids with relatively similar ecological requirements on the islands would have prevented their colonization by Hormiops. It is commonly accepted that the first successful colonization of an island or island group inhibits subsequent settlements of closely related taxa through niche pre-emption and interspecific competition. Only species from distant lineages and with different ecological adaptations are potentially able to survive and establish perennial populations on an island already inhabited by closely related taxa [85–88].
Therefore, the recent colonization of either the Côn Đảo or Seribuat Archipelagos from the mainland by Hormiops, as well as dispersal from one to the other, is highly unlikely. Evidence rather suggests that the presence of Hormiops species on the islands is ancient, preceding the formation of the South China Sea Basin and of the Gulf of Thailand. It is interesting to emphasize here that the widespread species L. australasiae is found in syntopy with H. infulcra sp. nov. on Pulau Tioman. Ecology of the Tioman hormurids strongly suggests that the presence of Hormiops probably predates the dispersal of L. australasiae to the island. The eurytopic L. australasiae indifferently colonizes holes in tree trunks and crevices in rock faces, at least in places where other hormurids do not occur (L. Monod, unpublished). On Pulau Tioman, however, L. australasiae predominantly inhabits trees, and rarely occurs in rock crevices. L. australasiae is approximately the same size as H. infulcra sp. nov., and both species have overlapping microhabitat requirements. It is thus postulated here that the presence of Hormiops on the island restricted the niches available to L. australasiae to trees, a habitat rarely used by H. infulcra sp. nov., which prefers rocks (see description). It is probable that L. australasiae would have colonized trees and rocks alike if H. infulcra sp. nov. was not already present on the island.
4.3 Ecology of Hormiops
The common ancestors of Australasian hormurids (Hormiops, Hormurus and Liocheles) were arguably crack-dwelling (colonizing trees and rocks), eurytopic, and restricted to everwet ecosystems, whereas the more derived hormurids comprise a higher proportion of more stenotopic taxa [5]. This indicates a transition from arboreal/lithophilous towards exclusively lithophilous or pelophilous ecomorphotypes.
The recent discovery of several stenotopic Hormurus from seasonally dry habitats of northern Australia [38] suggests that the decrease of tree density during phases of drier climates may be a preponderant factor responsible for the evolution of ecomorphological specializations, the corticolous scorpions seeking shelter and humidity on alternative substrates and eventually evolving specialized behaviours and characters in response to selective pressures exerted by these new substrates (Monod et al., in prep.).
The morphology and microhabitat of Hormiops are extremely similar to that of the Australian H. longimanus and Hormurus macrochela Monod 2013. Although Hormiops occurs in much wetter ecosystems than the two Australian species, it is postulated here that its morphology has evolved in response to environmental conditions similar to these of the two Australian taxa, i.e. seasonally dry forests. Therefore, I believe that the current evergreen rainforests of Côn Đảo and Pulau Tioman were previously drier and the tree cover more open than today, at least episodically.
This hypothesis is congruent with the accepted Sundaland palaeoclimate model (see supplementary material). During the Late Eocene, Sundaland's everwet forests were largely replaced by subhumid vegetation types [62,72,80,84,89–93]. Moreover, palynological evidence from the West Natuna Basin even suggests that during the Oligocene floral communities in the South China Sea region shared some similarities with today's seasonally dry open woodlands of SE Queensland [72].
The occurrence of several pelophilous Liocheles species in Asia, e.g. Liocheles nigripes (Pocock, 1897) in northern India, and several undescribed species from Malaysia, Vietnam (L. Prendini, pers. com.), and Thailand (L. Monod, unpublished), as well as numerous lithophilous species in the ancient Laurasian scorpion lineage Scorpiopidae Kraepelin, 1905 [94–98] suggest that the contraction of perhumid forests and the extension of drier vegetation types during the Caenozoic may have promoted the evolution of predominantly stenotopic scorpion lineages in continental South Asia.
4.4 Evolution of Hormiops on Sundaland
It is postulated that, during the Late Cretaceous–Early Caenozoic, the tectonic exhumation of Sundaland [69,83], the climate and vegetation alteration at the K–Pg boundary [92,99–102], or more likely a combination of these two large-scale events, induced in the hormurid lineage a phase of allopatric diversification that was probably coupled with the extinction of many species, eventually leading to the divergence of the extant genera Hormiops, Hormurus, and Liocheles.
The uplift of an extensive peri-Sundaland cordillera in the Late Cretaceous [40,62,63,66,67,69,72,73,83] probably resulted in severe disruptions of the landscape and consequently in the fragmentation of ecosystems across the region. Biological responses to this tectonic re-arrangements were probably also influenced by the climate and vegetation patterns. Floral communities were at the time rather diverse, from seasonally dry savannah in the north to perhumid rainforests in the south [62,72,92,93,103], and thus provided a wide range of habitats that may have facilitated ecological diversification. The K–Pg global deforestation abruptly altered these ecosystems, resulting in adverse environmental conditions and increasing ecological stresses on their biotas.
In the Mid Eocene, Hormiops had putatively already diverged from Liocheles by allopatric speciation/vicariance on Sundaland. Resumption of subduction at the margins [66,70] induced subsidence [63,66,74–76,78,79,83] and the formation of a fractured topography of horsts and grabens [62,63,72,83]. Furthermore, the expansion of sclerophyll vegetation in the Late Eocene (38–33 Ma) [62,72,80,84,89–93] resulted in the contraction of Southeast Asian everwet forests to Assam/Manmar [62,72,92,104] and to refugia along the southern and south-eastern coasts of Sundaland [62,72,92,93]. The landscape was then dominated by seasonally dry, open woodlands, and suitable habitat for humidity-dependent taxa such as hormurids were drastically reduced on the Sunda shelf, probably restricted to uplands and coastal areas [72]. These climatic and tectonic alterations are likely to have significantly affected Asian hormurid scorpions, possibly promoting a second period of diversification and extinction that reduced the distribution range of Hormiops to south-eastern refugia in the Sundaland palaeocordilleras.
Land connections between the Côn Đảo and Seribuat archipelagos were finally severed in the Oligocene with the formation of the Gulf of Thailand and South China Sea sedimentary basins (Fig. 6) [63,72,77,78,80–84]. Development of these basins also led to a dramatic reduction of emergent land and thus to further reduction of suitable habitats, resulting in the isolation of Hormiops on two island groups of the South China Sea. The two known Hormiops species thus diversified by vicariance following the disjunction of the ancestral range of the genus and are considered here as palaeoendemics. It must be emphasized that Hormiops possibly also survives on other archipelagos of the South China Sea and in the granitic massifs of south-western Borneo, all of which are vestiges of ancient Sundaland mountain ranges. The genus has so far not been recorded from these regions, and more field work is needed to check for its presence.
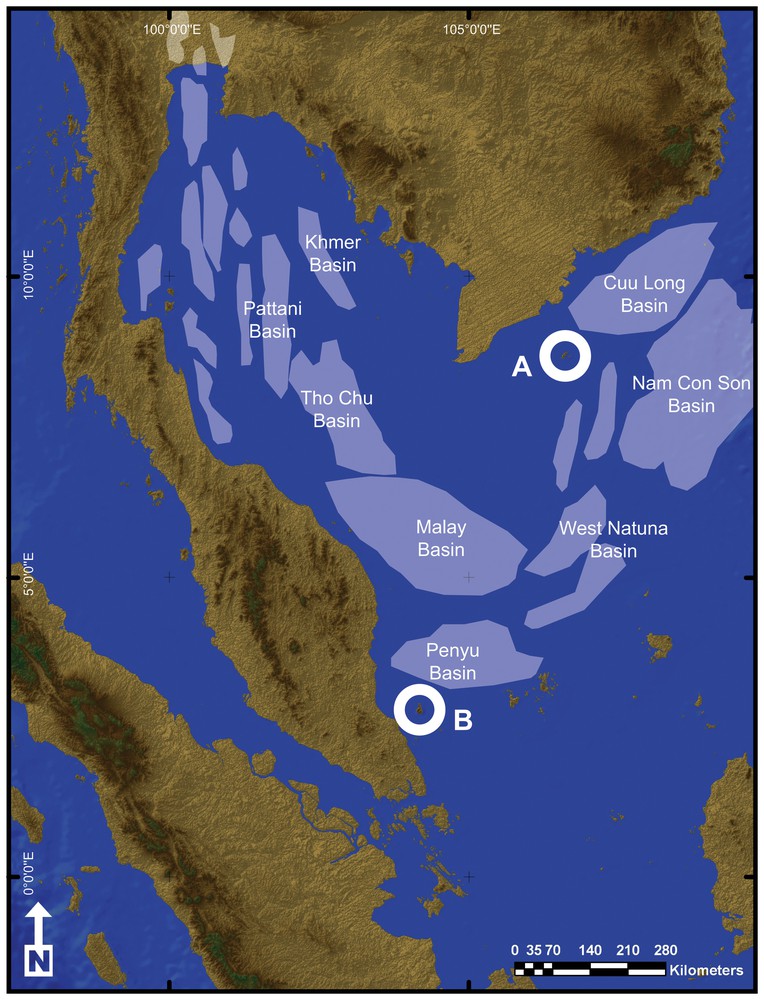
(Colour online.) Map of the Gulf of Thailand and South East China Sea Region showing the Côn Dáo (A) and Seribuat (B) archipelagos and the different sedimentary basins. All the basins started to develop in the Early Eocene as a result of faulting and rifting of the Sunda shelf, first forming an extensive network of palaeolakes in the Early Oligocene and finally becoming completely marine in the Middle to Late Miocene after Shoup et al. [70].
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
Field work in Malaysia and Vietnam was funded in part by the MHNG. The author wishes to express his gratitude to the following persons:
- • for granting access to the collections and preparing loans: Jacqueline Heurtault, Wilson Lourenço and Christine Rollard (MNHN), Lua Hui Keng, Peter Ng and Chang Man Wang (RMBR);
- • for helping with the administrative procedure of getting collecting permits for the Côn Đảo National Park: Dinh Sac Pham;
- • for providing scientific advice and constructive comments: Lorenzo Prendini and Peter Schwendinger;
- • for identifying the salticid spider: Dmitri Logunov;
- • for providing comments on an early draft of the manuscript: Peter Schwendinger;
- • for providing critical comments that helped improve and clarify the manuscript: Camilo Mattoni and an anonymous reviewer;
- • for assistance during fieldwork: Delphine Gaillard and Gwendolyn Romand.


