1 Introduction
Rice blast [Pyricularia oryzae Cavara (synonym P. grisea Sacc [teleomorph Magnaporthe grisea (Herbert) Barr])] is one of the most destructive diseases of rice (Oryza sativa L.) worldwide [1]. It is the most common disease in irrigated rice of both temperate and subtropical areas of East Asia [2]. Even in less blast conducive environments, such as rain-fed lowland rice areas in the sub-tropics, serious epidemics occur where aromatic cultivars are grown [1]. The pathogen is most common on leaves, causing leaf blast during the vegetative stage, or on nodes, neck and panicle branches during the reproductive stage, causing node and neck blast, respectively [3]. Leaf blast lesions reduce the net photosynthetic rate of individual leaves to an extent far beyond the visible diseased leaf fraction [4]. Neck blast is considered the most destructive phase of the disease and can occur without being preceded by severe leaf blast [5]. Blast epidemic causes complete loss of seedling [6] in nursery and epidemics in the field [7]. Outbreak of this disease is a serious and recurrent problem, and it is extremely difficult to control in all rice growing regions of the world, including Bangladesh [8,9]. Panicle infection causes complete yield loss [1]. Yield reduction by neck blast infection is twice as severe as leaf blast [10]. In India, 75% yield loss of grain occurred in 1950 in susceptible cultivars, while in the Philippines more than 50% yield loss has been recorded [1]. Neck blast causes direct yield losses, since filling of the grains on infected panicles is poor at best [11]. For this reason, neck blast is the more serious phase of the blast disease.
In Bangladesh, about 7000 varieties of rice are grown in different parts of the country. In general, rice is classified by its length, thickness, and aroma. Aromatic rice is known for its characteristics fragrance when cooked and it fetches higher price in rice market than non-aromatic rice [12]. It is to be mentioned here that aromatic rice is closely related to social and cultural heritage of Bengalis and it is consumed during different festivals, weddings, and for entertaining guests [13]. Locally adopted cultivars Kalijira, Sakkorkhora, and BRRI dhan34 have small grain and pleasant aroma. Aromatic rice varieties have occupied about 12.5% of total rain-fed lowland rice cultivation [14]. The production cost of fine rice per hectare is very low compared to that of coarse rice. The income potential is higher in aromatic fine rice cultivation, since its cultivation does not normally require additional expenditure on fertilizer, pesticides, and irrigation. The average yield of high-yielding rain-fed lowland rice is 3.4 t/ha, while the average yield of aromatic rice is 2.0–2.3 t/ha [15]. Several factors such as low yield potential variety, tendency to neck blast susceptibility due to high humidity and low temperature during flowering are responsible for low yield. In Bangladesh, almost all of the aromatic rice of rain-fed low land ecosystem is highly susceptible to neck blast due to a favourable environment during flowering [9]. Though most of the field research on rice blast disease has been conducted in tropical and subtropical environments [16], detailed information on the effect of neck blast disease on grain yield and seed quality of aromatic rice in Bangladesh is limited. In addition, information on pathogen transmission from neck-blast-infected panicle branches to seed is still confused. Therefore, the present study was undertaken to investigate the effect of blast disease on yield-contributing characters, and seed quality traits of aromatic rice in Bangladesh. Moreover, the percentage of blast pathogen transmission from panicles to seeds was also determined in this study.
2 Materials and methods
2.1 Plant materials, cultural conditions and disease assessment
Three aromatic rice cultivars, namely BRRI dhan34, Sakkorkhora and Kalijira, having superior grain quality and high-yielding potentiality, but being susceptible to blast, were selected in the present study. The total number of panicles including diseased and healthy of a one square meter of land were collected from three points diagonally of the same field for investigation during the wet season (July–December 2013). The collected panicles were initially graded into two groups, diseased and apparently healthy. The diseased groups were then classified according to their severity scale. Based on the number of panicles within each disease scale, panicle blast severity (PBS) was computed as follows [17]:

(Colour online.) Aromatic rice (var. Kalijira) plant with scale-9 neck blast disease severity.
2.2 Yield traits and grain size measurements
One hundred panicles from each group (either healthy or diseased) were separated randomly for yield traits and grain size measurements. Panicle length, filled and unfilled grains/panicle and grain sterility percentages of diseased and healthy panicles were determined during harvesting. Panicle weight, grain length, width and their ratio, and 1000-grain weight with the corresponding moisture of diseased and healthy panicles were determined after 3 days of sun drying. The lengths and widths of the grains were measured using digital slide callipers. The grain yields in healthy or diseased conditions were estimated by examining yield performance and breaking the yield into its components [18]. Where, grain yield (t/ha) = panicle number/m2 × grain number/panicle × % filled grains × 1000-grain weight (g) × l0−5. In case of healthy conditions, we used the average grain number/panicle, the % of filled grains and the 1000-grain weight of healthy panicles. In the same way, we used yield component data of diseased panicles for yield estimation of diseased condition. All the grain weights were adjusted to 14% moisture content calculated by dry weight basis.
2.3 Determination of grain quality and blast pathogen transmission to seeds
The grain quality of the freshly harvested air-dried seeds of the respective varieties was determined by yielding the seed-borne pathogen presence in filled and unfilled grains. The occurrence of seed-borne fungi on seeds was determined by the modified blotter method without seed surface sterilization [19,20]. Twenty-five seeds (either filled or unfilled grain) of each cultivar selected at random were spaced on damp 9-cm Whatman No. 1 filter paper in plastic Petri dishes and each one was replicated 16 times. The plates were incubated during 12-h-light and 12-h-darkness cycles at 25 ± 2 °C for 4 days to detect the blast pathogen and this was continued up to 8 days for other fungi detection. After the incubation period, conidia and hyphae of fungi growing on the seeds were picked off each infected seed with fine needles, mounted on a slide, and examined with a compound microscope (Fig. 2). Each fungus was identified on the basis of its conidia and/or hyphae characteristics [21] and the frequency of isolation was tabulated.
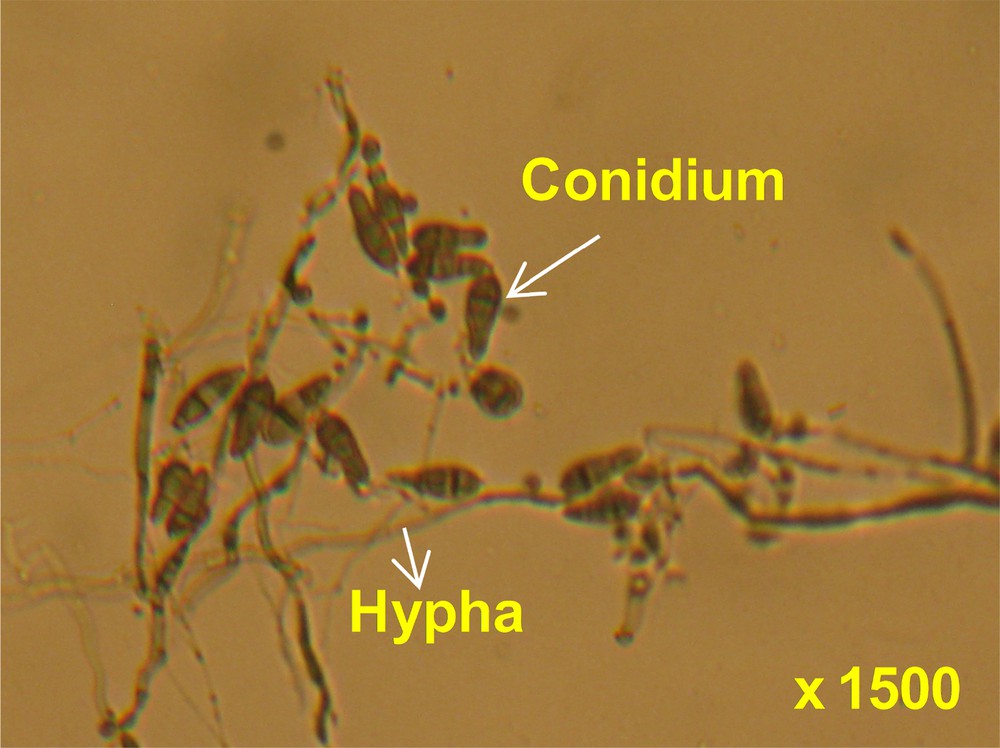
(Colour online.) Hyphae and conidia of Pyricularia oryzae observed under compound microscope (× 1500).
The transmission of the blast pathogen from the panicle base or branches to the seeds was determined by the isolation frequencies of the blast pathogen from freshly harvested seeds of neck-blast-infected panicles. In this case, seed samples were investigated without surface sterilization [20] because, as it is an airborne pathogen, it is still not clear whether blast pathogen exist on the seed's surface or as a systemic pathogen.
2.4 Data analyses
The data of apparently healthy and diseased panicles were compared through Student's t-test. Field data on neck blast incidence and severity were analysed using Crop Stat for Windows Version 7.0.2007.0 at the 5% level of significance.
3 Results
3.1 Neck blast incidence and severity in experimental field
All of the tested aromatic cultivars were highly affected by neck blast disease under natural conditions. Maximum disease incidence expressed by the percentage of panicle infection was found in Kalijira (76.2%), followed by Sakkorkhora (75.5%), and BRRI dhan34 (72.1%). In the same way, panicle blast severity (PBS) was also found to be the highest in Kalijira (47.0%), followed by Sakkorkhora (34.0%) and BRRI dhan34 (25.5%) (Table 1). Among the tested varieties, the highest disease incidence or severity was significantly found in Kalijira and the lowest one in BRRI dhan34.
Natural neck blast disease incidence and severity in the studied aromatic rice fields.
| Variety name | Percentage of panicle infection (%) | Panicle blast severity (PBS) (%) |
| BRRI dhan34 | 72.1 | 25.50 |
| Sakkorkhora | 75.5 | 34.00 |
| Kalijira | 76.2 | 47.00 |
| LSD (P = 0.05) | 0.708 | 0.498 |
3.2 Effect of neck blast on yield-contributing characters
Effect of neck blast disease on yield-contributing characters of aromatic rice was found significant. In the case of BRRI dhan34, grain sterility percentages increased and the weight per panicle decreased significantly due to neck blast infection. But this did not affect significantly panicle length and 1000-grain weight (Table 2). The mean data of all characters showed a negative trend due to neck blast infection. In the same way, the resting two varieties Sakkorkhora (Table 3) and Kalijira (Table 4) also showed an all-negative effect of neck blast disease on the yield-contributing characters. Though the mean value of the panicle length was reduced due to neck blast infection, there was no significant difference between healthy and diseased panicles for all the tested varieties.
Effect of neck blast on the yield-contributing characters of var. BRRI dhan34 aromatic rice.
| Panicle status | Yield-contributing characters | |||||
| Panicle length (cm) | Filled grain/panicle | Unfilled grain/panicle | Grain sterility (%) | 1000-grain weight (g) | Weight/panicle (g) | |
| Healthy | 23.72 | 207.20 | 23.60 | 10.07 | 10.50 | 2.37 |
| Diseased | 22.76 | 176.60 | 44.60 | 19.97 | 9.92 | 1.45 |
| Calculated t value | 0.83 | 1.66 | 3.03 | 4.83 | 1.66 | 2.84 |
| Tabulated t value | 2.78 | 2.78 | 2.78 | 2.78 | 2.78 | 2.78 |
| Significance level | NS | NS | a | b | NS | a |
a Indicates the significance levels at 5%.
b Indicates the significance levels at 1%.
Effect of neck blast on yield-contributing characters of aromatic rice var. Sakkorkhora.
| Panicle status | Yield-contributing characters | |||||
| Panicle length (cm) | Filled grain/panicle | Unfilled grain/panicle | Grain sterility (%) | 1000-grain weight (g) | Weight/panicle (g) | |
| Healthy | 29.42 | 199.00 | 16.20 | 7.49 | 11.76 | 2.76 |
| Diseased | 27.14 | 150.20 | 67.00 | 30.70 | 10.26 | 1.60 |
| Calculated t value | 1.85 | 10.82 | 10.13 | 19.66 | 15.02 | 5.49 |
| Tabulated t value | 2.78 | 2.78 | 2.78 | 2.78 | 2.78 | 2.78 |
| Significance level | NS | a | a | a | a | a |
a Indicates the significance level at 1%.
Effect of neck blast on yield-contributing characters of var. Kalijira aromatic rice.
| Panicle status | Yield-contributing characters | |||||
| Panicle length (cm) | Filled grain/panicle | Unfilled grain/panicle | Grain sterility (%) | 1000-grain weight (g) | Weight/panicle (g) | |
| Healthy | 25.64 | 141.20 | 32.20 | 18.60 | 11.39 | 1.98 |
| Diseased | 23.38 | 107.40 | 68.80 | 38.91 | 10.37 | 1.40 |
| Calculated t value | 1.61 | 5.91 | 8.25 | 13.93 | 7.11 | 2.29 |
| Tabulated t value | 2.78 | 2.78 | 2.78 | 2.78 | 2.78 | 2.78 |
| Significance level | NS | a | a | a | a | NS |
a Indicates the significance level at 1%.
A significant effect of neck blast on grain size and yield was also found to some extent. Length-to-width ratios of rice grains (dried paddy with husk) were affected significantly by neck blast disease, except Kalijira (Table 5). Though neck blast disease incidence and severity were higher in the Kalijira plot, it did not affect significantly grain size. The estimated grain yields of all tested aromatic rice varieties were affected significantly by neck blast disease (Table 6). The highest yield reduction (50.66%) compared to healthy samples due to neck blast infection was found in Sakkorkhora, followed by Kalijira (48.04%), and BRRI dhan34 (28.51%). Among the tested varieties, the highest estimated grain yield with the lowest yield reduction was found in BRRI dhan34.
Effect of neck blast disease on aromatic rice grain size.
| Panicle status | BRRI dhan34 | Sakkorkhora | Kalijira | ||||||
| Length (mm) | Width (mm) | L/W ratio | Length (mm) | Width (mm) | L/W ratio | Length (mm) | Width (mm) | L/W ratio | |
| Healthy | 6.00 | 1.97 | 3.08 | 5.88 | 2.62 | 2.26 | 6.17 | 2.20 | 2.82 |
| Diseased | 5.84 | 2.11 | 2.79 | 5.81 | 2.24 | 2.62 | 6.22 | 2.19 | 2.84 |
| Calculated t value | 1.02 | 1.98 | 2.57 | 0.81 | 4.54 | 4.17 | 0.50 | 0.11 | 0.31 |
| Tabulated t value | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 | 2.10 |
| Significance level | NS | NS | a | NS | b | b | NS | NS | NS |
a Indicates the significance levels at 5%.
b Indicates the significance levels at 1%.
Effect of neck blast disease on the estimated grain yield of aromatic rice.
| Panicle status | Estimated grain yield (t/ha) | ||
| BRRI dhan34 | Sakkorkhora | Kalijira | |
| Healthy | 5.37 ± 0.09 | 5.30 ± 0.12 | 3.14 ± 0.34 |
| Diseased | 3.84 ± 0.11 (−28.51) | 2.61 ± 0.27 (−50.66) | 1.63 ± 0.20 (−48.04) |
| Calculated t value | 3.15 | 27.12 | 9.51 |
| Tabulated t value | 2.13 | 2.13 | 2.13 |
| Significance level | a | b | b |
a Indicates the significance levels at 5%.
b Indicates the significance levels at 1%.
3.3 Transmission of P. oryzae from panicle base or branches to seeds
Transmission of P. oryzae from neck (panicle base) to seeds was determined by the frequency of isolation of blast fungus from freshly harvested seeds of test varieties (both filled and unfilled grains) collected from highly neck-blast-infected panicles. The occurrence frequencies of P. oryzae in freshly harvested seeds were very low compared to other seed-borne pathogens (Fig. 3). A maximum of 3.5% of diseased panicles infected by the blast pathogen was observed in unfilled grains (resp. 0.5% in filled grains). There was no blast pathogen (P. oryzae) isolated in filled grains of apparently healthy panicles.
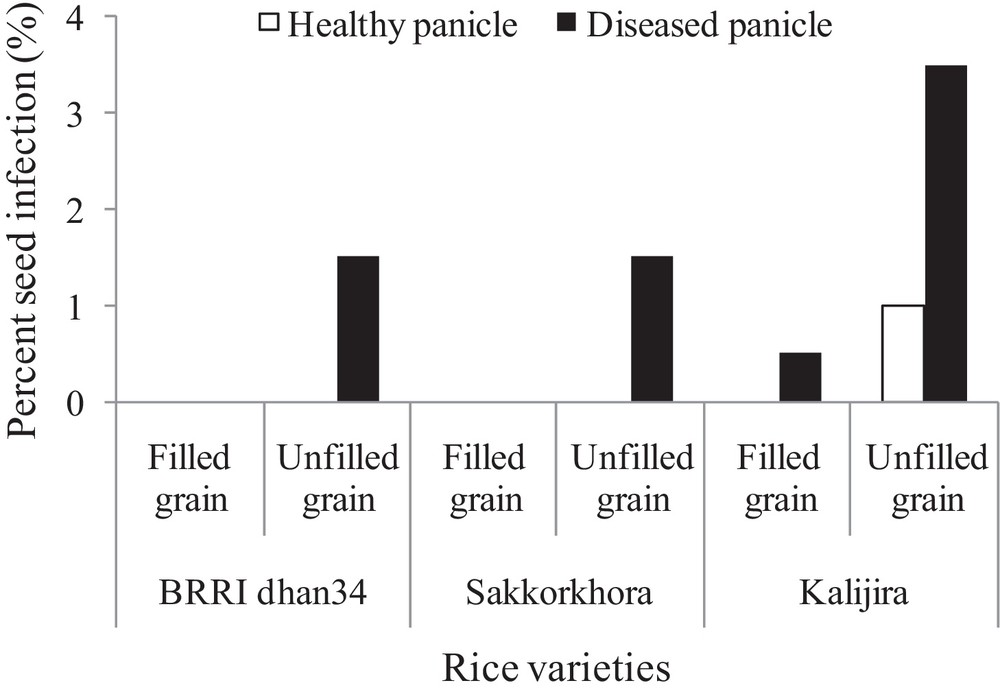
Frequencies (%) of association of seed-borne blast fungi (Pyricularia oryzae) with three freshly harvested cultivars of aromatic rice seeds.
3.4 Isolation and identification of seed-borne fungi other than P. oryzae
Frequencies of isolation of seed-borne fungi other than P. oryzae are presented in Table 7. Fusarium sp., Bipolaris oryzae, Curvularia lunata, Aspergillus niger, Alternaria sp. were the most frequent seed-borne pathogens in this study. The occurrence of these pathogens was more frequent in unfilled grains than in filled grains. In the same way, frequencies of these pathogens were higher in the grains of infected panicles compared to healthy panicles. On average, among the tested three aromatic varieties, seeds of Kalijira were more infected by the seed-borne fungi.
Frequencies (%) of association of fungi (other than Pyricularia oryzae) with three aromatic rice varieties.
| Variety | Panicle status | Fusarium sp. | Bipolaris sp. | Curvularia sp. | Sarocladium sp. | Trichoconis sp. | Alternaria sp. | ||||||
| Filled grain | Unfilled grain | Filled grain | Unfilled grain | Filled grain | Unfilled grain | Filled grain | Unfilled grain | Filled grain | Unfilled grain | Filled grain | Unfilled grain | ||
| BRRI dhan34 | Healthy panicle | 3.5 | 9.5 | 5.5 | 17.0 | 4.0 | 2.5 | 1.0 | 2.5 | 3.0 | 2.5 | 0.0 | 1.5 |
| Diseased panicle | 4.0 | 9.5 | 8.0 | 18.0 | 8.0 | 9.5 | 2.0 | 5.5 | 5.5 | 16 | 0.0 | 1.5 | |
| Sakkorkhora | Healthy panicle | 1.5 | 5.5 | 12.0 | 5.5 | 3.0 | 9.5 | 1.5 | 2.0 | 8.5 | 16 | 0.0 | 0.0 |
| Diseased panicle | 10.5 | 13.5 | 15.0 | 26.5 | 4.5 | 11.0 | 4.0 | 5.0 | 9.0 | 15.5 | 0.5 | 0.0 | |
| Kalijira | Healthy panicle | 5.5 | 13.0 | 3.5 | 7.5 | 11.5 | 18.0 | 0.0 | 1.0 | 4.5 | 6.5 | 0.5 | 1.5 |
| Diseased panicle | 8.0 | 19.0 | 4.5 | 7.5 | 15.0 | 21.0 | 0.0 | 5.0 | 21.0 | 15.0 | 2.0 | 3.0 |
4 Discussion
The differences in the blast infection levels observed among the tested varieties were most likely due to genetic differences. The soil properties, cultural management and also microenvironments were similar in healthy and diseased panicle cases, and therefore the differences found in agronomic and quality traits between two conditions (diseased and healthy) could be attributed to the effect of the disease, which causes the reduction of the 1000-grain weight and of the individual panicle weight and increases grain sterility percentages. The grain size was also affected by the disease, but it was influenced by the variety. Disease conditions were found to reduce the estimated grain yield up to 50.66% compared to healthy conditions. However, the magnitude of the reduction was depending on the variety, reflecting a variation in the genotype's response to the blast disease. Yield losses due to rice blast reported in the literature exceed 50% over large areas in some parts of world [1], and they have been ascribed to several different reasons. In Nepal, a 10–20% yield reduction in susceptible varieties is reported due to this disease, but in severe cases it goes up to an 80% yield reduction [20]. Koutroubas et al. [16] found that blast disease reduced the accumulation and remobilization of the pre-anthesis assimilates to the grains of rice. The associations between blast severity and grain yield reported in the literature are rather variable, since they are dependent on the environment and on the level of disease infection. Torres and Teng [22] reported that neck blast infection was less reduced by collar infections.
Katsube and Koshimizu [23] determined the relationship between the percentages of yield loss and neck blast disease incidence as Y = 0.57 X, where Y = percentage of yield loss and X = percentage of neck blast incidence. In this study, we found the average neck blast disease incidence and the estimated yield loss percentage to be 74.6 and 42.40%, respectively. We validated our estimated yield loss data using the above model and found a yield loss of 42.52% (yield loss percentage Y = 0.57 × 74.6 = 42.52). Our estimated yield loss was in full agreement with the above findings.
The disease caused a reduction in grain weight compared to the grains of healthy panicles. These results could be explained taking into account the specific nature of blast disease in rice. The infection of plants during the generative growth stages mainly results in panicle or neck infections that may cause the necrosis of the plant neck and an incomplete grain filling. Candole et al. [24] reported that rough rice from blast-infected panicles was drier and thinner than on blast-free panicles. The values of the grain length and of the grain length-to-width ratio were found to be similar in diseased and healthy panicles. One explanation for this could be that grain size and shape, as it is characterized by the grain length and the grain length-to-width ratio, are traits that are little affected by the environment [25]. It has been reported that grain shape is simultaneously controlled by the genes expressed in the triploid endosperm, the cytoplasm, the embryo, and the maternal tissues [26,27].
Although many researchers reported that there is a strong correlation between panicle symptoms and seed infection, it is not clear whether seeds (spikelets) become infected from the panicle base or branches. Puri et al. [28] found that some rice lines had no seed infection, even though they got high neck blast infection. Kakoly et al. [29] found a seed infection percentage of zero in P. grisea when seeds were collected from heavily neck-blast-infected fields and tested by blotter methods. Chadha and Gopalakrishna [30] did a PCR-based assay for detecting M. grisea in rice seed lots and found infestation rates as low as 0.2%. Our results were partially in agreement with the above findings. In our study, we found as much as 3.5% of infected unfilled grain from diseased panicles. Filled grains of healthy panicles were free from blast pathogen, though both diseased and healthy panicles were collected from the same fields. In Bangladesh, we still do not have any standardized data to certify seed lots against the blast pathogen. But we have general data on the acceptance label of seed-borne pathogen. It is 5%, 10%, and 20% for breeder, foundation, and certified seeds, respectively. The acceptance label varied from country to country and pathogen to pathogen.
We found that unfilled grains had a higher degree of infection by P. oryzae than filled grain. This was also observed by Singh and Mathur [31] for the infection of rice by Sarocladium oryzae. Early and severe infection of panicles by the fungus may have been the cause of most of these unfilled grains. Manandhar et al. [20] reported that less infection by P. oryzae was found in the samples that were subjected to winnowing and sun dried. The reason is that the unfilled grains, which are more likely to be infected, are easily removed by winnowing, and that sun drying may reduce the inoculum present on the seed's surface. Suzuki [32] reported that severe infection by P. oryzae may prevent rice seeds from developing completely. Occurrence frequencies of other seed-borne fungi such as Fusarium sp., Bipolaris sp., Curvularia sp., Sarocladium sp., Tricoconis sp., Alternaria sp. were also high in unfilled grains compared to filled grains. Du et al. [33] also found these pathogens to be seed-borne ones associated with rice seeds.
Another important observation made in the frame of this study was that seeds of healthy-looking panicles (either filled or unfilled grains) were less importantly attacked by seed-borne fungi than those from diseased panicles. Not only P. oryzae but also other seed-borne fungi followed the same pattern. It was maybe due to the strong positive relationship between neck blast severity and grain sterility percentages that were enhanced by the degrees of seed infection, because we found more seed-borne fungal infection in sterile grains compared to filled ones.
5 Conclusion
Blast is one of the most destructive and cosmopolitan disease with great potential threat for successful rice production, especially that of aromatic rice. To summarize, this study pointed three important things:
- • there is a tendency of aromatic rice (both high-yielding and local rice) to be susceptible to neck blast in favourable environments, even though no leaf blast symptoms appear on leaves;
- • neck blast disease reduces grain quality and yield traits, but it depends on the disease's severity and on the variety's genetic make-up. Unfilled grains are the main source of seed-borne pathogens in the seed lot, especially blast pathogen;
- • the transmission of blast pathogen from the panicle's base or branches to the seeds is very poor.
These findings are important, especially concerning a seed certification programme in which seed lots are certified on the basis of field inspection. Finally, controlled experiments are needed to draw more general conclusions.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
The authors are highly grateful to the Integrated Agricultural Productivity Project in the Bangladesh Rice Research Institute (BRRI), funded by the Ministry of Agriculture, for providing all necessary support.


