1 Introduction
Anthropogenic (human-made) noise is a form of pollution that is contributing increasingly to natural soundscapes on a global scale [1,2]. Anthropogenic noise causes physiological, neurological and endocrinological problems, increased risk of coronary disease, cognitive impairment and sleep disruption of many mammals, reptiles, fishes and invertebrates taxa [3–6]. For example, behavioural impacts on fishes (sharks and teleost) include lower feeding frequencies [7], increased movement and displacement [8,9], impaired orientation in larvae [10], and slower reaction times [7]. However, anthropogenic noises produced by boat traffic could have some positive effects for marine aquaculture if these sounds could be used as a deterrent to repel the predators of oysters, mussels or other aquaculture taxa [11,12].
For marine aquaculture industries worldwide, depredation is an important and long-standing issue [11,13,14]. Farmers and researchers have been testing different techniques (e.g., magnetic or electric field, acoustic barrier, bubble curtain, cages) to reduce predation on marine aquaculture [11,14,15]. For example, mussels are a favourite prey item for diving ducks in Scotland and Canada, and farmers know that they can use boats to scare the birds. Ross et al. [12] reproduced the boat motor sound with an underwater playback system to avoid using real boats and to reduce costs, thus demonstrating an effective alternative deterrent when the farmers were absent. In French Polynesia, the pearl farming industry is the second economic pillar of the country, representing 60% of all exports [16]. However, the oyster farmers convey that the industry has been heavily impacted by predation by teleost fishes, rays and other taxa for several years. Although other animals, such as triggerfish, turtles, and puffer fish prey on the black-lipped pearl oyster Pinctada margaritifera in French Polynesia, the white-spotted eagle ray, Aetobatus ocellatus, is one of the most detrimental predators [15]. The eagle rays were not only eating a large quantity of oysters, but they were also destroying oyster long-lines [15].
As many oyster farmers observed that the presence of a boat near long-lines disturbed rays, in the present study, we focussed on the use of an acoustic system to deter eagle rays from oyster farms. Specifically, our field experiments investigated behavioural responses of eagle rays to different sounds: boat motor's sound, white noise, and three single-frequency tones (40 Hz, 600 Hz, 1 kHz).
2 Methods
The present study was conducted on adults of white-spotted eagle rays A. ocellatus (range size: 1.0 to 1.3 m) foraging under anchored boats on the north coast of Moorea Island, French Polynesia (17°29′23.09′′S 149°51′6.06′′W) from October 2012 to November 2013. The acoustic system used in field experiments consisted of a 7 V battery (Yuasa NP2.1-1) connected to an amplifier (Formula F-102, CA, USA), connected to an mp3 player (Sansa Clip1, SanDisk, Milpitas, CA, USA). All the equipment was set up in a waterproof casing. The mp3 player was connected to an underwater loudspeaker (UW-30, frequency response 0.1–10 kHz, University Sound, Columbus, USA) with a 4 m long cable to allow the placement of the speaker as close to the rays as possible (about 1 m above the animal – depth of site: 5 m). The acoustic system was surrounded by a buoy, for positive buoyancy and pushed in the field by an observer. The observer played sound as soon as a ray was spotted. The sounds were played only while the ray was in full view and foraging over sand. When an eagle ray finished foraging, was waiting for prey, or was resting, the experiment was not conducted. If the ray was not feeding, it left the area if an observer approached [15,C. Berthe, unpublished data].
First, a control experiment was conducted on nine individuals in order to determine if the presence of the acoustic system and/or observer elicited flight behaviour in white-spotted eagle rays when they foraged over sand. The system with the loudspeaker switched off was placed near the ray (about 1 m above the animal) without playing any sound, and the observer was positioned 5 m from the ray. The ray's behaviour was noted during 5 minutes (i.e. escape or continue to forage). Secondly, five sounds were tested: boat motor, white noise and three single-frequency tones (40 Hz, 600 Hz, 1 kHz which correspond to, respectively, low, medium and high frequency perceived by elasmobranch fishes – [17,18]). The artificial white noise (signal of which its frequencies are randomly distributed within a specified frequency range resulting in a constant power spectral density – sound waves extending over a wide frequency range: 10 Hz to 22 kHz) and the three single-frequency tones were created with Avisoft SasLab Pro [10,19]. Ten 30-second replicate playbacks per sound (white noise, 40 Hz, 600 Hz, 1 kHz) were constructed.
For the boat motor sound, recordings were made at a depth of 5 m outside the barrier reef of Moorea (at 2 km away from the nearest reef) using a hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier; sensitivity – 165 dB re 1 V/μPa; frequency range 2 Hz–10 kHz; High Tech Inc., Gulfport MS) and a solid-state recorder (Edirol R-09HR 16-bit recorder; sampling rate 44.1 kHz; Roland Systems Group, Bellingham WA). A boat with a 25 horse power Yamaha engine started 50 m from the hydrophone and drove past in a straight line for 100 m; passing the hydrophone at a closest distance of 10 m. The recorder was fully calibrated using pure sine wave signals generated in SAS Lab (Avisoft, Germany), played on an mp3 player, and measured in line with an oscilloscope [6,10]. Recordings were clipped into 30-s samples so that when boat was present a whole pass was sampled. Ten 30-s replicate playbacks were then constructed.
As ambient noise at the experimental site was never above 80 dB re 1 μPa (across all frequency bands between 10 and 2000 Hz – [19]), the sound level of our five sound composites was calibrated at, at least 90 dB re 1 μPa RMS by placing a hydrophone at 1 m of high-speaker (Fig. 1) to ensure that it was above the local ambient noise floor. To check the quality of boat sound emitted by the loudspeaker, sound pressure and particle acceleration were measured using the hydrophone (described above) and a M30 accelerometer (sensitivity 0–3 kHz, manufactured and calibrated by GeoSpectrum Technologies, Dartmouth, Canada; recorded on a laptop via a USB soundcard, MAYA44, ESI Audiotechnik GmbH, Leonberg, Germany). Acoustic analyses performed in MATLAB v2010a were described by Holles et al. [10] and Nedelec et al. [6].

Sound envelope of boat playback, white noise and the three single-frequency tones (40 Hz, 6000 Hz, 1000 Hz) during 30 seconds with all sound calibrated at, at least 90 dB re 1 μPa RMS.
Overall, 59 rays were tested using only one sound per ray (i.e. 10 rays per sound type and 9 rays for the control experiment), and the duration of sound exposure was 5 minutes. Two behavioural responses were recorded in real-time for each eagle ray: no reaction (i.e. ray continued to forage over sand) or escape behaviour (i.e. swam away from the foraging site at, at least, 50 m). Rays are easily distinguishable due to the individual pattern on their dorsal side (C. Berthe, unpublished data). Thus, no ray was tested twice during the experiment. A first χ2 test was conducted on the five sound types in order to assess if rays had a significant homogeneous or heterogeneous behaviour according to the tested sounds. Then, a second χ2 test was conducted to compare the number of rays showing escape behaviour with one sound type compared to the control test (i.e. five χ2 tests conducted) to determine whether a particular sound had a significant effect (escape behaviour) on the rays. The statistical analyses were conducted using R software v2.11.1 (R Development Core Team, 2010).
3 Results
During the control experiment, rays did not exhibit escape behaviour. The 9 tested rays did not move and continued to forage during the five-minute observation period (Fig. 2). This result confirmed that rays were not disturbed by the presence of an observer.
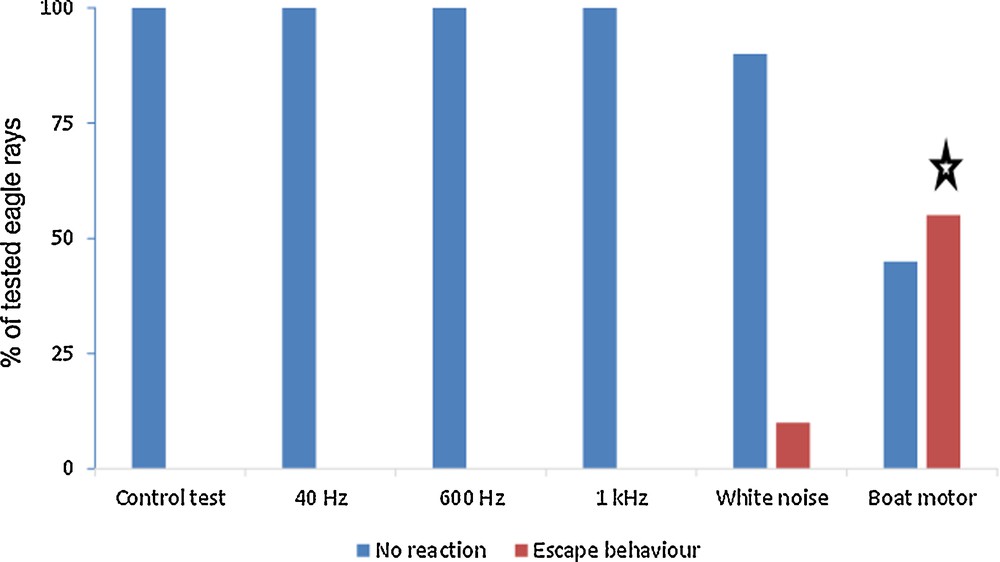
Eagle rays (Aetobatus ocellatus) behaviour related to the control test (without sound–switched-off loudspeaker) and the tested sounds: a boat motor sound, a white noise and three single-frequency tones (40 Hz, 600 Hz, 1 kHz). Ten rays were tested for each sound type and nine rays for control test. Two possible responses of eagle rays were observed: no reaction (i.e. continue to forage) or escape behaviour (i.e. swam away from the foraging site at, at least, 50 m). Stars indicate a significant difference between the number of rays showing escape behaviour with one sound type and the control test (χ2 test – P < 0.05).
During the sound tests, rays exhibited significant heterogeneous behaviour depending on the sound tested (χ20.05,4 = 18.2, P < 0.001, Fig. 2). For example, 60% of rays swam at least 50 m away from the foraging site when the boat motor sound was played. Among the six rays that fled, four swan away in the 10 first seconds, 1 between 1 and 4 min and 1 during the last minute of boat playback. On the contrary, no ray exhibited escape behaviour when the 600 Hz sound was played. Thus, none of the sounds at 40 Hz, 600 Hz, 1 kHz or the white noise resulted in altered ray behaviour (i.e. comparison between sound and control tests – χ20.05,1 < 3.84, P > 0.05), and the rays continued to forage over sand (Fig. 2). In contrast, the sounds of the boat motor elicited significantly more escape behaviours during foraging activity (χ20.05,1 = 37.7, P < 0.001). But, no significant relationship was highlighted between the sound intensities of boat (between 90 and 120 dB re 1 μPa RMS) and the time for the eagle rays to escape (i.e. 66% of rays exhibited an escape behaviour during the 10 first seconds, corresponding to the lowest sound intensities of boat noise – 90 and 98 dB re 1 μPa RMS, Fig. 2).
4 Discussion
Our study showed the negative effect of boat noises on the foraging activity of eagle rays. Thus, 60% of the rays tested stopped foraging and fled the area when the boat motor sound was played (Fig. 2). Local farmers in French Polynesia observed that eagle rays exhibited escape behaviour when a boat approached, and our findings confirmed this observation. Moreover, our field observations allowed us to identify that 66% of eagle rays fled between 1 to 10 seconds after the beginning of boat sound (sound characterized by low frequencies – < 350 Hz–at the beginning of recording). These field observations are consistent with those of Casper [17], who demonstrated that elasmobranches mainly perceived the particle motion, which is more prevalent at low frequencies. Sharks are thought to be attracted to low frequencies because stressed preys emit low frequency sounds [17,18,20]. Eagle rays are a favourite prey item of the hammerhead shark [21] and could be more reactive to low frequencies to avoid shark's predation. However, our study showed no escape behaviour when only a single-frequency tone was played (40 Hz, 600 Hz or 1 kHz). Therefore, it may not be just a specific low frequency (as the 40 Hz tested in the present study) that disturbs the eagle rays during their foraging activity, but rather a combination of sound low frequencies. This hypothesis should be validated by future studies on a whole group of rays, as the behavior, such as escape, is often dependent of the school density [22] and that a social organization was described in spotted eagle ray (same genus Aetobatus) [23].
Worldwide, a lot of research has been done on aquaculture predation [12,24–26], but aside from use of boat noise, few reliable and affordable solutions have been found [12,27]. Our study confirmed that anthropogenic noise, such as that produced by boat traffic could negatively affect the white-spotted eagle ray, similar to other marine species as indicated in earlier studies [1–3,6]. This negative effect on eagle rays could be used by humans to benefit marine aquaculture if such sounds deter the predators of pearl oysters [11,12]. Further experiments must be conducted in situ, near oyster long-lines and over a long-term period in order to determine if an underwater playback system could be used to efficiently deter eagle rays from oyster farms without negative effects on the growth of oysters and on the quality of the black pearls. Moreover, Newborough et al. [28] showed the problem of habituation effects with acoustic repulse systems that remove cetaceans from fishnets. To reduce the likelihood of habituation of eagle rays, local farmers would need to use different recordings, including sounds from different boat types or by using irregular temporal and spatial patterns when playing back sounds. Overall, our study showed, for the first time, that boat noise is detrimental to the eagle rays, but this negative effect could be used by Human to deter the rays from oyster farms.
Acknowledgements
This research was supported by a grant from the “Direction des ressources marines et minières” of French Polynesia. The authors would like to acknowledge Cédrik Lo for his help to build this study.


